Severe Hereditary Hypofibrinogenemia in Pregnancy: A Case Report of a Novel Obstetrical Management with Thromboelastometry Guided Fibrinogen Supplementation
Abstract
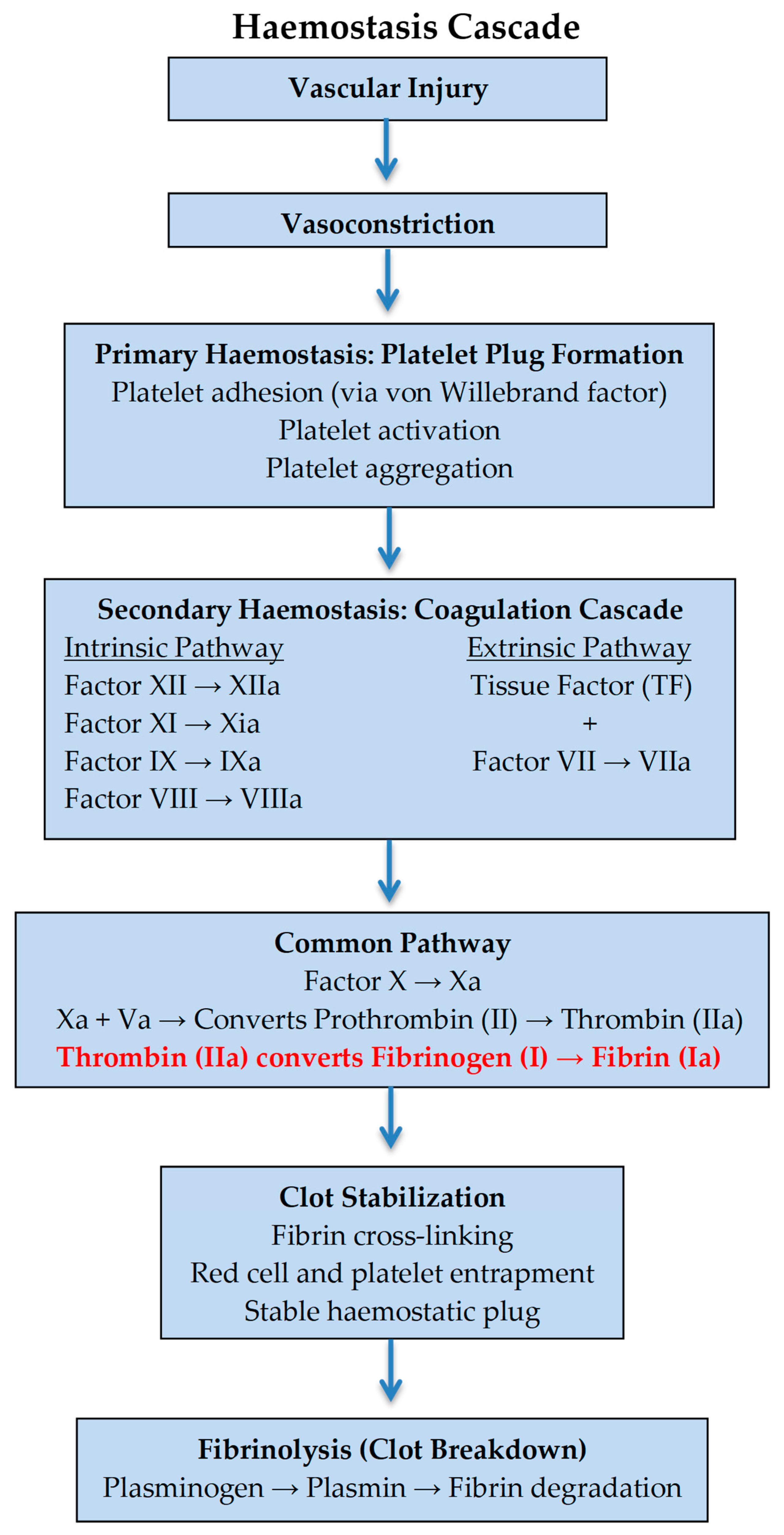
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFDs | Hereditary Fibrinogen Disorders |
| HH | Hereditary hypofibrinogenemia |
| SHH | Severe Hereditary hypofibrinogenemia |
| ROTEM® | Rotational thromboelastometry |
| FIBTEM® | Fibrin-based thromboelastometry |
| NATEM® | Non-activated thromboelastometry |
| CS | Cesarian Section |
| IV | Intravenously |
| FC | Fibrinogen Concentrate |
| MCF | Maximum clot firmness |
| FGR | Fetal growth restriction |
| FFP | Fresh frozen plasma |
| LMWH | Low-molecular-weight heparin |
| NICU | Neonatal Intensive Care Unit |
| PPH | Post-Partum Hemorrhage |
References
- Inbal, A.; Muszbek, L. Coagulation Factor Deficiencies and Pregnancy Loss. Semin. Thromb. Hemost. 2003, 29, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, X.; Teng, Y. Women With Congenital Hypofibrinogenemia/Afibrinogenemia: From Birth to Death. Clin. Appl. Thromb. 2020, 26, 1076029620912819. [Google Scholar] [CrossRef]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The Structure and Biological Features of Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.S.; Dimichele, D.M. Rare Inherited Disorders of Fibrinogen. Haemophilia 2008, 14, 1151–1158. [Google Scholar] [CrossRef]
- Casini, A.; De Moerloose, P.; Neerman-Arbez, M. Clinical Features and Management of Congenital Fibrinogen Deficiencies. Semin. Thromb. Hemost. 2016, 42, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; de Moerloose, P. Management of Congenital Quantitative Fibrinogen Disorders: A Delphi Consensus. Haemophilia 2016, 22, 898–905. [Google Scholar] [CrossRef]
- Valiton, V.; Hugon-Rodin, J.; Fontana, P.; Neerman-Arbez, M.; Casini, A. Obstetrical and Postpartum Complications in Women with Hereditary Fibrinogen Disorders: A Systematic Literature Review. Haemophilia 2019, 25, 747–754. [Google Scholar] [CrossRef]
- Drotarova, M.; Zolkova, J.; Belakova, K.M.; Brunclikova, M.; Skornova, I.; Stasko, J.; Simurda, T. Basic Principles of Rotational Thromboelastometry (ROTEM(®)) and the Role of ROTEM-Guided Fibrinogen Replacement Therapy in the Management of Coagulopathies. Diagnostics 2023, 13, 3219. [Google Scholar] [CrossRef]
- Kietaibl, S.; Ahmed, A.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of Severe Peri-Operative Bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second Update 2022. Eur. J. Anaesthesiol. EJA 2023, 40, 226–304. [Google Scholar]
- Agarwal, M.B.; Sanzgiri, P.S.; Bhanotra, P.C.; Rao, S.; Mehta, B.C.; Shah, M.D. Congenital Disorders of Fibrinogen. J. Postgrad. Med. 1981, 27, 178–183. [Google Scholar]
- Frenkel, E.; Duksin, C.; Herman, A.; Sherman, D.J. Congenital Hypofibrinogenemia in Pregnancy: Report of Two Cases and Review of the Literature. Obstet. Gynecol. Surv. 2004, 59, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Tiscia, G.L.; Margaglione, M. Human Fibrinogen: Molecular and Genetic Aspects of Congenital Disorders. Int. J. Mol. Sci. 2018, 19, 1597. [Google Scholar] [CrossRef]
- Wypasek, E.; Klukowska, A.; Zdziarska, J.; Zawilska, K.; Treliński, J.; Iwaniec, T.; Mital, A.; Pietrys, D.; Sydor, W.; Neerman-Arbez, M.; et al. Genetic and Clinical Characterization of Congenital Fibrinogen Disorders in Polish Patients: Identification of Three Novel Fibrinogen Gamma Chain Mutations. Thromb. Res. 2019, 182, 133–140. [Google Scholar] [CrossRef]
- Mohsenian, S.; Palla, R.; Menegatti, M.; Cairo, A.; Lecchi, A.; Casini, A.; Neerman-Arbez, M.; Asselta, R.; Scardo, S.; Siboni, S.M.; et al. Congenital Fibrinogen Disorders: A Retrospective Clinical and Genetic Analysis of the Prospective Rare Bleeding Disorders Database. Blood Adv. 2024, 8, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Neerman-Arbez, M.; Casini, A. Clinical Consequences and Molecular Bases of Low Fibrinogen Levels. Int. J. Mol. Sci. 2018, 19, 192. [Google Scholar] [CrossRef]
- Soares, A.W.; Maia, M.; Santo, J.E.; Costa, A.P.; Pereira, A.; Catarino, C. Hypofibrinogenaemia: A Case of Spontaneous Bleeding and Central Venous Thrombosis in the Same Lifetime. Eur. J. Case Rep. Intern. Med. 2020, 7, 001424. [Google Scholar] [CrossRef]
- Mumford, A.D.; Ackroyd, S.; Alikhan, R.; Bowles, L.; Chowdary, P.; Grainger, J.; Mainwaring, J.; Mathias, M.; O’Connell, N. Guideline for the Diagnosis and Management of the Rare Coagulation Disorders. Br. J. Haematol. 2014, 167, 304–326. [Google Scholar] [CrossRef]
- Georgiadou, P.; Sokou, R.; Tsantes, A.G.; Parastatidou, S.; Konstantinidi, A.; Houhoula, D.; Kokoris, S.; Iacovidou, N.; Tsantes, A.E. The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review. Diagnostics 2022, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.M. Congenital Hypofibrinogenemia in Pregnancy. Obstet. Gynecol. Surv. 1989, 44, 157–161. [Google Scholar] [CrossRef]
- Iwaki, T.; Castellino, F.J. Maternal Fibrinogen Is Necessary for Embryonic Development. Curr. Drug Targets 2005, 6, 535–539. [Google Scholar] [CrossRef]
- Ness, P.M.; Budzynski, A.Z.; Olexa, S.A.; Rodvien, R. Congenital Hypofibrinogenemia and Recurrent Placental Abruption. Obstet. Gynecol. 1983, 61, 519–523. [Google Scholar] [PubMed]
- Cai, H.; Liang, M.; Yang, J.; Zhang, X. Congenital Hypofibrinogenemia in Pregnancy: A Report of 11 Cases. Blood Coagul. Fibrinolysis 2018, 29, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bornikova, L.; Peyvandi, F.; Allen, G.; Bernstein, J.; Manco-Johnson, M.J. Fibrinogen Replacement Therapy for Congenital Fibrinogen Deficiency. J. Thromb. Haemost. 2011, 9, 1687–1704. [Google Scholar] [CrossRef]
- Bevan, D.H. Cryoprecipitate: No Longer the Best Therapeutic Choice in Congenital Fibrinogen Disorders? Thromb. Res. 2009, 124 (Suppl. S2), S12–S16. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakajima, Y.; Sakanashi, M. Perioperative Management of a Patient with Congenital Hypofibrinogenemia Who Underwent Myomectomy and Cesarean Section. J. Jpn. Soc. Clin. Anesth. 2013, 33, 242–246. [Google Scholar] [CrossRef]
- Diguisto, C.; Baker, E.; Stanworth, S.; Collins, P.W.; Collis, R.E.; Knight, M. Management and Outcomes of Women with Low Fibrinogen Concentration during Pregnancy or Immediately Postpartum: A UK National Population-Based Cohort Study. Acta Obstet. Gynecol. Scand. 2024, 103, 1339–1347. [Google Scholar] [CrossRef]
- Huq, F.Y.; Kadir, R.A. Management of Pregnancy, Labour and Delivery in Women with Inherited Bleeding Disorders. Haemophilia 2011, 17 (Suppl. S1), 20–30. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, Y.; Miyoshi, H.; Oshima, K.; Urabe, S.; Tanaka, N.; Kudo, Y. Prenatal and Peripartum Management of Patients with Hypofibrinogenemia Resulted in Two Successful Deliveries. Case Rep. Obstet. Gynecol. 2017, 2017, 9427359. [Google Scholar] [CrossRef]
- Khunakanan, S.; Akaraborworn, O.; Sangthong, B.; Thongkhao, K. Correlation between Maximum Clot Firmness in FIBTEM and Fibrinogen Level in Critical Trauma Patients. Crit. Care Res. Pract. 2019, 2019, 2756461. [Google Scholar] [CrossRef]
- Zhou, J.; Xin, Y.; Ding, Q.; Jiang, L.; Chen, Y.; Dai, J.; Lu, Y.; Wu, X.; Liang, Q.; Wang, H.; et al. Thromboelastography Predicts Risks of Obstetric Complication Occurrence in (Hypo)Dysfibrinogenemia Patients under Non-Pregnant State. Clin. Exp. Pharmacol. Physiol. 2016, 43, 149–156. [Google Scholar] [CrossRef]
- Treliński, J.; Pachniewska, K.; Matczak, J.; Robak, M.; Chojnowski, K. Assessment of Selected ROTEM Parameters, Kinetics of Fibrinogen Polymerization and Plasmin Amidolytic Activity in Patients with Congenital Fibrinogen Defects. Adv. Clin. Exp. Med. Off. Organ. Wroc. Med. Univ. 2016, 25, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Schöchl, H.; Cotton, B.; Inaba, K.; Nienaber, U.; Fischer, H.; Voelckel, W.; Solomon, C. FIBTEM Provides Early Prediction of Massive Transfusion in Trauma. Crit. Care 2011, 15, R265. [Google Scholar] [CrossRef] [PubMed]
- Bagot, C.N.; Leishman, E.; Onyiaodike, C.C.; Jordan, F.; Freeman, D.J. Normal pregnancy is associated with an increase in thrombin generation from the very early stages of the first trimester. Thromb. Res. 2017, 157, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ronenson, A.M.; Shifman, E.M.; Kulikov, A.V.; Raspopin, Y.S.; Görlinger, K.; Ioscovich, A.M.; Tikhova, G.P. Rotational Thromboelastometry Reference Range during Pregnancy, Labor and Postpartum Period: A Systematic Review with Meta-Analysis. J. Obstet. Anaesth. Crit. Care 2022, 12, 105–115. [Google Scholar] [CrossRef]
- Lee, J.; Wyssusek, K.H.; Kimble, R.M.N.; Way, M.; van Zundert, A.A.; Cohen, J.; Rowell, J.; Eley, V.A. Baseline parameters for rotational thromboelastometry (ROTEM®) in healthy pregnant Australian women: A comparison of labouring and non-labouring women at term. Int. J. Obs. Anesth. 2020, 41, 7–13. [Google Scholar] [CrossRef]
- Amgalan, A.; Allen, T.; Othman, M.; Ahmadzia, H.K. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 1813–1838. [Google Scholar] [CrossRef]
| Pregnancy | Age (Years) | Gravidity | Parity | Medical History | Labour Week | Mode of Delivery | Anaesthesia or Analgesia Method | Birth Weight (g) | Apgar Score | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 33 | 1 | 0 | - | - | - | - | - | - | Misscarriage at 6th week |
| 2nd | 34 | 2 | 0 | - | - | - | - | - | - | Misscarriage at 8th week |
| 3st | 35 | 3 | 1 | - | 40 + 1 w | ECS | GA | 3350 | 91 105 | - |
| 4nd | 37 | 4 | 1 | - | 38 + 1 w | ECS | GA | 3370 | 91 105 | - |
| Week | Fibrinogen | FIBTEM | ||||||
|---|---|---|---|---|---|---|---|---|
| (mg/dL) 180–450 | CT (sec) 38–62 | CFT (sec) 30–70 | A10 (mm) 7–23 | A20 (mm) 8–24 | MCF (mm) 9–25 | LI30 (%) <15% | LI60 (%) <15% | |
| 8 + 3 | 69.1 | 89 | - | 11 | 13 | 14 | 100 | 100 |
| 16 + 3 | 78.2 | 102 | - | 9 | 11 | 13 | 100 | 100 |
| 21 + 4 | 69.0 | 98 | - | 8 | 9 | 10 | 100 | 100 |
| 23 + 0 | 67.6 | 91 | - | 8 | 10 | 10 | 100 | 100 |
| 24 + 0 | 93.2 | 91 | - | 13 | 14 | 14 | 100 | 100 |
| 26 + 1 | 63.2 | 94 | - | 8 | 10 | 13 | 100 | 100 |
| 29 + 0 | 77.4 | 90 | - | 10 | 11 | 13 | 100 | 100 |
| 31 + 3 | 100.5 | 90 | 2516 | 15 | 18 | 21 | 100 | 100 |
| 34 + 1 | 70.8 | 77 | - | 11 | 12 | 15 | 100 | 98 |
| 36 + 0 | 68.5 | 78 | - | 9 | 11 | 14 | 100 | 100 |
| 37 + 1 | 68.8 | 128 | - | 9 | 12 | 14 | 100 | 100 |
| 37 + 6 10:25 | 79.5 | 83 | - | 9 | 11 | 14 | 100 | 100 |
| 37 + 6 17:03 | 95.4 | - | - | - | - | - | - | - |
| 38 + 0 | 88.0 | - | - | - | - | - | - | - |
| 38 + 1 | 82.3 | - | - | - | - | - | - | - |
| 38 + 2 AP | 97.2 | - | - | - | - | - | - | - |
| 38 + 2 10:53 PP | 94.0 | - | - | - | - | - | - | - |
| 38 + 2 13:38 PP | 103.6 | 89 | - | 11 | 13 | 15 | 100 | 100 |
| 38 + 2 18:54 PP | 95.7 | - | - | - | - | - | - | - |
| 1st day PP | 105.3 | 59 | 3998 | 14 | 16 | 21 | 100 | 100 |
| 2nd day PP | 128.9 | - | - | - | - | - | - | - |
| 4th day PP | 106.4 | 72 | 3533 | 15 | 17 | 20 | 100 | 100 |
| 7th day PP | 94.2 | 87 | - | 12 | 13 | 15 | 100 | 100 |
| Week | Fibrinogen | NATEM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/dL) 180–450 | CT (sec) 300–999 | CFT (sec) 150–700 | Alp (α-angle) 30–70 | A10 (mm) 35–60 | A20 (mm) 35–60 | MCF (mm) 40–65 | LI30 (%) <15% | LI60 (%) <15% | |
| 8 + 3 | 69.1 | 717 | 198 | 54 | 40 | 50 | 54 | 100 | 99 |
| 16 + 3 | 78.2 | 555 | 167 | 59 | 42 | 51 | 54 | 100 | 99 |
| 21 + 4 | 69.0 | 293 | 169 | 59 | 42 | 51 | 53 | 100 | 97 |
| 23 + 0 | 67.6 | 467 | 186 | 56 | 40 | 49 | 52 | 100 | 97 |
| 24 + 0 | 93.2 | 588 | 144 | 62 | 47 | 55 | 57 | 100 | 97 |
| 26 + 1 | 63.2 | 529 | 148 | 61 | 45 | 53 | 55 | 100 | |
| 29 + 0 | 77.4 | 535 | 151 | 61 | 46 | 54 | 56 | 100 | 97 |
| 31 + 3 | 100.5 | 582 | 129 | 65 | 53 | 60 | 62 | 100 | 98 |
| 34 + 1 | 70.8 | 487 | 152 | 61 | 45 | 53 | 55 | 100 | 98 |
| 36 + 0 | 68.5 | 474 | 177 | 57 | 42 | 51 | 54 | 100 | 99 |
| 37 + 1 | 68.8 | 444 | 150 | 62 | 43 | 52 | 54 | 100 | 100 |
| 37 + 6 10:25 | 79.5 | - | - | - | - | - | - | - | - |
| 37 + 6 17:03 | 95.4 | - | - | - | - | - | - | - | - |
| 38 + 0 | 88.0 | - | - | - | - | - | - | - | - |
| 38 + 1 | 82.3 | - | - | - | - | - | - | - | - |
| 38 + 2 AP | 97.2 | - | - | - | - | - | - | - | - |
| 38 + 2 10:53 PP | 94.0 | - | - | - | - | - | - | - | - |
| 38 + 2 13:38 PP | 103.6 | 529 | 161 | 59 | 45 | 53 | 57 | 100 | 100 |
| 38 + 2 18:54 PP | 95.7 | - | - | - | - | - | - | - | - |
| 1st day PP | 105.3 | 243 | 286 | 49 | 44 | 56 | 61 | 100 | 100 |
| 2nd day PP | 128.9 | - | - | - | - | - | - | - | - |
| 4th day PP | 106.4 | 605 | 194 | 57 | 50 | 60 | 63 | 100 | 98 |
| 7th day PP | 94.2 | 288 | 124 | 66 | 52 | 59 | 60 | 100 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampas, G.; Karkalemis, K.; Bagiasta, A.; Lefaki, M.-E.; Metallinou, D.; Staikou, C.; Iliodromiti, Z.; Sokou, R.; Tataropoulou, K.; Boutsikou, T.; et al. Severe Hereditary Hypofibrinogenemia in Pregnancy: A Case Report of a Novel Obstetrical Management with Thromboelastometry Guided Fibrinogen Supplementation. Diagnostics 2025, 15, 1671. https://doi.org/10.3390/diagnostics15131671
Karampas G, Karkalemis K, Bagiasta A, Lefaki M-E, Metallinou D, Staikou C, Iliodromiti Z, Sokou R, Tataropoulou K, Boutsikou T, et al. Severe Hereditary Hypofibrinogenemia in Pregnancy: A Case Report of a Novel Obstetrical Management with Thromboelastometry Guided Fibrinogen Supplementation. Diagnostics. 2025; 15(13):1671. https://doi.org/10.3390/diagnostics15131671
Chicago/Turabian StyleKarampas, Grigorios, Konstantinos Karkalemis, Anastasia Bagiasta, Maria-Ekaterini Lefaki, Dimitra Metallinou, Chryssoula Staikou, Zoi Iliodromiti, Rozeta Sokou, Kassandra Tataropoulou, Theodora Boutsikou, and et al. 2025. "Severe Hereditary Hypofibrinogenemia in Pregnancy: A Case Report of a Novel Obstetrical Management with Thromboelastometry Guided Fibrinogen Supplementation" Diagnostics 15, no. 13: 1671. https://doi.org/10.3390/diagnostics15131671
APA StyleKarampas, G., Karkalemis, K., Bagiasta, A., Lefaki, M.-E., Metallinou, D., Staikou, C., Iliodromiti, Z., Sokou, R., Tataropoulou, K., Boutsikou, T., Eleftheriades, M., Vlahos, N., Christopoulos, P., & Politou, M. (2025). Severe Hereditary Hypofibrinogenemia in Pregnancy: A Case Report of a Novel Obstetrical Management with Thromboelastometry Guided Fibrinogen Supplementation. Diagnostics, 15(13), 1671. https://doi.org/10.3390/diagnostics15131671











