Branch Duct IPMN-Associated Acute Pancreatitis in a Large Single-Center Cohort Study
Abstract
1. Introduction
2. Methods
- The inclusion criteria were as follows:
- -
- At least one episode of AP based on the Atlanta classification, which requires the presence of two of the following three criteria: typical abdominal pain (pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back), serum amylase and/or lipase > 3 times the upper normal limit, and high-definition imaging (contrast-enhanced CT scan and/or contrast-enhanced MRI scan) results suggestive of pancreatitis.
- -
- At least one available set of high-resolution imaging results (contrast-enhanced CT scan and/or contrast-enhanced MRI scan) obtained within 7 days of the clinical onset of the first AP attack, showing the presence of a pancreatic cyst suggestive of BD-IPMN.
- -
- At least a second round of high-resolution imaging performed > 3 months later.
- The exclusion criteria were as follows:
- -
- Signs of chronic pancreatitis observed using the first high-resolution imaging technique (relevant signs include pancreatic atrophy, dilation of the main pancreatic duct ≥ 5 mm, and ductal or parenchymal calcifications).
- -
- Signs of MD-IPMN (mixed type) on the first high-resolution imaging technique (main pancreatic duct ≥ 5 mm).
- -
- The presence of symptoms or cystic features observed via the first high-resolution imaging technique, suggesting upfront surgical resection, such as mural nodules, thickened cystic walls, jaundice, or solid neoplastic tissue near the cystic lesion.
- -
- History of previous pancreatic surgery or pancreatic sphincterotomy.
- -
- Less than one year of follow-up.
- -
- Other causes of acute pancreatitis are defined as follows:
- (a)
- Biliary pancreatitis: bile duct stones are detected on imaging or a transient elevation of transaminase is detected at the clinical onset of acute pancreatitis.
- (b)
- Alcohol-related pancreatitis: 20 g/day of chronic alcohol consumption and exclusion of other causes of acute pancreatitis.
- (c)
- Hypertriglyceridemic pancreatitis: serum triglycerides at clinical onset > 500 mg/dL.
- (d)
- Genetic pancreatitis: detection of gene mutations (CFTR, SPINK-1, PRSS-1), which are conventionally investigated if the first attack of acute pancreatitis occurred before the age of 40 years.
- (e)
- Other causes of pancreatitis: in cases of tumor or significant ductal or parenchymal abnormalities on imaging, clinical, demographic, radiological, and pathological data were analyzed. Diabetes was defined according to the American Diabetes Association (ADA) criteria [18]. The presence of pancreatic necrosis was confirmed using high-resolution imaging.
Statistical Analysis
3. Results
3.1. Clinical and Radiological Features of the Population
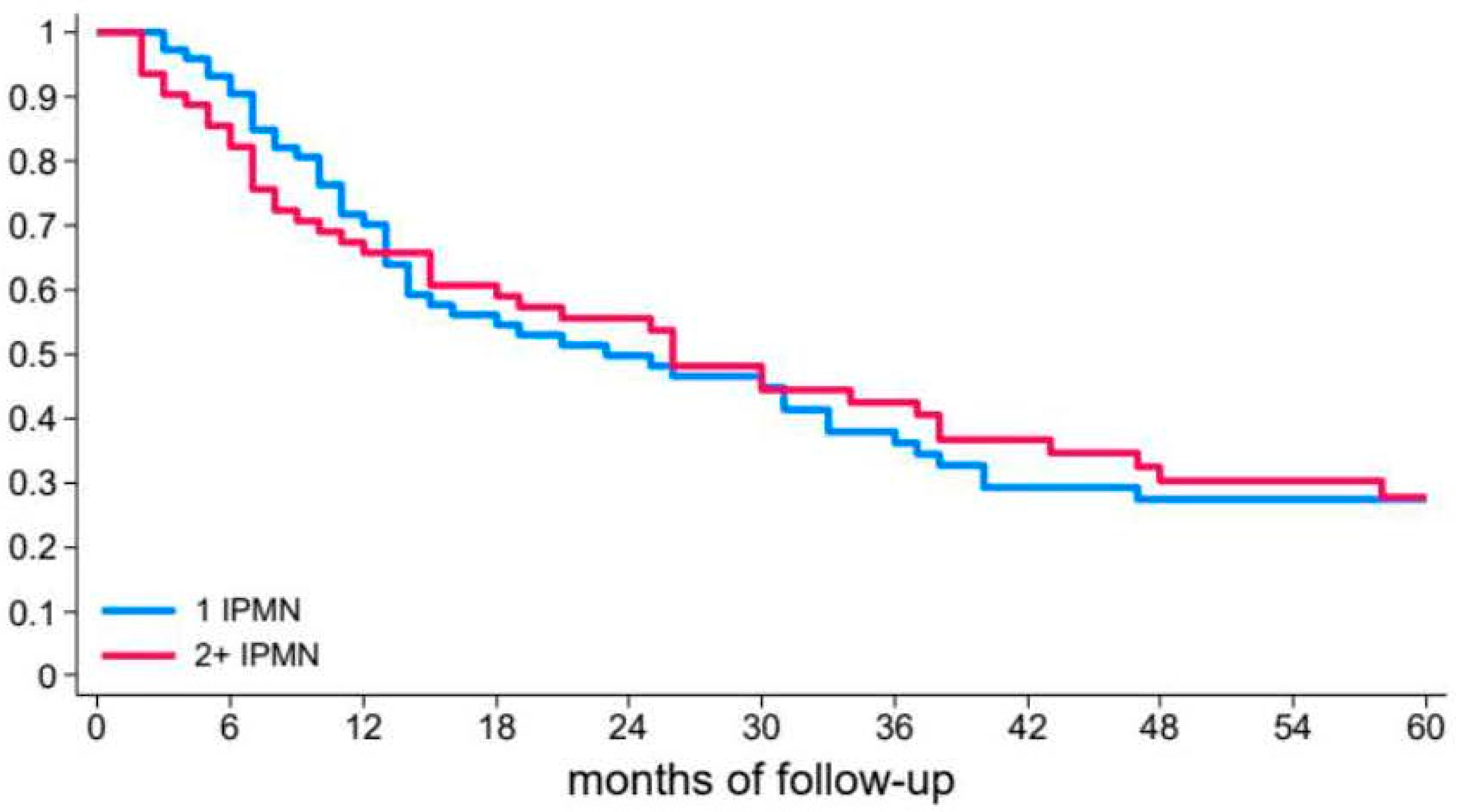
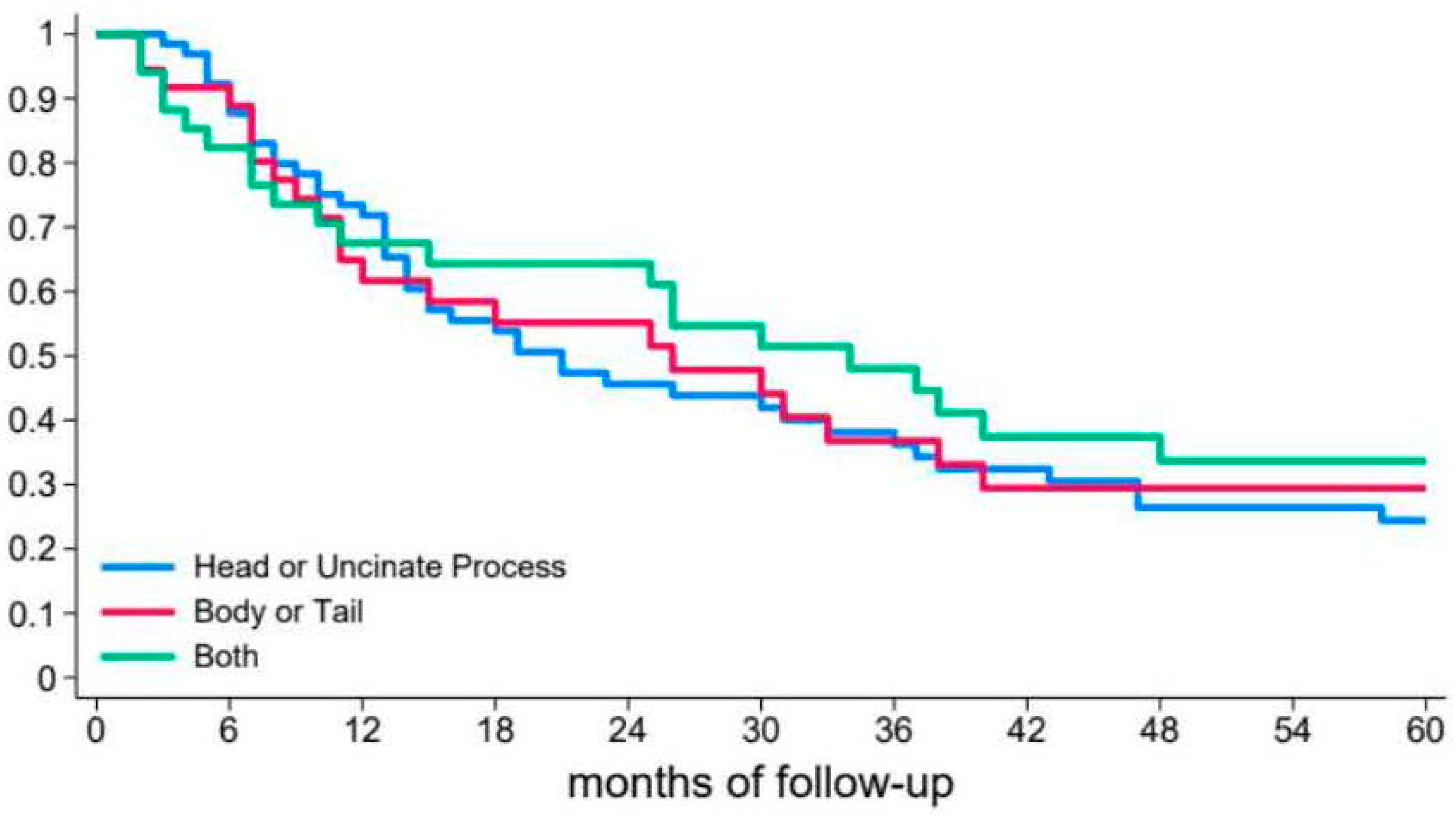
3.2. Risk of Recurrent Pancreatitis
3.3. Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, M. International consensus on the management of intraductal papillary mucinous neoplasm of the pancreas. Ann. Transl. Med. 2015, 3, 286. [Google Scholar]
- Crippa, S.; Piccioli, A.; Salandini, M.C.; Cova, C.; Aleotti, F.; Falconi, M. Treatment of branch-duct intraductal papillary mucinous neoplasm of the pancreas: State of the art. Updates Surg. 2016, 68, 265–271. [Google Scholar] [CrossRef] [PubMed]
- European Study Group on Cystic Tumors of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Crippa, S.; Capurso, G.; Cammà, C.; Fave, G.D.; Castillo, C.F.-D.; Falconi, M. Risk of pancreatic malignancy and moratlity in branch-duct IPMNs undergoing surveillance: A systematic review and meta-analysis. Dig. Liver Dis. 2016, 48, 473–479. [Google Scholar] [CrossRef]
- Crippa, S.; Arcidiacono, P.G.; De Coberlli, F.; Falconi, M. Review of the diagnosis and management of intraductal papillary mucinosu neoplasms. United Eur. Gastroenterol. J. 2020, 8, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Klöppel, G.; Adsay, N.V.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. 2005, 447, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.G.; Navaneethan, U.; Vege, S.S. Intraductal papillary mucinous neoplasm and acute pancreatitis. J. Clin. Gastroenterol. 2011, 45, 755–758. [Google Scholar] [CrossRef]
- Tanaka, M.; Kobayashi, K.; Mizumoto, K.; Yagamuchi, K. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J. Gastroenterol. 2005, 40, 669–675. [Google Scholar] [CrossRef]
- Bernardoni, L.; Crinò, S.F.; De Conti, G.; Bellocchi, M.C.C.; de Pretis, N.; Amodio, A.; Frulloni, L.; Gabbrielli, A. Preliminary experience with pancreatic shincterotomy as treatment for intraductal papillary mucinous neoplasm-associated recurrent pancreatitis. Endosc. Int. Open 2017, 5, E1144–E1150. [Google Scholar]
- Jablonska, B.; Szmigiel, P.; Mrowiec, S. Pancreatic intraductal papillary mucinous neoplasm’s: Current diagnosis and management. World J. Gastrointest. Oncol. 2021, 13, 1880–1895. [Google Scholar] [CrossRef]
- Schepis, T.; Tringali, A.; D’Aversa, F.; Perri, V.; Familiari, P.; Boškoski, I.; Nista, E.C.; Costamagna, G. Endoscopic pancreatic sphincterotomy in patients with IPMN-related recurrent pancreatitis: A single center experience. Dig. Liver Dis. 2023, 55, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Lorenzo, D.; Ratone, J.P.; Culetto, A.; Maire, F.; Levy, P.; Giovannini, M.; Barthet, M. Pancreatic sphincterotomy improves pain symptoms due to branch-duct intrapapillary mucinous neoplasia without worrisome features: A multicenter study. Endosc. Int. Open. 2019, 7, E1130–E1134. [Google Scholar] [CrossRef]
- Shin, S.H.; Han, D.J.; Park, K.T.; Kim, Y.H.; Park, J.B.; Kim, S.C. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasm’s of the pancreas. World J. Surg. 2010, 34, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Castillo, C.F.-D.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Castillo, C.F.-D.; Furukawa, T.; Hijioka, S.; Jang, J.Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef]
- Hamada, T.; Oyama, H.; Tange, S.; Hakuta, R.; Ishigaki, K.; Kanai, S.; Kawaguchi, Y.; Noguchi, K.; Saito, T.; Sato, T.; et al. The Revised Kyoto Criteria and Risk of Malignancy Among Patients With Intraductal Papillary Mucinous Neoplasms. Clin. Gastroenterol. Hepatol. 2024, 22, 2413–2423.e18. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Aslanian, H.R.; Laine, L.; Jamidar, P.A.; Farrell, J.F.; Mitchell, K.A.; Salem, R.R. Resection of pancreatic cystic neoplasm’s in recurrent acute pancreatitis prevents recurrent pancreatitis but does not identify more malignancies. World J. Gastroenterol. 2021, 27, 1630–1642. [Google Scholar]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Mukewar, S.; de Pretis, N.; Aryal-Khanal, A.; Ahmed, N.; Sah, R.; Enders, F.; Larson, J.J.; Levy, M.J.; Takahashi, N.; Topazian, M.; et al. Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut 2017, 66, 1811–1817. [Google Scholar] [CrossRef]
- Sahora, K.; Mino-Kenudson, M.; Brugge, W.; Thayer, S.P.; Ferrone, C.R.; Sahani, D.; Pitman, M.B.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F.F.-D. Branch duct papillary mucinous neoplasm: Does cyst size change the tip of the scale? A critical analysis of the revised International consensus guidelines in a large single-institutional series. Ann. Surg. 2013, 258, 466–475. [Google Scholar] [CrossRef]
- Aso, T.; Ohtsuka, T.; Matsunaga, T.; Kimura, H.; Watanabe, Y.; Tamura, K.; Ideno, N.; Osoegawa, T.; Takahata, S.; Shindo, K.; et al. “High-risk stigmata” of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary neoplasms of the pancreas. Pancreas 2014, 43, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Toste, P.A.; Farrell, J.J.; Clerkin, B.M.; Williams, J.; Muthusamy, V.R.; Watson, R.R.; Tomlinson, J.S.; Hines, O.J.; Reber, H.A.; et al. Current recommendations for surveillance and surgery of intraductal papillary mucinous neoplasm’s may overlook some patients witgh cancer. J. Gastrointest. Surg. 2015, 19, 258–265. [Google Scholar] [CrossRef]
- Ohno, E.; Balduzzi, A.; Hijioka, S.; De Pastena, M.; Marchegiani, G.; Kato, H.; Takenaka, M.; Haba, S.; Salvia, R. Association of high-risk stigmata and worrisome features with advanced neoplasia in intraductal papillary mucinous neoplasms (IPMN): A systematic review. Pancreatology 2024, 24, 48–61. [Google Scholar] [CrossRef]
- Xu, J.H.; Ni, C.Y.; Zhuang, Y.Y.; Li, L.; Lin, Y.; Xia, Z.S.; Wu, W.R.; Chen, Q.K.; Zhong, W. Acute pancreatitis in intraductal papillary mucinous neoplasm: A single-center retrospective cohort study with systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 424. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, A.L.; Hammel, P.; Rebours, V.; Couvelard, A.; Vullierme, M.P.; Maire, F.; Hentic, O.; Aubert, A.; Sauvanet, A.; Lévy, P.; et al. Acute pancreatitis in patients operated on for intraductal papillary mucinous neoplasms of the pancreas: Frequency, severity, and clinicopathologic correlations. Pancreas 2010, 39, 658–661. [Google Scholar] [CrossRef]
- Marchegiani, G.; Pollini, T.; Burelli, A.; Han, Y.; Jung, H.S.; Kwon, W.; Castellanos, D.M.R.; Crippa, S.; Belfiori, G.; Arcidiacono, P.G.; et al. Surveillance for Presumed BD-IPMN of the Pancreas: Stability, Size, and Age Identify Targets for Discontinuation. Gastroenterology 2023, 165, 1016–1024.e5. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.S.; Sharma, A.; Phillip, N.; Gupta, R.; Aryal-Khanal, A.; de Pretis, N.; Anani, V.; Enders, F.T.; Larson, J.J.; Takahashi, N.; et al. Risk of Pancreatic Cancer in Patients With Pancreatic Cysts and Family History of Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2018, 16, 1123–1130.e1. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar]
- Aziz, H.; Acher, A.W.; Krishna, S.G.; Cloyd, J.M.; Pawlik, T.M. Comparison of Society Guidelines for the Management and Surveillance of Pancreatic Cysts: A Review. JAMA Surg. 2022, 157, 723–730. [Google Scholar] [CrossRef]
| Patients nr. (%) | 135 (100) |
| Males nr. (%) | 74 (54.8) |
| Age (years) mean (SD) | 55.8 (12.5) |
| Diabetes nr. (%) | 16 (11.8) |
| Alcohol nr. (%) | 41 (30.4) |
| Smoke nr. (%) | 64 (47.4) |
| Recurrent pancreatitis nr. (%) | 102 (76.6) |
| Recurrence time (months) median (p25–p75) | 15 (7–37) |
| Pancreatic necrosis nr. (%) | 15 (11.1) |
| Intensive Care Admission nr. (%) | 1 (0.7) |
| Follow-up (years) median (p25–p75) | 5.2 (3.9–6.0) |
| Characteristics | Unadjusted Hazard Ratio (95% CI) | p Value | Adjusted Hazard Ratio (95% CI) | p Value | |
|---|---|---|---|---|---|
| Characteristics of Patients | Age at 1st AP | 1.00 (0.99–1.02) | 0.830 | 1.00 (0.99–1.02) | 0.650 |
| Sex | |||||
| Female | 1# | 0.396 | 1# | 0.539 | |
| Male | 0.84 (0.56–1.26) | 0.86 (0.54–1.38) | |||
| Smoking | |||||
| No | 1# | 0.626 | 1# | 0.846 | |
| Yes | 0.91 (0.61–1.34) | 1.04 (0.68–1.61) | |||
| Alcohol | |||||
| No | 1# | 0.214 | 1# | 0.407 | |
| Yes | 0.76 (0.50–1.17) | 0.80 (0.47–1.35) | |||
| Diabetes | |||||
| No | 1# | 0.722 | 1# | 0.052 | |
| Yes | 0.50 (0.26–0.95) | 0.50 (0.25–1.01) | |||
| Necrosis | |||||
| No | 1# | 0.952 | 1# | 0.899 | |
| Yes | 0.97 (0.54–1.74) | 0.96 (0.50–1.86) | |||
| Characteristics of BD–IPMN | Number of cysts | ||||
| 1 | 1# | 0.242 | 1# | 0.252 | |
| >1 | 1.09 (0.73–1.61) | 1.31 (0.82–2.10) | |||
| Cyst location | |||||
| Head/uncinate | 1# | 0.631 | 1# | 0.310 | |
| Body/tail | 0.86 (0.54–1.39) | 1.02 (0.61–1.70) | |||
| Diffuse | 0.80 (0.49–1.30) | 0.65 (0.36–1.18) | |||
| Cyst size | 1.02 (1.00–1.04) | 0.099 | 1.01 (0.99–1.04) | 0.218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pretis, N.; Martinelli, L.; Amodio, A.; Caldart, F.; Crucillà, S.; Battan, M.S.; Zorzi, A.; Crinò, S.F.; Conti Bellocchi, M.C.; Bernardoni, L.; et al. Branch Duct IPMN-Associated Acute Pancreatitis in a Large Single-Center Cohort Study. Diagnostics 2025, 15, 1676. https://doi.org/10.3390/diagnostics15131676
de Pretis N, Martinelli L, Amodio A, Caldart F, Crucillà S, Battan MS, Zorzi A, Crinò SF, Conti Bellocchi MC, Bernardoni L, et al. Branch Duct IPMN-Associated Acute Pancreatitis in a Large Single-Center Cohort Study. Diagnostics. 2025; 15(13):1676. https://doi.org/10.3390/diagnostics15131676
Chicago/Turabian Stylede Pretis, Nicolò, Luigi Martinelli, Antonio Amodio, Federico Caldart, Salvatore Crucillà, Maria Sole Battan, Alberto Zorzi, Stefano Francesco Crinò, Maria Cristina Conti Bellocchi, Laura Bernardoni, and et al. 2025. "Branch Duct IPMN-Associated Acute Pancreatitis in a Large Single-Center Cohort Study" Diagnostics 15, no. 13: 1676. https://doi.org/10.3390/diagnostics15131676
APA Stylede Pretis, N., Martinelli, L., Amodio, A., Caldart, F., Crucillà, S., Battan, M. S., Zorzi, A., Crinò, S. F., Conti Bellocchi, M. C., Bernardoni, L., De Marchi, G., Campagnola, P., Salvia, R., Gabbrielli, A., Marcon, A., & Frulloni, L. (2025). Branch Duct IPMN-Associated Acute Pancreatitis in a Large Single-Center Cohort Study. Diagnostics, 15(13), 1676. https://doi.org/10.3390/diagnostics15131676










