Ultra-Widefield Retinal Optical Coherence Tomography (OCT) and Angio-OCT Using an Add-On Lens
Abstract
1. Introduction
2. Materials and Methods
3. Results
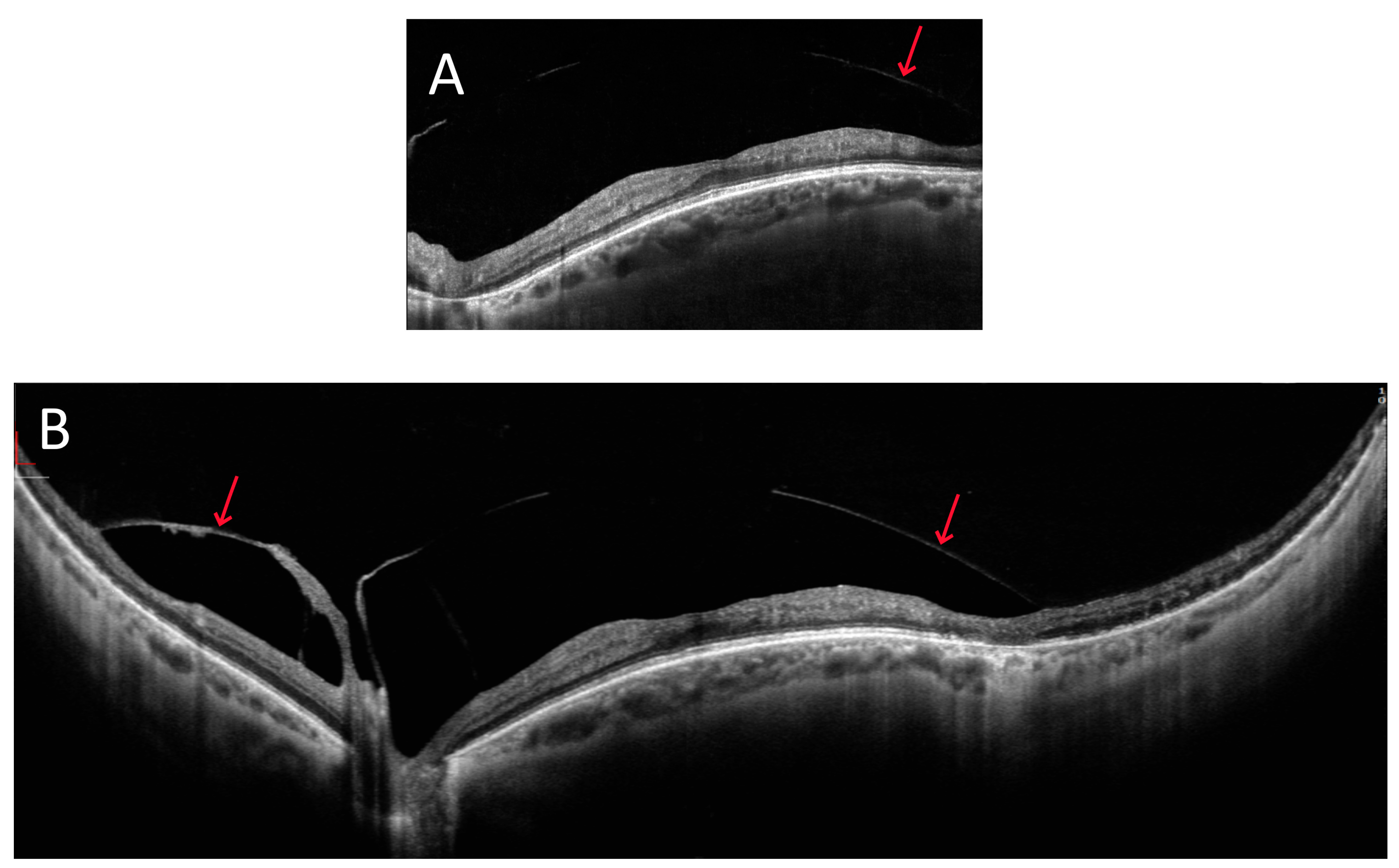
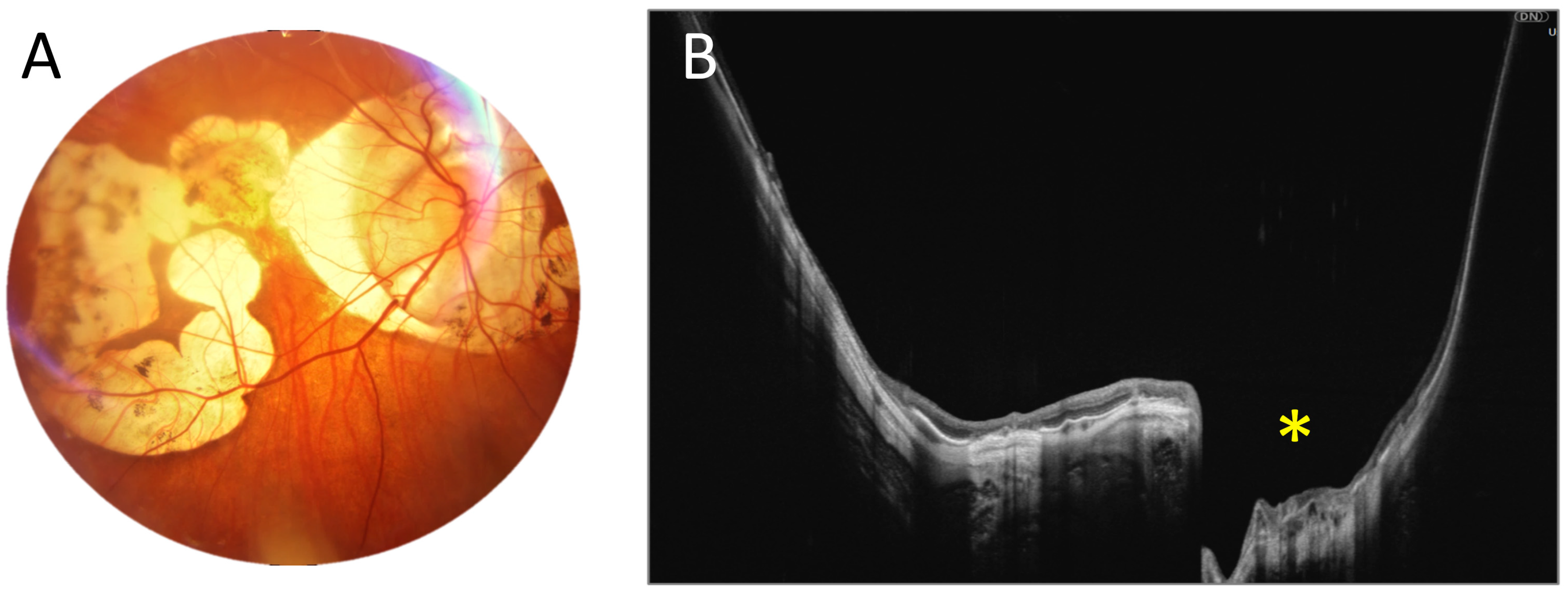
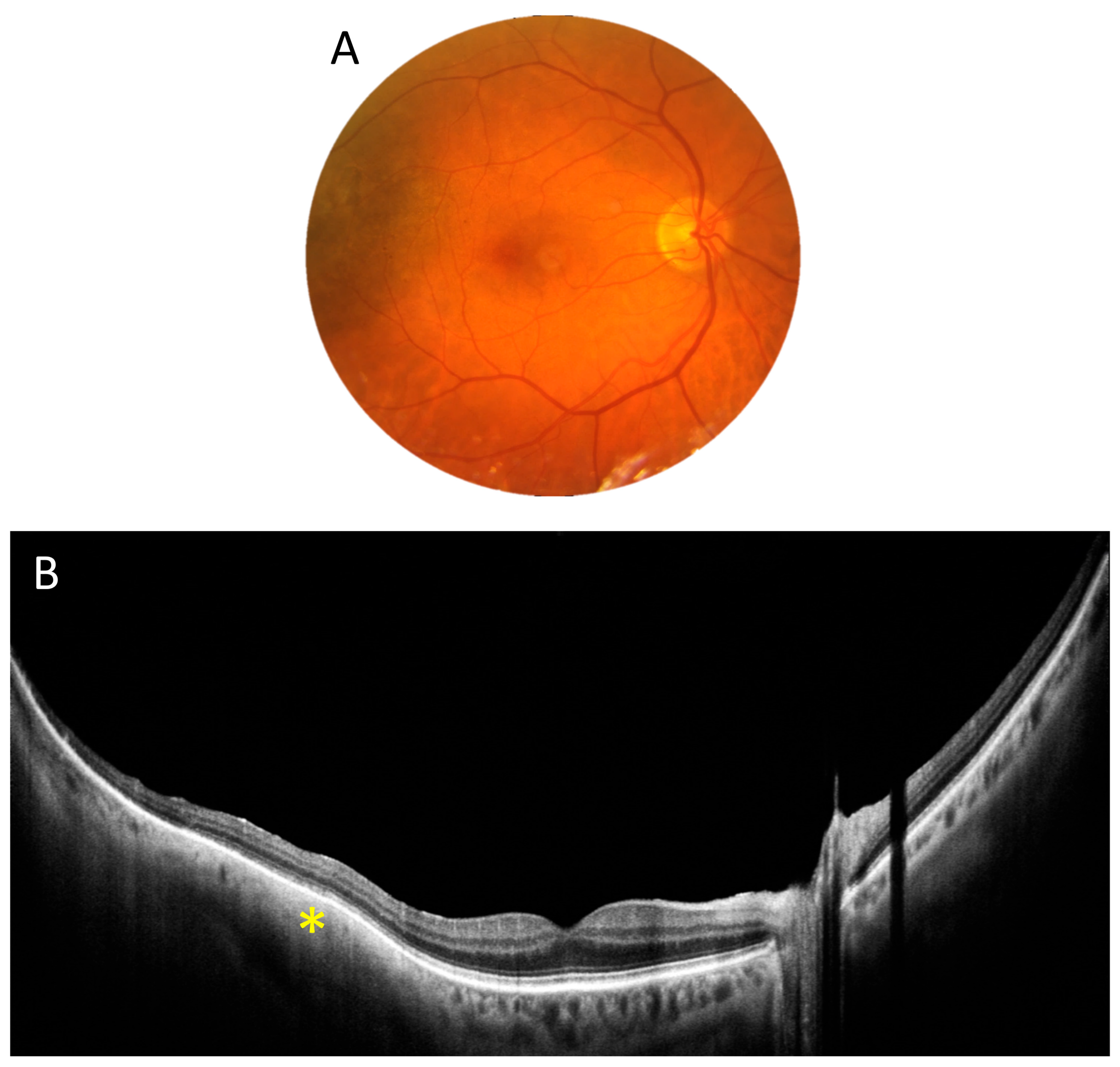
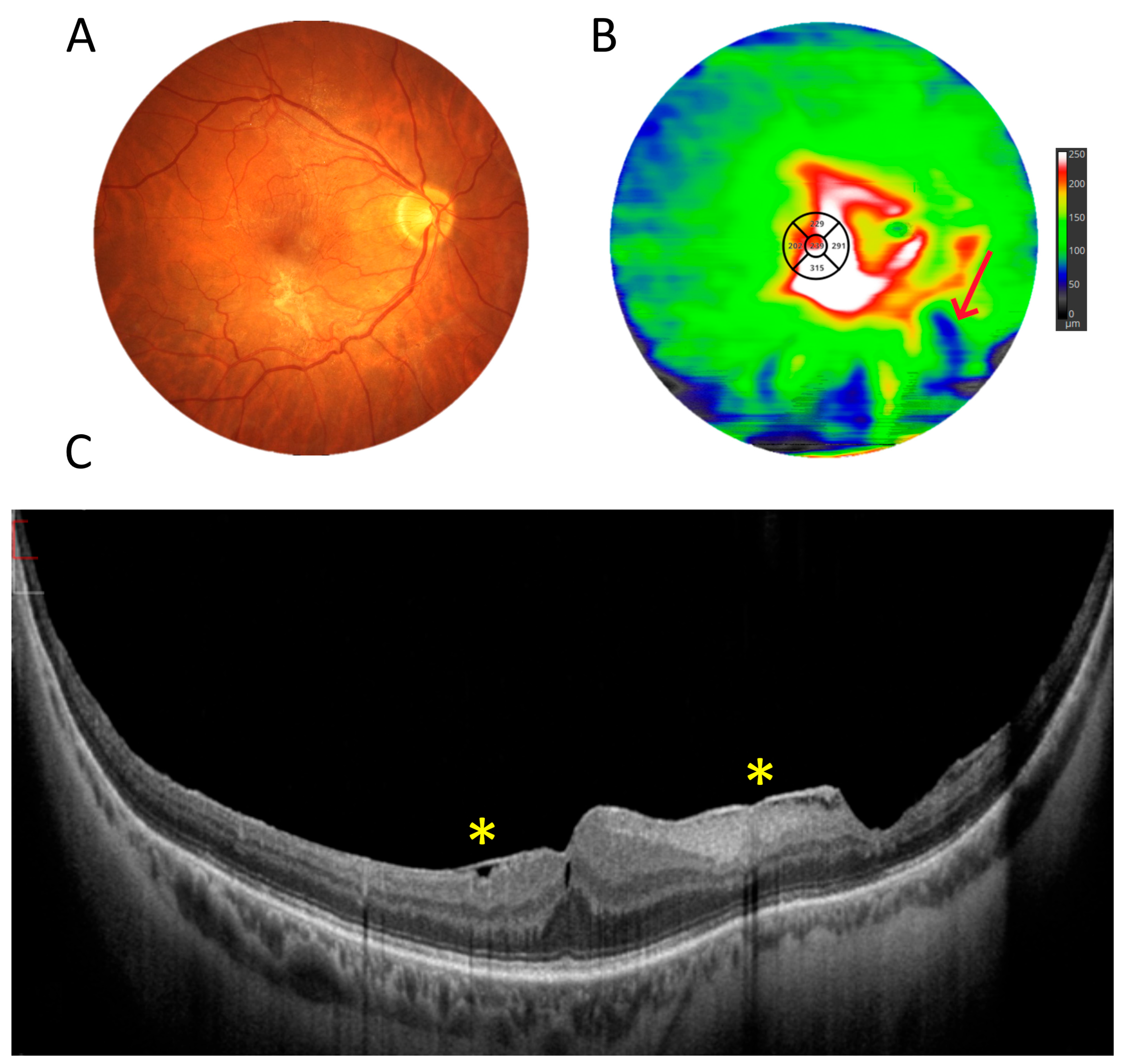
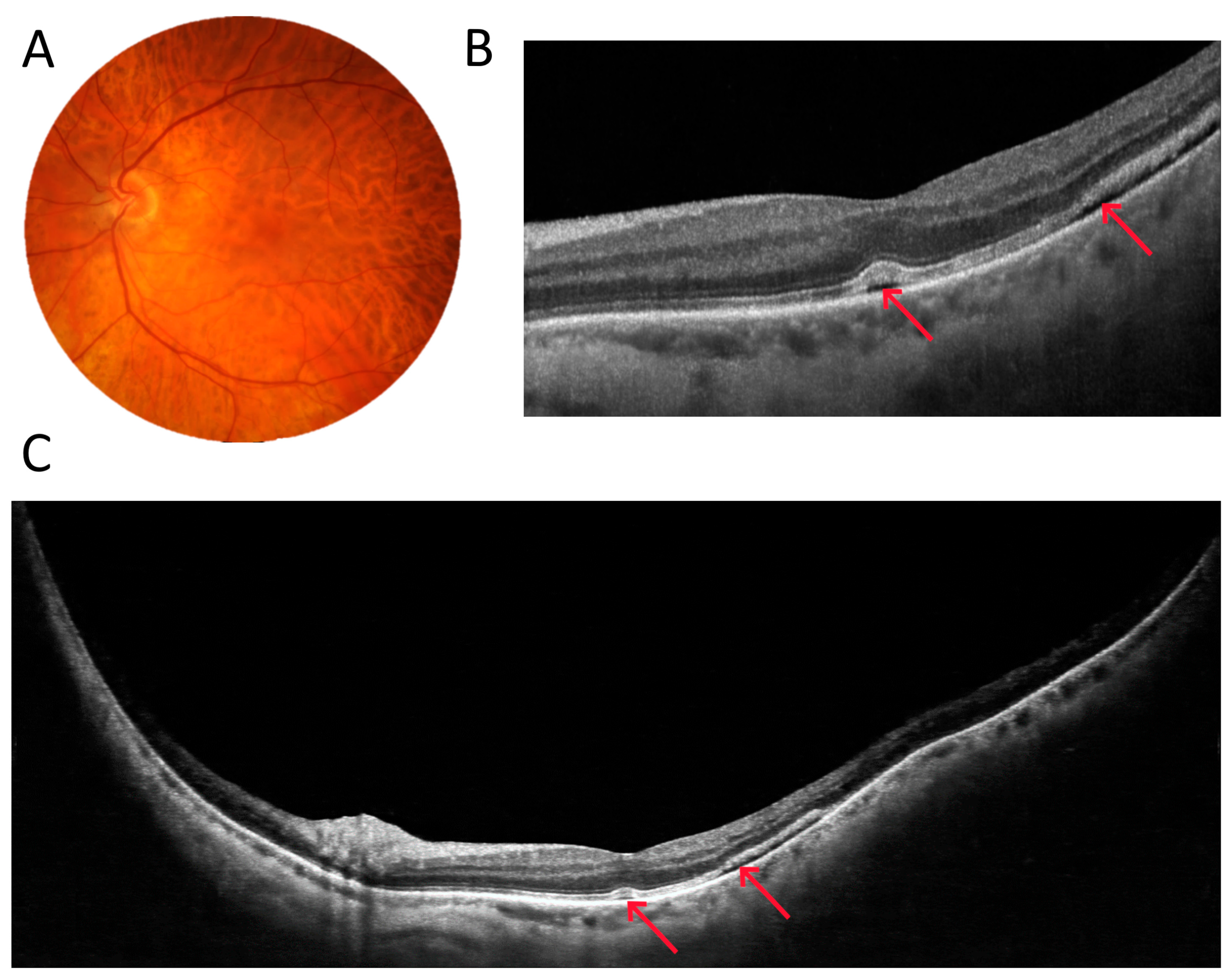
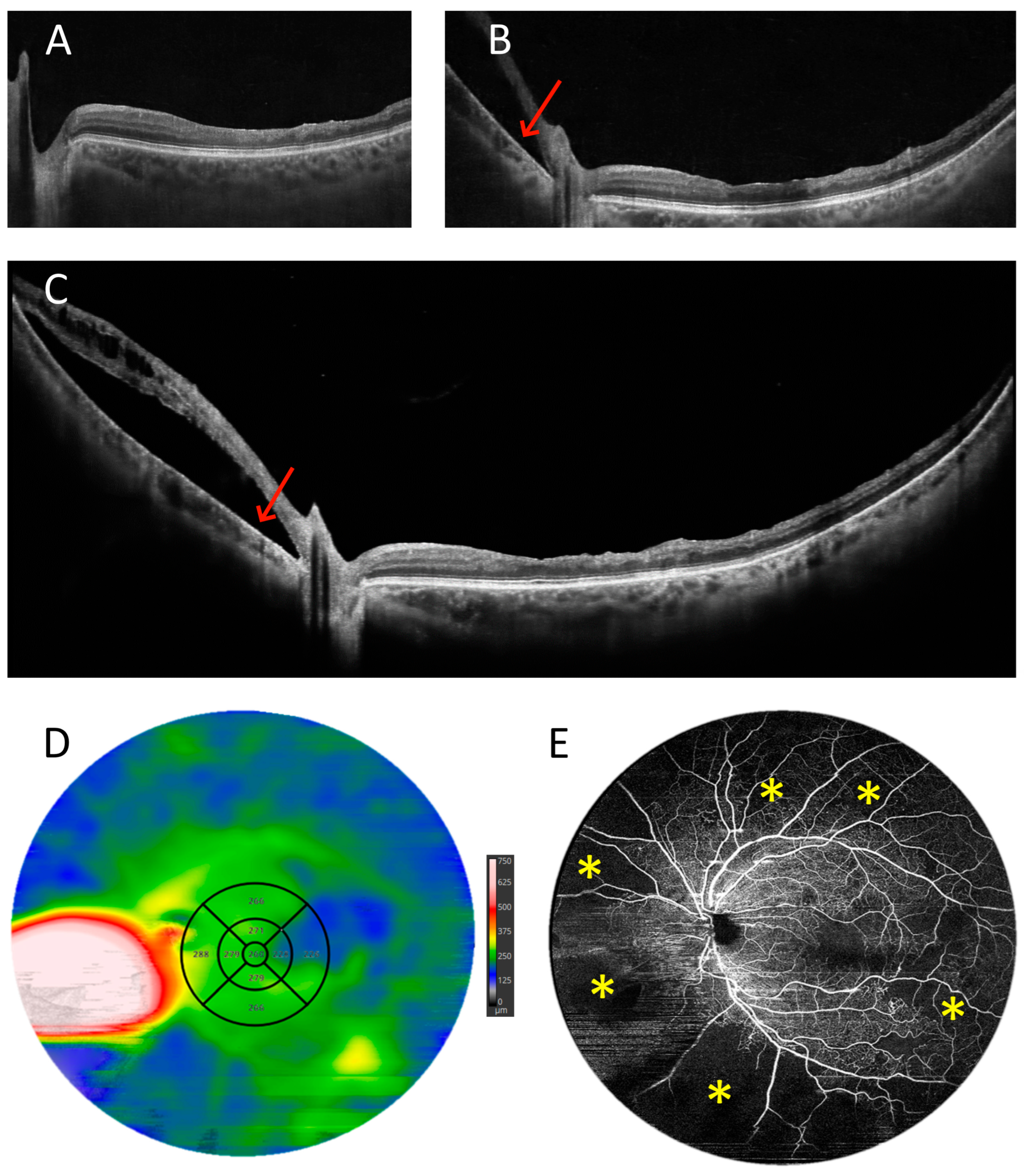
3.1. OCT Measurements
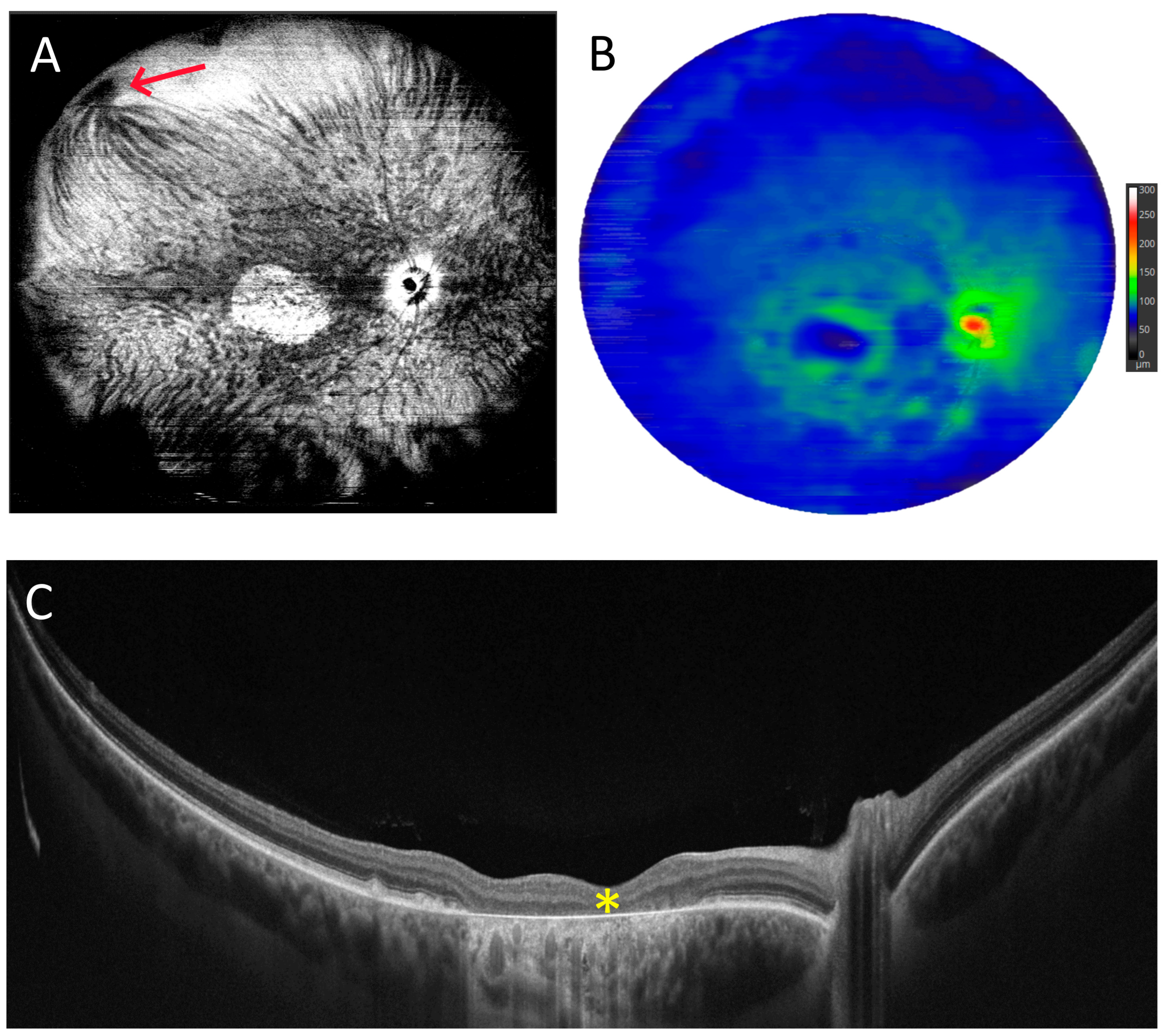
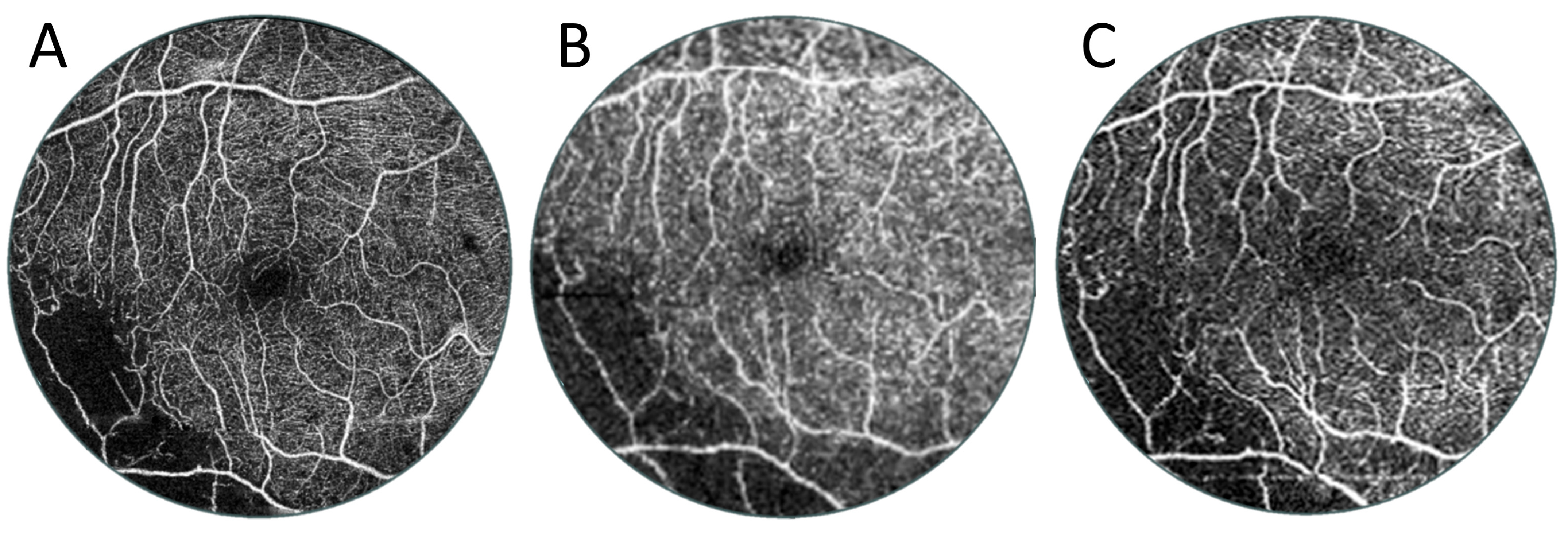
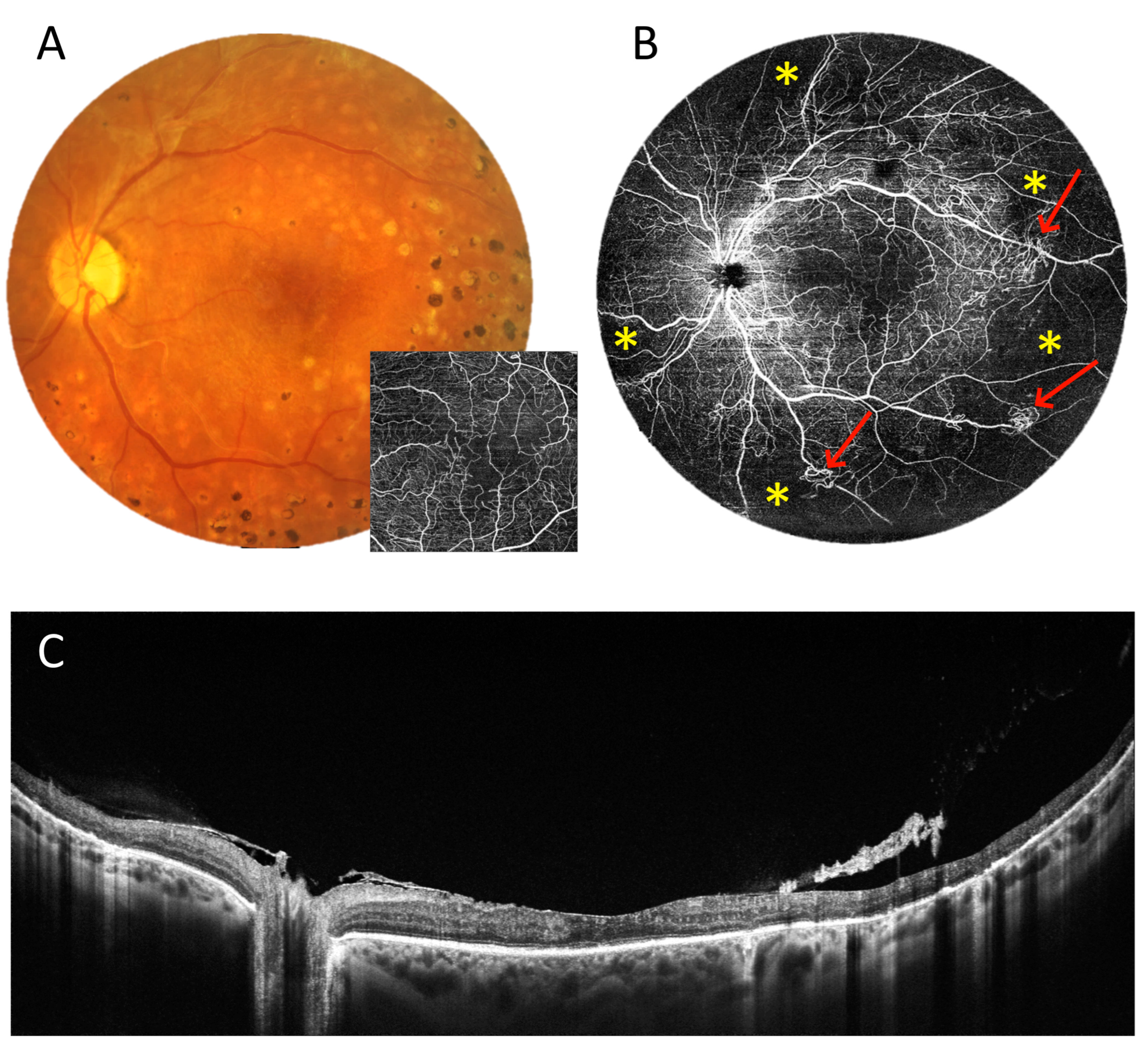
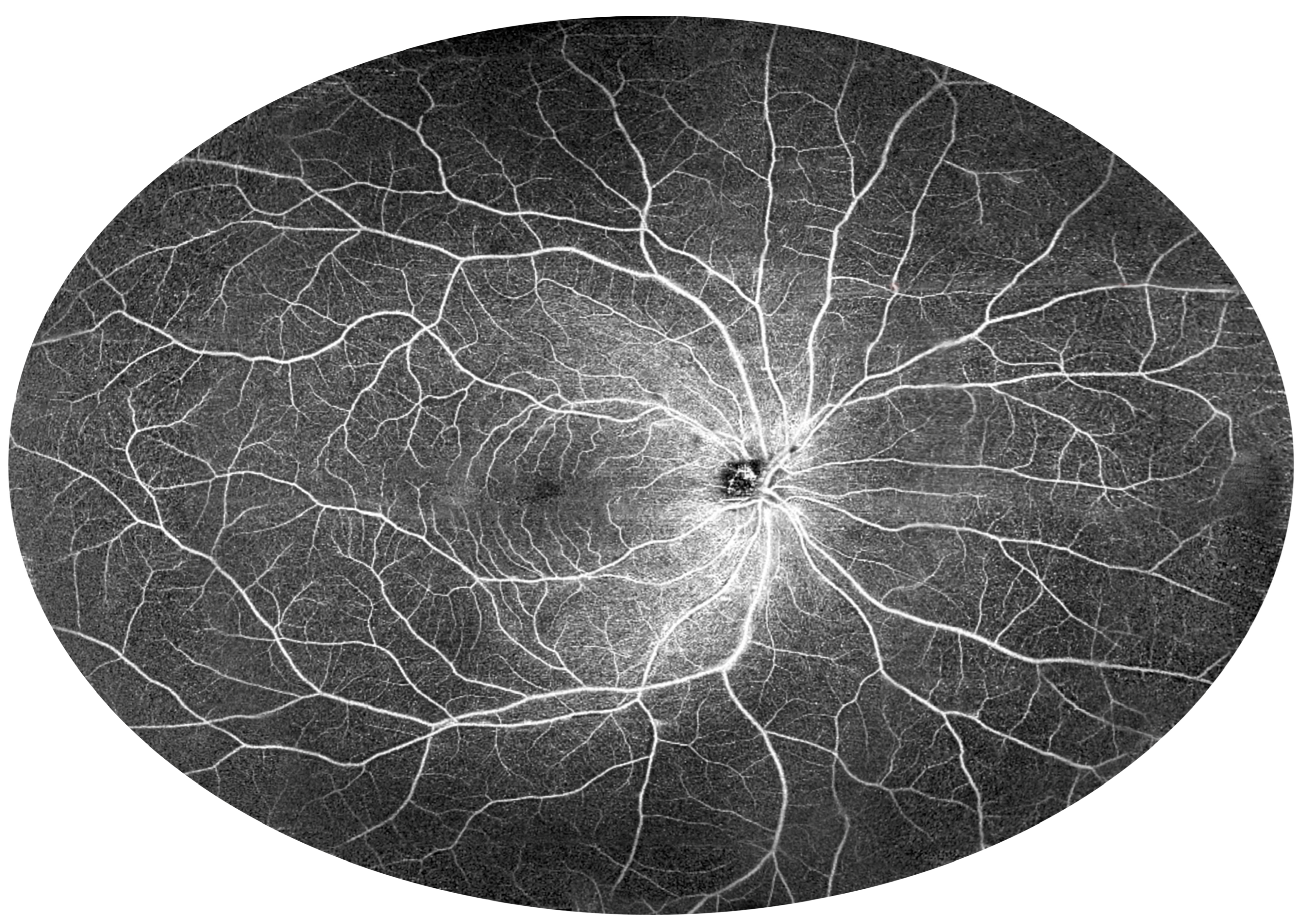
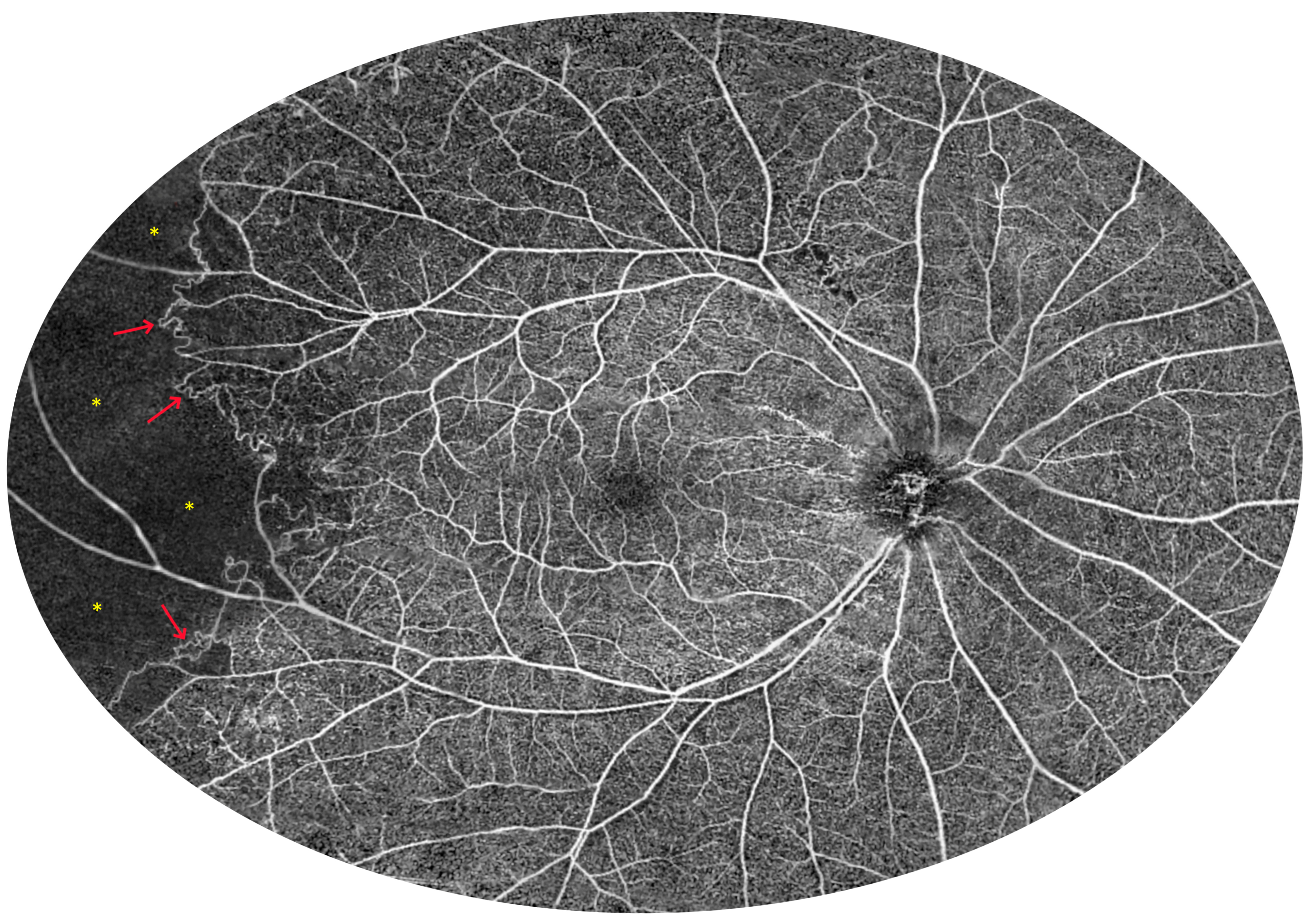
3.2. Angio-OCT Measurements
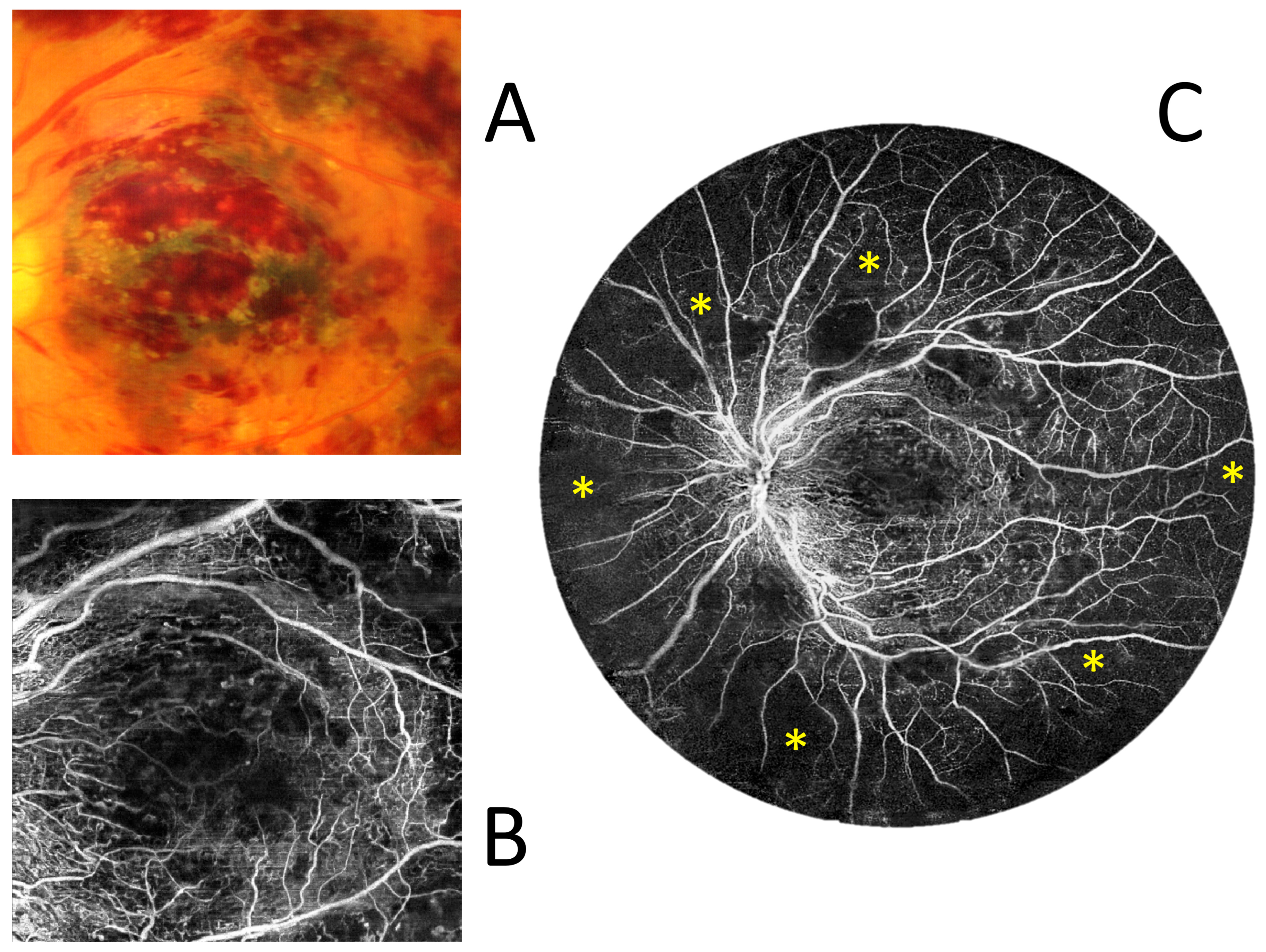
3.3. OCT and Angio-OCT Measurements
3.4. UWF Angio-OCT Mosaics
3.5. Retinal Thickness Measurements
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzaridis, S.; Friedlander, M. Optical coherence tomography: When a picture is worth a million words. J. Clin. Investig. 2023, 133, e174951. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Srinivasan, V.; Fujimoto, J.G.; Ko, T.; Schuman, J.S.; Kowalczyk, A.; Duker, J.S. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2005, 112, 1734–1746. [Google Scholar] [CrossRef]
- Sikorski, B.L.; Malukiewicz, G.; Stafiej, J.; Lesiewska-Junk, H.; Raczynska, D. The diagnostic function of OCT in diabetic maculopathy. Mediat. Inflamm. 2013, 2013, 434560. [Google Scholar] [CrossRef]
- Sikorski, B.L.; Gorczynska, I.; Bukowska, D.; Gorczynska, I.; Szkulmowski, M.; Krawiec, K.; Malukiewicz, G.; Wojtkowski, M. Noninvasive Visualization of 3-D Retinal Microcapillary Network Using OCT. PO 263. In Proceedings of the American Academy of Ophthalmology Meeting, Chicago, IL, USA, 10–13 November 2012. [Google Scholar]
- Ruminski, D.; Sikorski, B.L.; Bukowska, D.; Szkulmowski, M.; Krawiec, K.; Malukiewicz, G.; Bieganowski, L.; Wojtkowski, M. OCT angiography by absolute intensity difference applied to normal and diseased human retinas. Biomed. Opt. Express 2015, 6, 2738–2754. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Jia, Y.; Zhang, M.; Su, J.P.; Liu, G.; Hwang, T.S.; Bailey, S.T.; Huang, D. Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT27-36. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Vingopoulos, F.; Le, R.; Laíns, I.; Wu, D.M.; et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 577–581. [Google Scholar] [CrossRef]
- Tan, T.E.; Ibrahim, F.; Chandrasekaran, P.R.; Teo, K.Y.C. Clinical utility of ultra-widefield fluorescein angiography and optical coherence tomography angiography for retinal vein occlusions. Front. Med. 2023, 10, 1110166. [Google Scholar] [CrossRef]
- Amato, A.; Nadin, F.; Borghesan, F.; Cicinelli, M.V.; Chatziralli, I.; Sadiq, S.; Mirza, R.; Bandello, F.; Sugawara, A. Widefield Optical Coherence Tomography Angiography in Diabetic Retinopathy. J. Diabetes Res. 2020, 2020, 8855709. [Google Scholar] [CrossRef]
- Bajka, A.; Bacci, T.; Wiest, M.R.J.; Brinkmann, M.; Hamann, T.; Toro, M.; Zweifel, S.A. Feasibility and Clinical Utility of Wide-Field Optical Coherence Tomography Angiography Compared to Ultrawide-Field Fluorescein Angiography in Patients with Diabetic Retinopathy. Klin. Monbl Augenheilkd. 2023, 240, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Murakami, T.; Kawai, K.; Nishikawa, K.; Ishihara, K.; Mori, Y.; Tsujikawa, A. Inference of Capillary Nonperfusion Progression on Widefield OCT Angiography in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 24. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Toslak, D.; Son, T.; Ma, J. Understanding the relationship between visual-angle and eye-angle for reliable determination of the field-of-view in ultra-wide field fundus photography. Biomed Opt Express. 2021, 30, 6651–6659. [Google Scholar] [CrossRef]
- Aiello, L.P.; Odia, I.; Glassman, A.R.; Melia, M.; Jampol, L.M.; Bressler, N.M.; Kiss, S.; Silva, P.S.; Wykoff, C.C.; Diabetic Retinopathy Clinical Research Network; et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging with Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Marcus, D.M.; Liu, D.; Aiello, L.P.; Antoszyk, A.; Elman, M.; Friedman, S.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; et al. Association of Ultra-Widefield Fluorescein Angiography-Identified Retinal Nonperfusion and the Risk of Diabetic Retinopathy Worsening Over Time. JAMA Ophthalmol. 2022, 140, 936–945. [Google Scholar] [CrossRef]
- Marcus, D.M.; Silva, P.S.; Liu, D.; Aiello, L.P.; Antoszyk, A.; Elman, M.; Friedman, S.; Glassman, A.R.; Googe, J.M.; DRCR Retina Network; et al. Association of Predominantly Peripheral Lesions on Ultra-Widefield Imaging and the Risk of Diabetic Retinopathy Worsening Over Time. JAMA Ophthalmol. 2022, 140, 946–954. [Google Scholar] [CrossRef]
- Nicholson, L.; Vazquez-Alfageme, C.; Patrao, N.V.; Triantafyllopolou, I.; Bainbridge, J.W.; Hykin, P.G.; Sivaprasad, S. Retinal Nonperfusion in the Posterior Pole Is Associated with Increased Risk of Neovascularization in Central Retinal Vein Occlusion. Am. J. Ophthalmol. 2017, 182, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Cavallerano, J.D.; Haddad, N.M.N.; Kwak, H.; Dyer, K.H.; Omar, A.F.; Shikari, H.; Sun, J.K.; Aiello, L.P. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015, 122, 949–956. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Gong, Y.; Liu, N.; Cao, Y.; Li, Z.; Zhang, Y. Advanced ultrawide-field optical coherence tomography angiography identifies previously undetectable changes in biomechanics-related parameters in nonpathological myopic fundus. Front. Bioeng. Biotechnol. 2022, 10, 920197. [Google Scholar] [CrossRef]
- Russell, J.F.; Flynn, H.W., Jr.; Sridhar, J.; Townsend, J.H.; Shi, Y.; Fan, K.C.; Scott, N.L.; Hinkle, J.W.; Lyu, C.; Gregori, G.; et al. Distribution of Diabetic Neovascularization on Ultra-Widefield Fluorescein Angiography and on Simulated Widefield OCT Angiography. Am. J. Ophthalmol. 2019, 207, 110–120. [Google Scholar] [CrossRef]
- Hwang, T.S.; Gao, S.S.; Liu, L.; Lauer, A.K.; Bailey, S.T.; Flaxel, C.J.; Wilson, D.J.; Huang, D.; Jia, Y. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016, 134, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hormel, T.T.; Guo, Y.; Tsuboi, K.; Flaxel, C.J.; Huang, D.; Hwang, T.S.; Jia, Y. Perfused and Nonperfused Microaneurysms Identified and Characterized by Structural and Angiographic OCT. Ophthalmol. Retin. 2023, 8, 108–115. [Google Scholar] [CrossRef]
- Yeung, L.; Lee, Y.-C.; Lin, Y.-T.; Lee, T.-W.; Lai, C.-C. Macular Ischemia Quantification Using Deep-Learning Denoised Optical Coherence Tomography Angiography in Branch Retinal Vein Occlusion. Transl. Vis. Sci. Technol. 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef]
- Khodabandeh, A.; Shahraki, K.; Roohipoor, R.; Riazi-Esfahani, H.; Yaseri, M.; Faghihi, H.; Bazvand, F. Quantitative measurement of vascular density and flow using optical coherence tomography angiography (OCTA) in patients with central retinal vein occlusion: Can OCTA help in distinguishing ischemic from non-ischemic type? Int. J. Retin. Vitr. 2018, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Kaizu, Y.; Nakao, S.; Sekiryu, H.; Wada, I.; Yamaguchi, M.; Hisatomi, T.; Ikeda, Y.; Kishimoto, J.; Sonoda, K.-H. Retinal flow density by optical coherence tomography angiography is useful for detection of nonperfused areas in diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2275–2282. [Google Scholar] [CrossRef]
- Lahme, L.; Marchiori, E.; Panuccio, G.; Nelis, P.; Schubert, F.; Mihailovic, N.; Torsello, G.; Eter, N.; Alnawaiseh, M. Changes in retinal flow density measured by optical coherence tomography angiography in patients with carotid artery stenosis after carotid endarterectomy. Sci. Rep. 2018, 8, 17161. [Google Scholar] [CrossRef]
- Lujan, B.J.; Calhoun, C.T.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; Melia, M.; Schlossman, D.K.; Sun, J.K.; DRCR Retina Network. Optical Coherence Tomography Angiography Quality Across Three Multicenter Clinical Studies of Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef]
- Gao, S.S.; Jia, Y.; Liu, L.; Zhang, M.; Takusagawa, H.L.; Morrison, J.C.; Huang, D. Compensation for Reflectance Variation in Vessel Density Quantification by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4485–4492. [Google Scholar] [CrossRef]
- Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 786–806. [CrossRef]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Hagag, A.M.; Wang, J.; Zhang, M.; Smith, A.; Wilson, D.J.; Huang, D.; Jia, Y. Automated Quantification of Nonperfusion Areas in 3 Vascular Plexuses with Optical Coherence Tomography Angiography in Eyes of Patients with Diabetes. JAMA Ophthalmol. 2018, 136, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Atik, A.; Barton, K.; Azuara-Blanco, A.; Kerr, N.M. Health economic evaluation in ophthalmology. Br. J. Ophthalmol. 2021, 105, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Framme, C.; Dittberner, M.; Rohwer-Mensching, K.; Gottschling, J.; Buley, P.; Hufendiek, K.; Hufendiek, K.; Junker, B.; Tode, J.; Lammert, F.; et al. Performance and cost calculation for a university ophthalmological outpatient clinic. Ophthalmologe 2022, 119, 46–54. [Google Scholar] [CrossRef]
- Murphy, A.; McElnea, E.; Byrne, S. Health technology assessment: A primer for ophthalmology. Eur. J. Ophthalmol. 2018, 28, 358–364. [Google Scholar] [CrossRef]
- Konno, A.; Ishibazawa, A.; De Pretto, L.; Shimouchi, A.; Omae, T.; Song, Y.-S. Relationship between nonperfusion area from widefield optical coherence tomography angiography and macular vascular parameters in diabetic retinopathy. Int. Ophthalmol. 2023, 43, 4803–4814. [Google Scholar] [CrossRef]




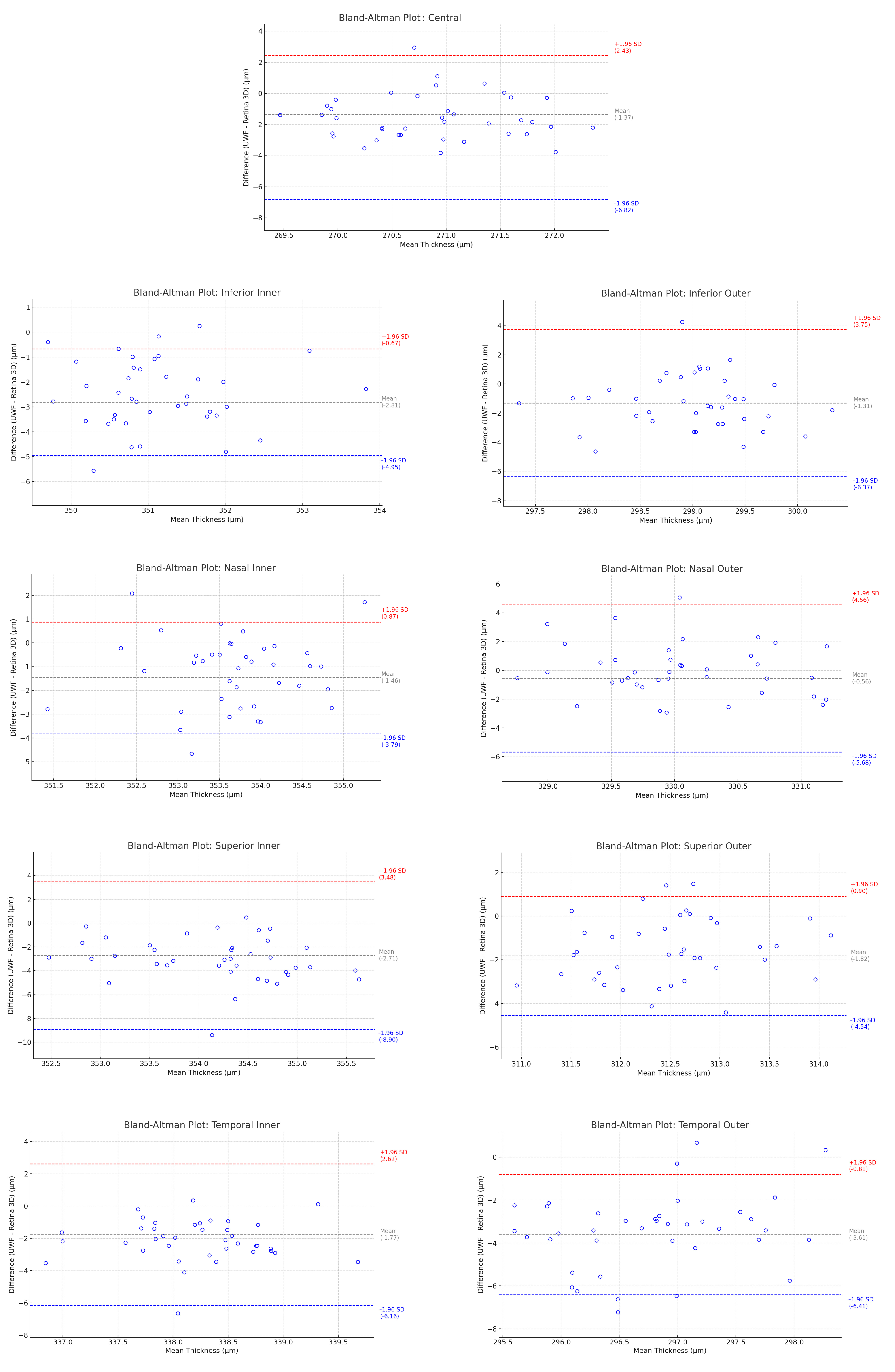
| ETDRS Subfields n = 39 | Mean UWF Thickness (µm) | Mean Retina 3D Thickness (µm) | Mean Difference (µm) | Mean Difference SD | Lower LoA (µm) | 95% CI of Lower LoA | Upper LoA (µm) | 95% CI of Upper LoA |
|---|---|---|---|---|---|---|---|---|
| Central | 270.02 | 271.39 | −1.37 | 2.96 | −6.82 | −2.78 to −10.87 | 2.43 | −0.32 to 22.48 |
| Inferior Inner | 349.73 | 352.54 | −2.81 | 1.09 | −4.94 | −6.42 to −3.45 | −0.68 | −2.17 to 0.80 |
| Inferior Outer | 298.24 | 299.56 | −1.31 | 2.58 | −6.38 | −9.91 to −2.85 | 3.75 | 0.22 to 7.28 |
| Nasal Inner | 352.97 | 354.43 | −1.46 | 1.19 | −3.79 | −5.42 to −2.16 | 0.88 | −0.75 to 2.51 |
| Nasal Outer | 329.76 | 330.32 | −0.56 | 2.61 | −5.67 | −9.24 to −2.11 | 4.55 | 0.99 to 8.12 |
| Superior Inner | 352.78 | 355.49 | −2.71 | 3.16 | −8.91 | −13.23 to −4.59 | 3.48 | −0.84 to 7.81 |
| Superior Outer | 311.55 | 313.37 | −1.82 | 1.39 | −4.55 | −6.45 to −2.64 | 0.91 | −0.99 to 2.82 |
| Temporal Inner | 337.16 | 338.93 | −1.77 | 2.24 | −6.16 | −9.22 to −3.10 | 2.62 | −0.44 to 5.68 |
| Temporal Outer | 295.19 | 298.80 | −3.61 | 1.43 | −6.41 | −8.37 to −4.46 | −0.81 | −2.76 to 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorski, B.L. Ultra-Widefield Retinal Optical Coherence Tomography (OCT) and Angio-OCT Using an Add-On Lens. Diagnostics 2025, 15, 1697. https://doi.org/10.3390/diagnostics15131697
Sikorski BL. Ultra-Widefield Retinal Optical Coherence Tomography (OCT) and Angio-OCT Using an Add-On Lens. Diagnostics. 2025; 15(13):1697. https://doi.org/10.3390/diagnostics15131697
Chicago/Turabian StyleSikorski, Bartosz L. 2025. "Ultra-Widefield Retinal Optical Coherence Tomography (OCT) and Angio-OCT Using an Add-On Lens" Diagnostics 15, no. 13: 1697. https://doi.org/10.3390/diagnostics15131697
APA StyleSikorski, B. L. (2025). Ultra-Widefield Retinal Optical Coherence Tomography (OCT) and Angio-OCT Using an Add-On Lens. Diagnostics, 15(13), 1697. https://doi.org/10.3390/diagnostics15131697






