Multimodal Imaging of Diabetic Retinopathy: Insights from Optical Coherence Tomography Angiography and Adaptive Optics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ophthalmic Examination
2.3. Image Processing
2.3.1. Optical Coherence Tomography Angiography
2.3.2. Adaptive Optics
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DR | diabetic retinopathy |
| NPDR | non-proliferative diabetic retinopathy |
| PDR | proliferative diabetic retinopathy |
| IRMAs | intraretinal microvascular abnormalities |
| DME | diabetic macular edema |
| DMI | diabetic macular ischemia |
| OCT | optical coherence tomography |
| OCTA | optical coherence tomography angiography |
| FA | fluorescein angiography |
| FAZ | foveal avascular zone |
| BCVA | best-corrected visual acuity |
| VD | vessel density |
| VLD | vessel length density |
| FD | fractal dimension |
| AO | adaptive optics |
| TVD | total vessel diameter |
| LD | lumen diameter |
| WT | mean wall thickness |
| WLR | wall-to-lumen ratio |
| WCSA | cross-sectional area of the vascular wall |
| AO-FC | adaptive optics fundus camera |
| SCP | superficial capillary plexus |
| ILM | internal limiting membrane |
| IPL | inner plexiform layer |
| INL | inner nuclear layer |
| ROIs | regions of interest |
| anti-VEGF | anti-vascular endothelial growth factor |
| PRP | panretinal photocoagulation |
| PPV | pars plana vitrectomy |
References
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of Quantitative Optical Coherence Tomography Angiography Metrics in Patients with Diabetes. Sci. Rep. 2017, 7, 2575. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, D.; Tang, Z.; Ng, D.S.; Cheung, C.Y. Optical coherence tomography angiography in diabetic retinopathy: An updated review. Eye 2021, 35, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Waheed, N.K.; Rosen, R.B.; Jia, Y.; Munk, M.R.; Huang, D.; Fawzi, A.; Chong, V.; Nguyen, Q.D.; Sepah, Y.; Pearce, E. Optical coherence tomography angiography in diabetic retinopathy. Prog. Retin. Eye Res. 2023, 97, 101206. [Google Scholar] [CrossRef]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Fortun, J.A. Diabetic Macular Edema: Current Understanding, Pharmacologic Treatment Options, and Developing Therapies. Asia Pac. J. Ophthalmol. 2018, 7, 28–35. [Google Scholar] [CrossRef]
- Sim, D.A.; Keane, P.A.; Fung, S.; Karampelas, M.; Sadda, S.R.; Fruttiger, M.; Patel, P.J.; Tufail, A.; Egan, C.A. Quantitative analysis of diabetic macular ischemia using optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 417–423. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Spaide, R.F. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization With Antiangiogenic Therapy for Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Z.; Shi, J.; Ran, A.; Tang, F.; Tang, Z.; Lok, J.; Szeto, S.; Chan, J.; Yip, F.; et al. A Multitask Deep-Learning System for Assessment of Diabetic Macular Ischemia on Optical Coherence Tomography Angiography Images. Retina 2022, 42, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Klancnik, J.M.; Jr Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Weinhaus, R.S.; Burke, J.M.; Delori, F.C.; Snodderly, D.M. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Exp. Eye Res. 1995, 61, 1–16. [Google Scholar] [CrossRef]
- Mendis, K.R.; Balaratnasingam, C.; Yu, P.; Barry, C.J.; McAllister, I.L.; Cringle, S.J.; Yu, D.Y. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5864–5869. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Jia, Y.; Gao, S.S.; Bailey, S.T.; Lauer, A.K.; Flaxel, C.J.; Wilson, D.J.; Huang, D. Optical coherence tomography angiography features of diabetic retinopathy. Retina 2015, 35, 2371–2376. [Google Scholar] [CrossRef]
- Rosen, R.B.; Andrade Romo, J.S.; Krawitz, B.D.; Mo, S.; Fawzi, A.A.; Linderman, R.E.; Carroll, J.; Pinhas, A.; Chui, T.Y.P. Earliest Evidence of Preclinical Diabetic Retinopathy Revealed Using Optical Coherence Tomography Angiography Perfused Capillary Density. Am. J. Ophthalmol. 2019, 203, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Schottenhamml, J.; Moult, E.M.; Ploner, S.; Lee, B.; Novais, E.A.; Cole, E.; Dang, S.; Lu, C.D.; Husvogt, L.; Waheed, N.K.; et al. An automatic, intercapillary area-based algorithm for quantifying diabetes-related capillary dropout using optical coherence tomography angiography. Retina 2016, 36 (Suppl. 1), S93–S101. [Google Scholar] [CrossRef]
- Yasin Alibhai, A.; Moult, E.M.; Shahzad, R.; Rebhun, C.B.; Moreira-Neto, C.; McGowan, M.; Lee, D.; Lee, B.; Baumal, C.R.; Witkin, A.J.; et al. Quantifying Microvascular Changes Using OCT Angiography in Diabetic Eyes without Clinical Evidence of Retinopathy. Ophthalmol. Retina 2018, 2, 418–427. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Bonini Filho, M.A.; Baumal, C.R.; Reichel, E.; Rogers, A.; Witkin, A.J.; Duker, J.S.; Waheed, N.K. Evaluation of Preretinal Neovascularization in Proliferative Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 115–119. [Google Scholar] [CrossRef]
- Hwang, T.S.; Hagag, A.M.; Wang, J.; Zhang, M.; Smith, A.; Wilson, D.J.; Huang, D.; Jia, Y. Automated Quantification of Nonperfusion Areas in 3 Vascular Plexuses With Optical Coherence Tomography Angiography in Eyes of Patients With Diabetes. JAMA Ophthalmol. 2018, 136, 929–936. [Google Scholar] [CrossRef]
- Salz, D.A.; de Carlo, T.E.; Adhi, M.; Moult, E.; Choi, W.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Fujimoto, J.G.; Waheed, N.K. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared With Fluorescein Angiography and Normal Eyes. JAMA Ophthalmol. 2016, 134, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Pearce, E.; Fenner, B.; Sen, P.; Chong, V.; Sivaprasad, S. Looking Ahead: Visual and Anatomical Endpoints in Future Trials of Diabetic Macular Ischemia. Ophthalmologica 2021, 244, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Fawzi, A.; Teo, K.Y.; Fukuyama, H.; Sen, S.; Tsai, W.S.; Sivaprasad, S. Diabetic macular ischaemia—A new therapeutic target? Prog. Retin. Eye Res. 2022, 89, 101033. [Google Scholar] [CrossRef]
- Bresnick, G.H.; Condit, R.; Syrjala, S.; Palta, M.; Groo, A.; Korth, K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch. Ophthalmol. 1984, 102, 1286–1293. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Inoue, M.; Ahn, S.; McCann, J.; Dhrami-Gavazi, E.; Yannuzzi, L.A.; Freund, K.B. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology 2016, 123, 2352–2367. [Google Scholar] [CrossRef]
- Safi, H.; Anvari, P.; Naseri, D.; Shenazandi, H.; Kazemi, P.; Farsi, P.; Jafari, S.; Sedaghat, A.; Yaseri, M.; Ghasemi Falavarjani, K. Quantitative analysis of optical coherence tomography angiography metrics in diabetic retinopathy. Ther. Adv. Ophthalmol. 2020, 12, 2515841419897459. [Google Scholar] [CrossRef] [PubMed]
- Krawitz, B.D.; Phillips, E.; Bavier, R.D.; Mo, S.; Carroll, J.; Rosen, R.B.; Chui, T.Y.P. Parafoveal Nonperfusion Analysis in Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2018, 7, 4. [Google Scholar] [CrossRef]
- Sim, D.A.; Keane, P.A.; Zarranz-Ventura, J.; Fung, S.; Powner, M.B.; Platteau, E.; Bunce, C.V.; Fruttiger, M.; Patel, P.J.; Tufail, A.; et al. The effects of macular ischemia on visual acuity in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2353–2360. [Google Scholar] [CrossRef]
- Custo Greig, E.; Brigell, M.; Cao, F.; Levine, E.S.; Peters, K.; Moult, E.M.; Fujimoto, J.G.; Waheed, N.K. Macular and Peripapillary Optical Coherence Tomography Angiography Metrics Predict Progression in Diabetic Retinopathy: A Sub-analysis of TIME-2b Study Data. Am. J. Ophthalmol. 2020, 219, 66–76. [Google Scholar] [CrossRef]
- Han, R.; Gong, R.; Liu, W.; Xu, G. Optical coherence tomography angiography metrics in different stages of diabetic macular edema. Eye Vis. 2022, 9, 14. [Google Scholar] [CrossRef]
- Okumichi, H.; Itakura, K.; Yuasa, Y.; Fukuto, A.; Kiuchi, Y. Foveal structure in nanophthalmos and visual acuity. Int. Ophthalmol. 2021, 41, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Say, E.A.; Khoo, C.T.; Higgins, T.P.; Magrath, G.; Ferenczy, S.; Shields, C.L. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef]
- Shiihara, H.; Terasaki, H.; Sonoda, S.; Kakiuchi, N.; Shinohara, Y.; Tomita, M.; Sakamoto, T. Objective evaluation of size and shape of superficial foveal avascular zone in normal subjects by optical coherence tomography angiography. Sci. Rep. 2018, 8, 10143. [Google Scholar] [CrossRef]
- Krawitz, B.D.; Mo, S.; Geyman, L.S.; Agemy, S.A.; Scripsema, N.K.; Garcia, P.M.; Chui, T.Y.P.; Rosen, R.B. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vis. Res. 2017, 139, 177–186. [Google Scholar] [CrossRef]
- You, Q.; Freeman, W.R.; Weinreb, R.N.; Zangwill, L.; Manalastas, P.I.C.; Saunders, L.J.; Nudleman, E. Reproducibility of vessel density measurement with optical coherence tomography angiography in eyes with and without retinopathy. Retina 2017, 37, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 66008. [Google Scholar] [CrossRef]
- Hirano, T.; Kitahara, J.; Toriyama, Y.; Kasamatsu, H.; Murata, T.; Sadda, S. Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br. J. Ophthalmol. 2019, 103, 216–221. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Falavarjani, K.G.; Tepelus, T.C.; Sadda, S.R. Quantitative Comparison of Swept-Source and Spectral-Domain OCT Angiography in Healthy Eyes. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 385–391. [Google Scholar] [CrossRef]
- Szewczuk, A.; Zaleska-Żmijewska, A.; Dziedziak, J.; Szaflik, J.P. Clinical Application of Adaptive Optics Imaging in Diagnosis, Management, and Monitoring of Ophthalmological Diseases: A Narrative Review. Med. Sci. Monit. 2023, 29, e941926. [Google Scholar] [CrossRef]
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive optics: Principles and applications in ophthalmology. Eye 2021, 35, 244–264. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.M.; Dąbrowska, A.; Szaflik, J.P. Adaptive Optics (rtx1) High-Resolution Imaging of Photoreceptors and Retinal Arteries in Patients with Diabetic Retinopathy. J. Diabetes Res. 2019, 2019, 9548324. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Dikland, F.A.; van Bakel, R.; Andrade De Jesus, D.; Sánchez Brea, L.; Klein, S.; van Walsum, T.; Rossant, F.; Farías, D.C.; Grieve, K.; et al. Adaptive optics ophthalmoscopy: A systematic review of vascular biomarkers. Surv. Ophthalmol. 2022, 67, 369–387. [Google Scholar] [CrossRef]
- Luo, T.; Gast, T.J.; Vermeer, T.J.; Burns, S.A. Retinal Vascular Branching in Healthy and Diabetic Subjects. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2685–2694. [Google Scholar] [CrossRef]
- Ueno, Y.; Iwase, T.; Goto, K.; Tomita, R.; Ra, E.; Yamamoto, K.; Terasaki, H. Association of changes of retinal vessels diameter with ocular blood flow in eyes with diabetic retinopathy. Sci. Rep. 2021, 11, 4653. [Google Scholar] [CrossRef]
- Untracht, G.R.; Durkee, M.S.; Zhao, M.; Kwok-Cheung Lam, A.; Sikorski, B.L.; Sarunic, M.V.; Andersen, P.E.; Sampson, D.D.; Chen, F.K.; Sampson, D.M. Towards standardising retinal OCT angiography image analysis with open-source toolbox OCTAVA. Sci. Rep. 2024, 14, 5979. [Google Scholar] [CrossRef]
- Mehta, N.; Braun, P.X.; Gendelman, I.; Alibhai, A.Y.; Arya, M.; Duker, J.S.; Waheed, N.K. Repeatability of binarization thresholding methods for optical coherence tomography angiography image quantification. Sci. Rep. 2020, 10, 15368. [Google Scholar] [CrossRef]
- Couturier, A.; Rey, P.A.; Erginay, A.; Lavia, C.; Bonnin, S.; Dupas, B.; Gaudric, A.; Tadayoni, R. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascularendothelial growth factor. Ophthal-Mology 2019, 126, 1685–1694. [Google Scholar][Green Version]
- Kim, Y.J.; Yeo, J.H.; Son, G.; Kang, H.; Sung, Y.S.; Lee, J.Y.; Kim, J.G.; Yoon, Y.H. Efficacy of intravitreal Aflibercept injection for Improvement of retinal Nonperfusion in diabetic retinopathy (AFFINITY study). BMJ Open Diabetes Res. Care 2020, 8, e001616. [Google Scholar][Green Version]
- Massengill, M.T.; Cubillos, S.; Sheth, N.; Sethi, A.; Lim, J.I. Response of Diabetic Macular Edema to Anti-VEGF Medications Correlates with Improvement in Macular Vessel Architecture Measured with OCT Angiography. Ophthalmol. Sci. 2024, 4, 100378. [Google Scholar] [CrossRef]
- Abdelhalim, A.S.; Abdelkader, M.; Mahmoud, M.S.E.; Mohamed Mohamed, A.A. Macular vessel density before and after panretinal photocoagulation in patients with proliferative diabetic retinopathy. Int. J. Retina Vitreous 2022, 8, 21. [Google Scholar] [CrossRef]
- Chatziralli, I.; Dimitriou, E.; Agapitou, C.; Kazantzis, D.; Kapsis, P.; Morogiannis, N.; Kandarakis, S.; Theodossiadis, G.; Theodossiadis, P. Optical Coherence Tomography Angiography Changes in Macular Area in Patients with Proliferative Diabetic Retinopathy Treated with Panretinal Photocoagulation. Biomedicines 2023, 11, 3146. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, A.A.; Fayed, A.E.; Linsenmeier, R.A.; Gao, J.; Yu, F. Improved Macular Capillary Flow on Optical Coherence Tomography Angiography After Panretinal Photocoagulation for Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 2019, 206, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nagaoka, T.; Sato, E.; Yoshida, A. Effect of panretinal photocoagulation on choroidal circulation in the foveal region in patients with severe diabetic retinopathy. Br. J. Ophthalmol. 2008, 92, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, Y.; Liu, D.; Stewart, J.M. Optical Coherence Tomography Angiography Assessment of Macular Choriocapillaris and Choroid Following Panretinal Photocoagulation in a Diverse Population with Advanced Diabetic Retinopathy. Asia Pac. J. Ophthalmol. 2020, 10, 203–207. [Google Scholar] [CrossRef]
- Lorusso, M.; Milano, V.; Nikolopoulou, E.; Ferrari, L.M.; Cicinelli, M.V.; Querques, G.; Ferrari, T.M. Panretinal Photocoagulation Does Not Change Macular Perfusion in Eyes with Proliferative Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 174–178. [Google Scholar] [CrossRef]
- Stefánsson, E. Physiology of vitreous surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 147–163. [Google Scholar] [CrossRef]
- Petrou, P., Sr.; Angelidis, C.D.; Andreanos, K.; Kanakis, M.; Kandarakis, S.; Karamaounas, A.; Papakonstantinou, E.; Mamas, N.; Droutsas, K.; Georgalas, I. Reduction of Foveal Avascular Zone After Vitrectomy Demonstrated by Optical Coherence Tomography Angiography. Cureus 2021, 13, e13757. [Google Scholar] [CrossRef]
- Russell, J.F.; Scott, N.L.; Townsend, J.H.; Shi, Y.; Gregori, G.; Crane, A.M.; Flynn, H.W., Jr.; Sridhar, J.; Rosenfeld, P.J. Wide-Field Swept-Source Optical Coherence Tomography Angiography of Diabetic Tractional Retinal Detachments before and after Surgical Repair. Retina 2021, 41, 1587–1596. [Google Scholar] [CrossRef]
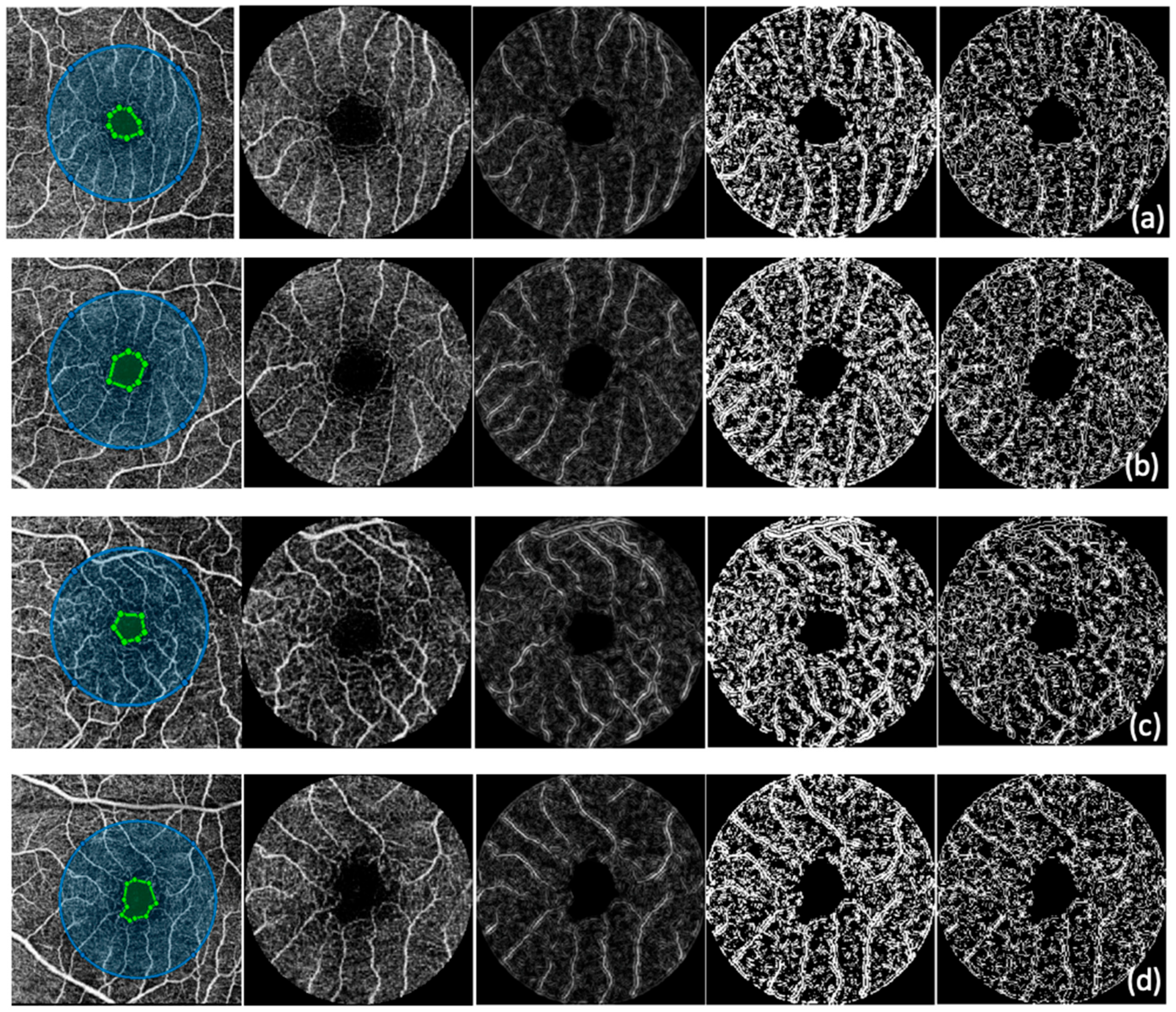

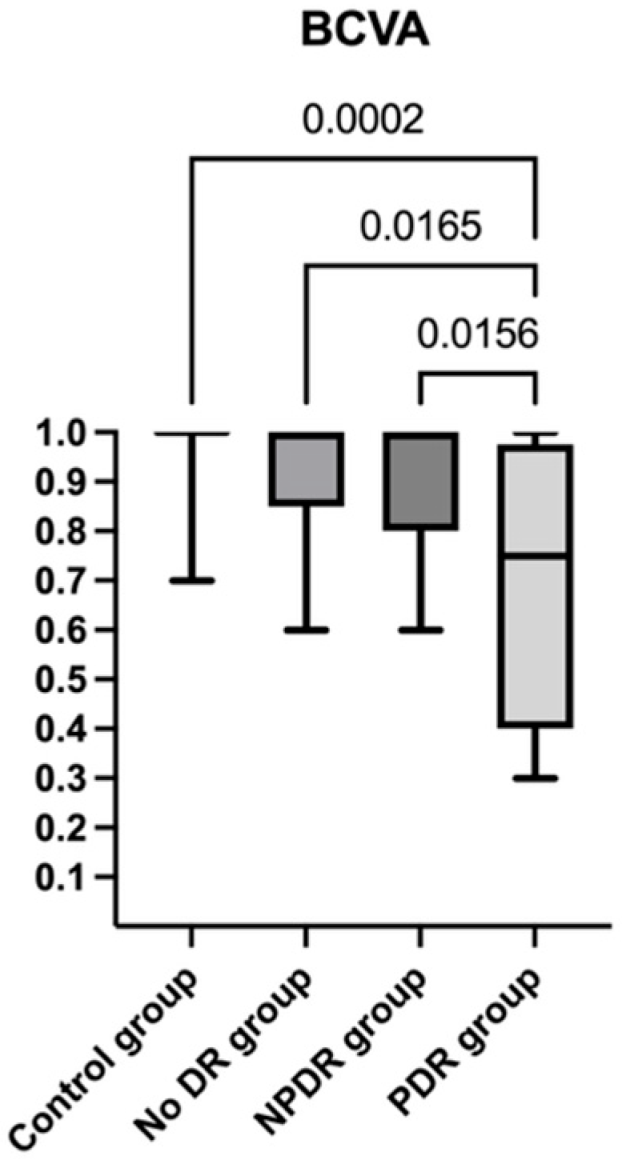
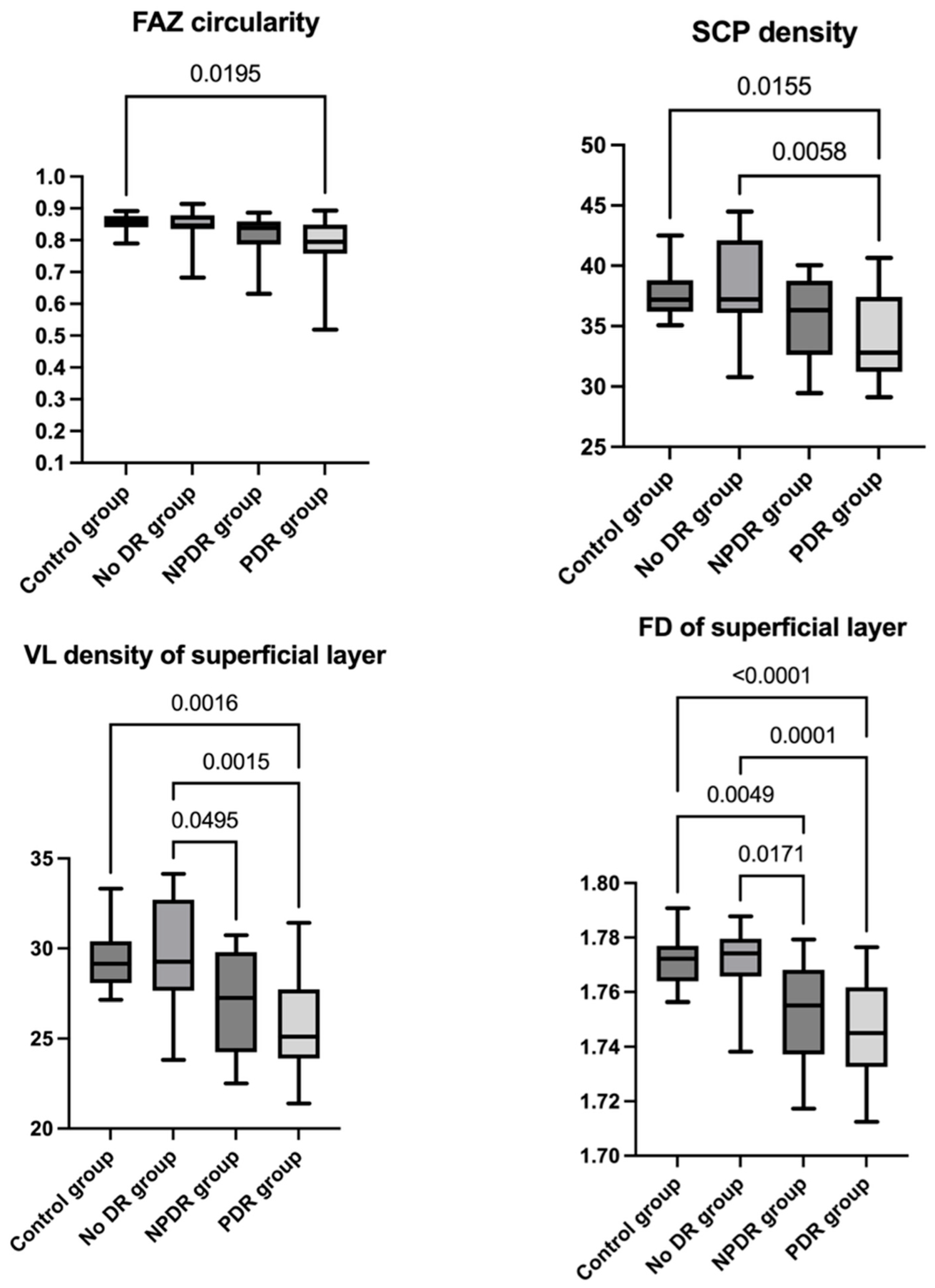
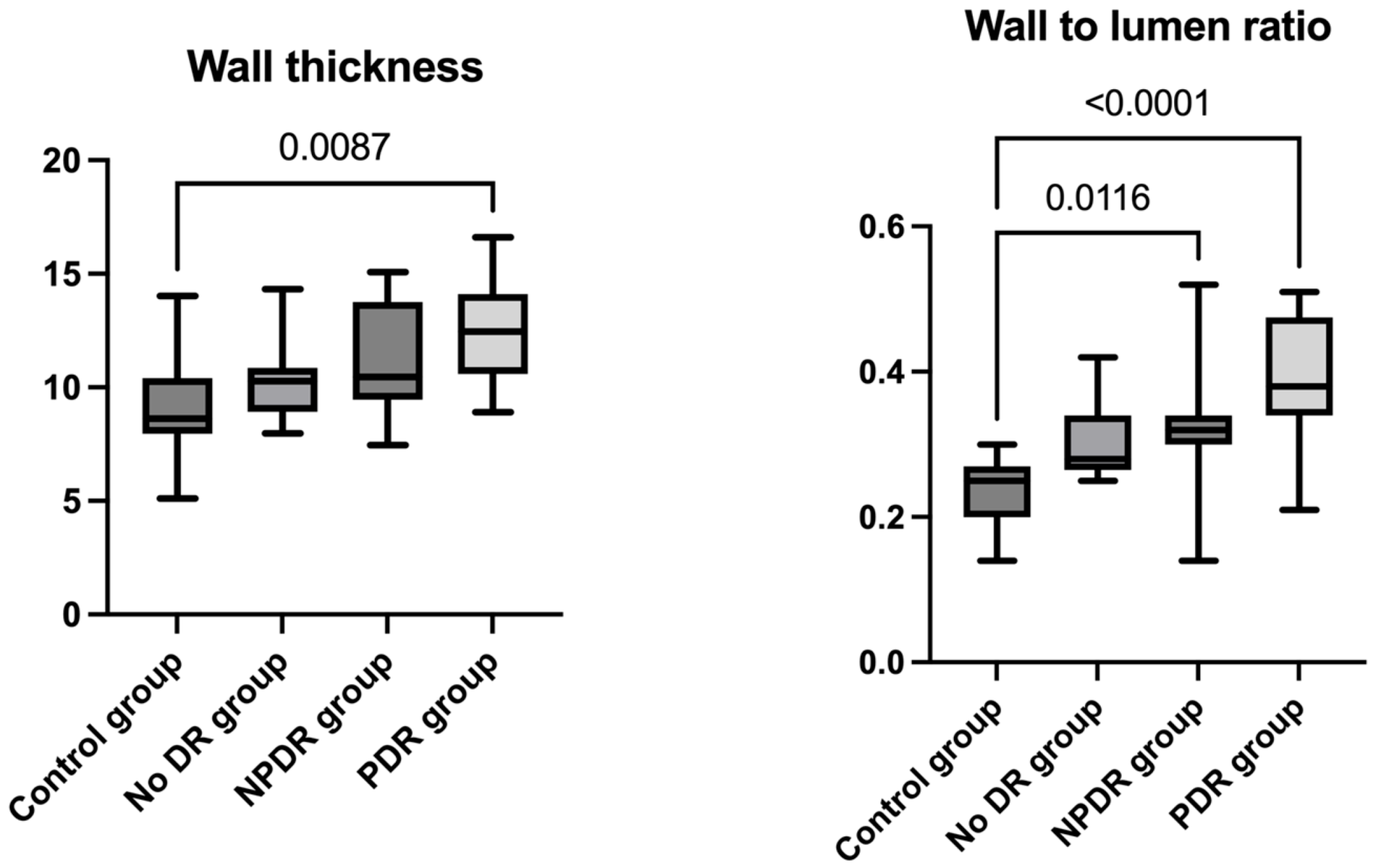
| Control Group | No DR Group | NPDR Group | PDR Group | |
|---|---|---|---|---|
| Number of patients (eyes) | 17 | 14 | 18 | 20 |
| Sex (male/female) | 11/6 | 5/9 | 10/8 | 15/5 |
| Age (years) | 52.29 ± 13.13 | 54.07 ± 21.36 | 50.27 ± 12.31 | 56 ± 13.33 |
| Diabetes type (type I/type II) | - | 4/10 | 3/15 | 5/15 |
| Insulin dependence (independent/dependent) | - | 4/10 | 11/7 | 11/9 |
| Diabetes duration (years) | - | 12.11 ± 6.81 | 15.55 ± 6.80 | 15.55 ± 9.25 |
| Eye laterality (right/left) | 7/10 | 6/8 | 11/7 | 10/10 |
| Axial length (mm) | 23.68 ± 1.00 | 23.89 ± 0.79 | 23.34 ± 0.38 | 23.42 ± 0.74 |
| Control Group | No DR Group | NPDR Group | PDR Group | |
|---|---|---|---|---|
| FAZ area of superficial layer (mm2) | 0.226 ± 0.100 | 0.214 ± 0.060 | 0.248 ± 0.084 | 0.248 ± 0.087 |
| FAZ perimeter of superficial layer (mm) | 1.774 ± 0.446 | 1.772 ± 0.293 | 1.933 ± 0.354 | 1.958 ± 0.378 |
| FAZ circularity index of superficial layer | 0.853 ± 0.028 | 0.845 ± 0.054 | 0.816 ± 0.062 | 0.792 ± 0.081 |
| SCP density (%) | 37.693 ± 1.950 | 38.274 ± 3.799 | 35.685 ± 3.455 | 34.338 ± 3.670 |
| VL density of superficial layer (%) | 29.385 ± 1.711 | 29.586 ± 3.020 | 27.049 ± 2.837 | 25.971 ± 2.992 |
| FD superficial layer | 1.771 ± 0.009 | 1.770 ± 0.013 | 1.753 ± 0.018 | 1.745 ± 0.018 |
| TVD (μm) | 100.341 ± 20.234 | 89.173 ± 14.913 | 96.458 ± 26.842 | 88.237 ± 23.184 |
| LD (μm) | 81.085 ± 16.323 | 68.542 ± 12.510 | 73.820 ± 25.796 | 60.787 ± 17.906 |
| WT (μm) | 9.670 ± 2.676 | 10.323 ± 1.737 | 11.042 ± 2.256 | 12.08 ± 2.610 |
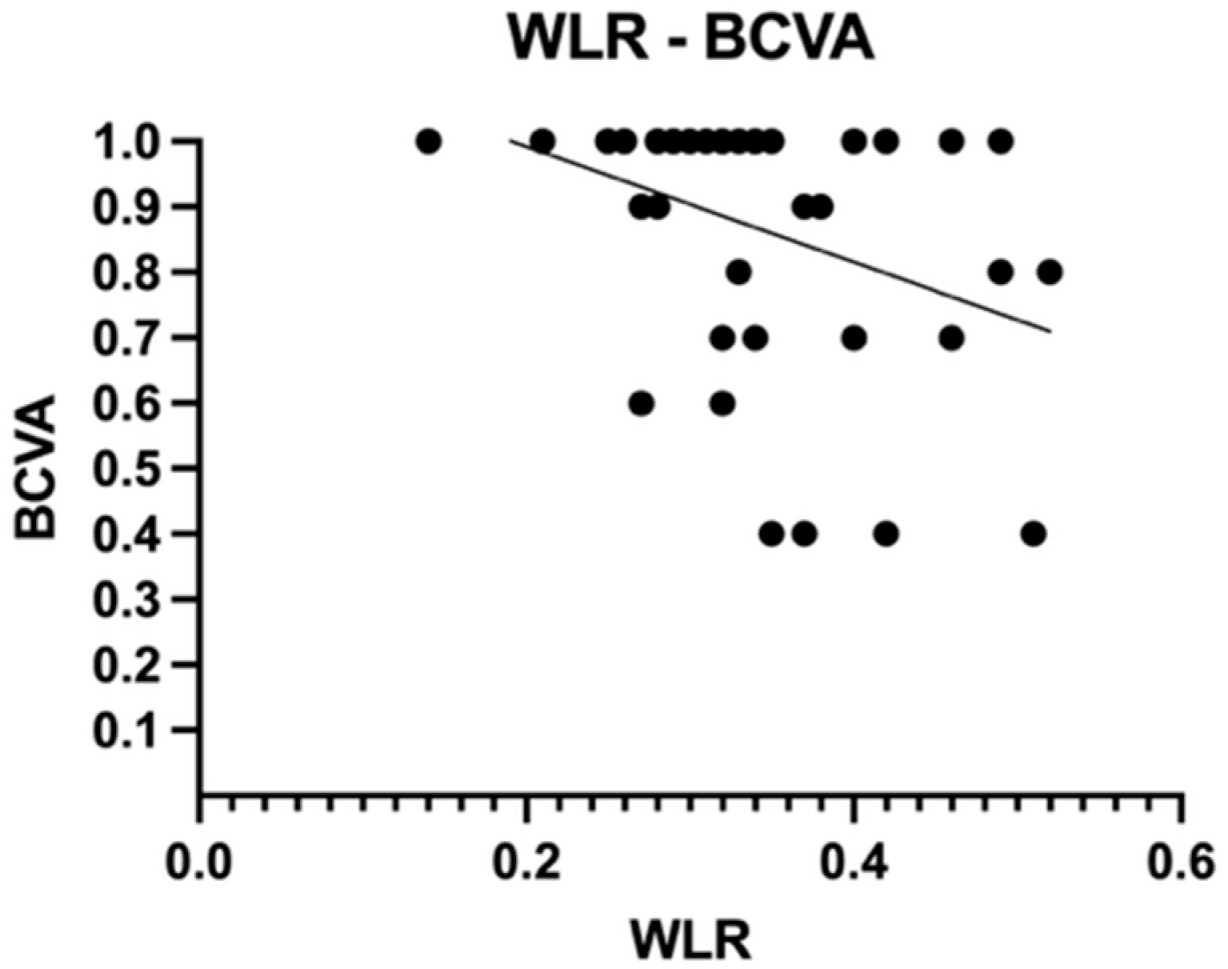
| WLR | 0.237 ± 0.048 | 0.305 ± 0.054 | 0.320 ± 0.080 | 0.390 ± 0.090 |
| WCSA (μm2) | 2842.17 ± 1285.05 | 2606.04 ± 839.31 | 3070.66 ± 1199.36 | 3002.85 ± 1316.49 |
| Control Group and No DR Group | Control Group and NPDR Group | Control Group and PDR Groups | No DR Group and NPDR Group | No DR Group and PDR Group | NPDR Group and PDR Group | |
|---|---|---|---|---|---|---|
| FAZ area of superficial layer (mm2) (Tukey’s test) | p adj = 0.977 | p adj = 0.870 | p adj = 0.867 | p adj = 0.670 | p adj = 0.661 | p adj > 0.999 |
| FAZ perimeter of superficial layer (mm) (Tukey’s test) | p adj > 0.999 | p adj = 0.593 | p adj = 0.450 | p adj = 0.627 | p adj = 0.491 | p adj = 0.997 |
| FAZ circularity index of superficial layer (Dunn’s test) | p adj > 0.999 | p adj = 0.385 | p adj = 0.019 | p adj > 0.999 | p adj = 0.104 | p adj > 0.999 |
| SCP density (%) (Tukey’s test) | p adj = 0.961 | p adj = 0.282 | p adj = 0.015 | p adj = 0.133 | p adj = 0.005 | p adj = 0.593 |
| VL density of superficial layer (%) (Tukey’s test) | p adj = 0.996 | p adj = 0.059 | p adj = 0.001 | p adj = 0.049 | p adj = 0.001 | p adj = 0.609 |
| FD superficial layer (Tukey’s test) | p adj = 0.994 | p adj = 0.004 | p adj < 0.0001 | p adj = 0.017 | p adj = 0.0001 | p adj = 0.432 |
| TVD (μm) (Dunn’s test) | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 |
| LD (μm) (Dunn’s test) | p adj = 0.756 | p adj = 0.967 | p adj = 0.069 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 |
| WT (μm) (Dunn’s test) | p adj > 0.999 | p adj = 0.328 | p adj = 0.008 | p adj > 0.999 | p adj = 0.169 | p adj = 0.917 |
| WLR (Dunn’s test) | p adj = 0.190 | p adj = 0.011 | p adj < 0.0001 | p adj > 0.999 | p adj = 0.064 | p adj = 0.514 |
| WCSA (μm2) (Dunn’s test) | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 | p adj > 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirescu, A.-E.; Deleanu, D.G.; Jurja, S.; Popa-Cherecheanu, A.; Balta, F.; Garhofer, G.; Balta, G.; Cristescu, I.-E.; Tofolean, I.T. Multimodal Imaging of Diabetic Retinopathy: Insights from Optical Coherence Tomography Angiography and Adaptive Optics. Diagnostics 2025, 15, 1732. https://doi.org/10.3390/diagnostics15141732
Mirescu A-E, Deleanu DG, Jurja S, Popa-Cherecheanu A, Balta F, Garhofer G, Balta G, Cristescu I-E, Tofolean IT. Multimodal Imaging of Diabetic Retinopathy: Insights from Optical Coherence Tomography Angiography and Adaptive Optics. Diagnostics. 2025; 15(14):1732. https://doi.org/10.3390/diagnostics15141732
Chicago/Turabian StyleMirescu, Andrada-Elena, Dan George Deleanu, Sanda Jurja, Alina Popa-Cherecheanu, Florian Balta, Gerhard Garhofer, George Balta, Irina-Elena Cristescu, and Ioana Teodora Tofolean. 2025. "Multimodal Imaging of Diabetic Retinopathy: Insights from Optical Coherence Tomography Angiography and Adaptive Optics" Diagnostics 15, no. 14: 1732. https://doi.org/10.3390/diagnostics15141732
APA StyleMirescu, A.-E., Deleanu, D. G., Jurja, S., Popa-Cherecheanu, A., Balta, F., Garhofer, G., Balta, G., Cristescu, I.-E., & Tofolean, I. T. (2025). Multimodal Imaging of Diabetic Retinopathy: Insights from Optical Coherence Tomography Angiography and Adaptive Optics. Diagnostics, 15(14), 1732. https://doi.org/10.3390/diagnostics15141732






