Abstract
Background and Clinical Significance: Prader–Willi syndrome (PWS) is a rare genetic disease caused by imprinted gene dysfunction, typically involving deletion of the chromosome 15q11.2-q13 region, balanced translocation, or related gene mutations in this region. PWS presents with complex and varied clinical manifestations. Abnormalities can be observed from the fetal stage and change with age, resulting in growth, developmental, and metabolic issues throughout different life stages. Case Presentation: We report the prenatal characteristics observed from the second to third trimester of pregnancy in a neonate with PWS. Prenatal ultrasound findings included a single umbilical artery, poor abdominal circumference growth from 26 weeks, normal head circumference and femur length growth, increased amniotic fluid volume after 30 weeks, undescended fetal testicles in the third trimester, small kidneys, and reduced fetal movement. The male infant was born at 38 weeks of gestation with a birth weight of 2580 g. He had a weak cry; severe hypotonia; small eyelid clefts; bilateral cryptorchidism; low responsiveness to medical procedures such as blood drawing; and poor sucking, necessitating tube feeding. Blood methylation-specific multiple ligation-dependent probe amplification (MS-MLPA) showed paternal deletion PWS. Notably, this case revealed two previously unreported prenatal features in PWS: a single umbilical artery and small kidneys. Conclusions: Through literature review and our case presentation, we suggest that a combination of specific sonographic features, including these newly identified markers, may aid clinicians in the early diagnosis of PWS.
1. Introduction
Prader–Willi syndrome (PWS), first described in 1956 by Prader, Labhart, and Willi, is characterized by lifelong, unremitting hyperphagia with accompanying endocrine disorders, cognitive deficits, sensory abnormalities, and behavioral difficulties [1]. It is one of the first confirmed genetic disorders involving genomic imprinting. The prevalence of PWS ranges from 1 in 10,000 to 1 in 30,000 across different populations [2,3,4], with most cases being sporadic. The pathogenesis of PWS is absence of paternal gene expression in the 15q11.2-q13 region, which can arise from paternal chromosome microdeletion, maternal uniparental disomy, or defects in the imprinting center [3,4,5,6,7]. The pathogenic cause and genetic mechanism of PWS are complex, and the vast majority of cases are de novo. PWS can be more readily identified by postnatal features including severe hypotonia; feeding difficulties with sucking deficit; and characteristic facial traits such as a narrow forehead, almond-shaped eyes, a narrow nasal bridge, a thin upper lip, and downturned mouth corners. These features tend to become more pronounced over time. The clinical manifestations of PWS vary greatly with age. Low muscle tone and inability to suckle can lead to feeding difficulties in infancy; bulimia beginning in childhood can lead to obesity; developmental retardation, short stature, cognitive impairment, gonadal dysplasia, and behavioral problems may appear with age [6,7,8,9,10,11]. Obesity and its complications are the leading causes of death among patients with PWS.
Prenatal diagnosis of PWS is challenging due to the lack of known specific prenatal phenotypes. Some cases of prenatally diagnosed PWS were identified through whole-genome noninvasive prenatal screening (NIPS), followed by confirmatory invasive testing via amniocentesis [12,13]. Few studies have reported the antenatal clinical features of fetuses with PWS, including polyhydramnios, reduced fetal movement, and abnormal fetal presentation, which are not unique to PWS [14,15,16,17,18,19]. This report presents a case of fetal PWS, showcasing changing prenatal ultrasound findings between the second and third trimesters. Notable findings include a single umbilical artery, poor growth of abdominal circumference, polyhydramnios, cryptorchidism, slightly smaller kidneys, and reduced fetal movement.
2. Case Presentation
A healthy 37-year-old multipara was referred to the Prenatal Diagnosis Center of HKU-SZH due to the detection of a single umbilical artery at 23 weeks of gestation in March 2022. She had previously given birth to a healthy 3.5 kg male newborn at term by cesarean section in 2011. The patient’s medical and family history were unremarkable. The current pregnancy was a natural conception. At 13 weeks of gestation, nuchal translucency thickness was 1.1 mm, crown-rump length was 70 mm, and first-trimester Down syndrome combined screening and non-invasive prenatal testing (NIPT) indicated low risk. An ultrasound scan at 23 weeks revealed an estimated fetal weight (EFW) of 590 g (47th percentile), an abdominal circumference (AC) of 184 mm (51st percentile), a single umbilical artery (SUA), normal fetal morphology, and normal amniotic fluid volume.
Subsequent ultrasounds showed slow growth of the abdominal circumference with normal head circumference and femur length growth (Figure 1), as well as a gradual increase in amniotic fluid volume. At 26 weeks, EFW and AC were, respectively, 860 g (15th percentile) and 204 mm (9th percentile), with normal amniotic fluid volume. By 29 weeks and 6 days, EFW and AC were, respectively, 1320 g (15th percentile) and 234 mm (7th percentile), with mild polyhydramnios (single deepest pocket: 96 mm; amniotic fluid index [AFI] of 233 mm). At 34 weeks and 4 days of gestation, EFW and AC were, respectively, 2160 g (15th percentile) and 273 mm (4th percentile), with bilateral cryptorchidism (Figure 2) and slightly smaller kidneys (both measuring 31 × 15 mm, below the third percentile) (Figure 3) [20,21,22]. Moderate polyhydramnios was also observed (single deepest pocket: 127 mm; AFI: 376 mm). Fetal movement was minimal during each ultrasound scan, characterized by only a few general movements of the head, trunk, and extremities, with no distinctive sequencing of the body parts and with variance in participating body parts (so-called complexity [23]). This was noted even after additional stimulation, such as pushing on the maternal abdomen or using sound. However, the pregnant woman consistently reported feeling significant fetal movement.
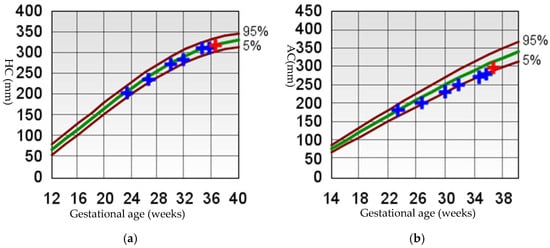
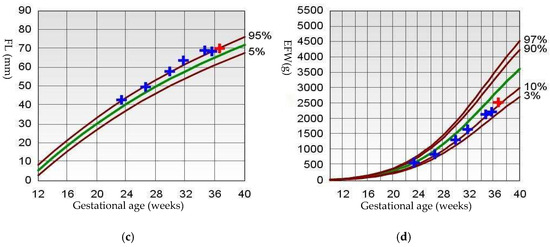
Figure 1.
Fetal growth curve [24,25]. (a) HC, head circumference; (b) AC, abdomen circumference; (c) FL, femur length; (d) EFW, estimated fetal weight. For (a–c) The brown curves represent the 5th and 95th percentile lines, and the green curve represents the 50th percentile line. For (d) The brown curves represent the 3rd, 10th, 90th, and 97th percentile lines, and the green curve represents the 50th percentile line. The crosses indicate the values from each ultrasound measurement, with blue crosses representing previous measurements and red crosses representing the last ultrasound measurement.
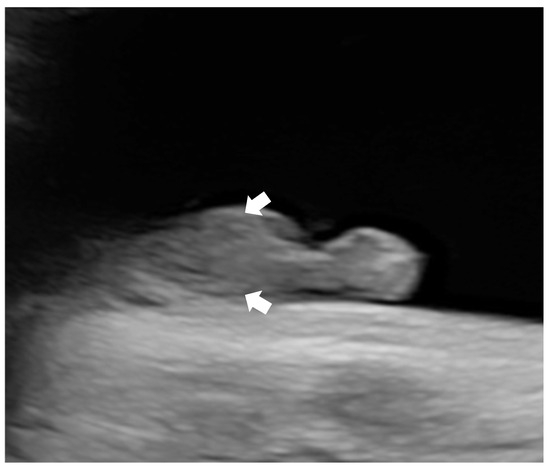
Figure 2.
Ultrasonographic image of bilateral crytorchidism (arrows).
Figure 3.
Ultrasonographic images of small kidneys (length of the kidneys are below the 3rd percentile for 34 weeks weeks) [20]: (a) left kidney; (b) right kidney.
Given the emerging ultrasound features, including a consistently small abdominal circumference, progressive polyhydramnios, bilateral cryptorchidism, small kidneys, and persistently reduced fetal movement, amniocentesis was recommended for genetic study. In China, there are no legal restrictions on termination of pregnancy due to fetal abnormalities, even in late gestation, provided that the diagnosis is confirmed and the parents are fully informed of the potential outcomes. However, the couple declined amniocentesis. A multidisciplinary team discussed the potential perinatal complications associated with a possible diagnosis of PWS in the fetus, and a perinatal management plan was developed at 36 weeks and 1 day of gestation that included strategies for preventing and treating delivery-related complications, managing possible neonatal issues such as asphyxia and poor sucking, and providing counseling about genetic testing for early diagnosis, as well as considerations for short- and long-term complications and follow-up. A 2580 g male newborn was delivered at 38 weeks and 1 day of gestation by Cesarean section because of previous Cesarean section. The newborn displayed several concerning features, including a small eyelid cleft, a weak cry, slightly low muscle tone, bilateral cryptorchidism, and a diminished response to medical procedures like blood draws, and tube feeding was necessary due to poor sucking. Ultrasound showed slightly small kidneys. Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) for the Prader–Willi/Angelman on 15q11-q13 region (SALSA MLPA Probemix ME028, MRC-Holland) was performed, according to the manufacturer’s instructions, on the genomic DNA extracted from the peripheral blood of the patient. The result showed a single copy loss (or heterozygous deletion) at the 15q11.2-q13.1 chromosomal region, which was shown to be methylated, suggesting a paternal deletion of PWS (Figure 4). A lactation specialist instructed breastfeeding function exercise during hospitalization. Before discharge, a long-term management plan was created and discussed with the parents, which included an endocrine assessment, lactation support, and pediatric surgical follow-up.
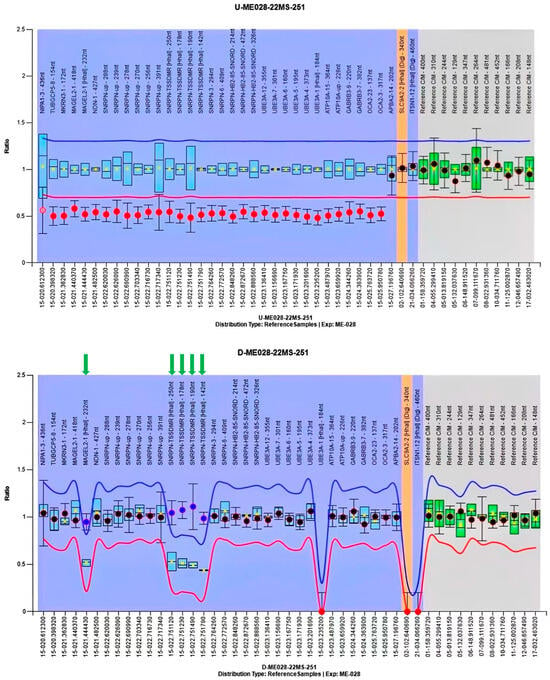
Figure 4.
Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) result of the proband. The upper panel presents the copy number analysis, while the lower panel illustrates the methylation status analysis. In the upper panel, the ratio of the copy number probes (indicated by red dots) of the undigested DNA of the proband was ~0.5, indicating presence of a single copy number (heterozygous deletion). In the lower panel, the ratio of the methylation-specific probes (denoted by green arrows) of the digested DNA of the proband was ~1.0, meaning that the single copy number region was not digested by the methylation-sensitive restriction enzyme HhaI; this implies that the single copy region is methylated. The MS-MLPA findings confirmed the diagnosis of a paternal deletion of PWS.
3. Literature Review
A literature search was conducted using PubMed with the keywords “fetal and PWS”, “prenatal and PWS”, and “PWS and fetal ultrasound” from the database’s inception until June 2024. Table 1 summarizes the prenatal characteristics associated with PWS. Most common prenatal manifestations of PWS include decreased fetal movements, polyhydramnios, and fetal growth restriction (FGR). FGR [7,16,26,27,28,29,30,31,32,33], typically asymmetrical, was observed in 21.1% to 65% of cases, characterized by an increased head-to-abdomen circumference ratio or decreased abdominal circumference [26,27,30,31,33]. Decreased fetal movements were reported in 27% to 92% of cases [7,16,26,27,28,29,30,31,32,33]. Polyhydramnios was present in 23% to 46% of cases [7,16,26,27,28,29,30,31,32,33]. Breech presentation was reported in 25.7% to 63.6% of fetuses [16,26,27,28,29,30,31], and cryptorchidism was observed in 87% to 96.7% of male fetuses [26,29,30]. Unusual hand and foot positioning was noted in 9.1% to 25.7% of cases [16,29,32,34,35]. Other less common findings including enlarged lateral ventricles [34,35,36], non-reactive cardiotocography (CTG) pattern [14,31], fetal cardiac rhabdomyoma [33], large biparietal diameter [14], and hydronephrosis [26] have also been seen in different individual cases. Small kidneys and single umbilical artery (SUA) have not been previously reported in PWS cases.

Table 1.
Summary of prenatal features of PWS.
4. Discussion
PWS presents with complex and varied clinical manifestations, with abnormalities emerging in the fetal stage and evolving with age. This progression leads to multisystem complications, including respiratory and sleep disorders (e.g., obstructive sleep apnea, obesity hypoventilation syndrome), endocrine and metabolic dysfunction (e.g., adrenal insufficiency, diabetes), and orthopedic issues (e.g., scoliosis, hip dysplasia). Behavioral challenges such as hyperphagia and psychiatric comorbidities further complicate management. The syndrome carries a high mortality rate due to respiratory failure, sudden death during sleep, and severe infections, culminating in a poor prognosis [36,37,38,39,40,41,42,43]. Instead of curative treatment, patients receive symptomatic care. Without early diagnosis and care, patients often experience excessive weight gain [44]. Various nutritional problem cases have been documented, often resulting in severe obesity that usually begins between the ages of 3 and 4 [9,10].
Early diagnosis facilitates parental counseling, helps anticipate and prevent obstetric complications during labor, and ensures better preparation for neonatal care. Comprehensive multidisciplinary care is essential for improving outcomes [45,46,47]. This offers parents guidance and support to prevent aspiration and asphyxia in infancy, reduce obesity later in childhood, and stimulate cognitive and adaptive skills, thereby enhancing the patient’s quality of life [48]. This approach can significantly reduce the severe short- and long-term effects of PWS.
PWS arises from the loss of expression of paternally inherited genes in the 15q11.2-q13 chromosomal region. About 70% of PWS cases are caused by errors in genomic imprinting resulting from a paternal deletion, while maternal uniparental disomy accounts for roughly 25% of cases. Fewer cases stem from defects in the imprinting center, such as microdeletions or epimutations on chromosome 15. Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) can diagnose PWS by detecting maternal-only imprinting at 15q11.2-q13. It can also identify the size of the paternally inherited deletion in this region, as well as detect deletions in the imprinting center and SNORD116 [7,17,47,49]. Therefore, MS-MLPA should be the preferred diagnostic method for PWS. While MS-MLPA cannot differentiate between uniparental disomy (UPD) 15 and an imprinting defect caused by epimutation, this limitation only impacts the assessment of recurrence risk and not the patient’s diagnosis [19,50].
However, the lack of a clear fetal phenotype for PWS and limited awareness of it has resulted in very few prenatal diagnoses, which mostly occur accidentally through cytogenetic molecular techniques. In a study of 38 children with PWS, none received a prenatal diagnosis, even though 9 underwent invasive tests that returned normal karyotypes; however, specific genetic tests for PWS were not conducted [27]. Therefore, understanding the fetal features of PWS is crucial for facilitating requests for specific prenatal genetic tests for diagnosis.
While prenatal symptoms cannot guarantee a definitive diagnosis, they can assist clinicians in diagnosing PWS and should prompt early parental counseling and careful decision making during pregnancy and delivery. The literature review identifies decreased fetal movements, FGR, and polyhydramnios as the most common prenatal features of PWS. FGR typically presents as asymmetrical, which can be identified by a higher head-to-abdomen circumference ratio or a smaller abdominal circumference. Among male fetuses, the prevalence of cryptorchidism ranges from 87% to 96.7% [26,29,30]. Breech presentation occurs in 25.7% to 63.6% of fetuses, likely due to increased movement from excessive amniotic fluid. Polyhydramnios is a common finding in fetuses with congenital neuromuscular diseases, probably caused by the weakness of swallowing movements, in analogy with the sucking problems encountered in neonatal life [14]. Additionally, abnormal extremities were noted in 9.1% to 25.7% of cases, possibly related to fetal central nervous system disease [16,29]. It is unclear whether certain uncommon ultrasound findings—such as enlarged lateral ventricles [26,34,35], non-reactive CTG patterns [14,31], fetal cardiac rhabdomyoma [33], large biparietal diameter [14], and hydronephrosis [26]—are characteristic of PWS. Further studies are necessary to identify specific prenatal phenotypes associated with PWS.
Our case illustrates the characteristics of a PWS fetus from the middle to the late stages of pregnancy. We noted that the clinical features mainly present after 26 weeks of gestation, similar with previous reports [29]. Regarding estimated weight, although the fetus did not meet the diagnostic criteria for FGR, poor growth in abdominal circumference aligns with literature findings. It shows that PWS is often associated with asymmetrical FGR, which is characterized by either an increased head-to-abdomen circumference ratio or a decreased abdominal circumference. A small abdominal circumference may be a more significant characteristic of PWS than reduced fetal weight. In our case, the fetal movements observed with ultrasound were inconsistent with those reported by the pregnant woman, suggesting that in the case of polyhydramnios combined with FGR, attention should be paid to the observation of fetal movement during ultrasound scanning, rather than relying on maternal perception which is subjective. While the presence of a single umbilical artery in our PWS fetus could be coincidental, conducting thorough and regular ultrasound assessments of fetuses with soft markers is crucial. This approach may enhance the chances of discovering new clinical clues for early disease diagnosis. In our case, smaller kidneys were first noted on ultrasound examination. Because measuring fetal kidneys is not standard in routine ultrasound examinations, we cannot determine if smaller kidneys are common in PWS fetuses or merely coincidental. However, it is reasonable to consider a potential association, and documenting this combination may aid future investigations into the genotype–phenotype correlation of PWS. To validate the inclusion of a single umbilical artery and small kidneys as markers for PWS in prenatal scans, a large sample size study is needed to confirm their consistency across PWS fetuses.
The complex pathogenesis of PWS, combined with the limitations of genetic tests, means that relying on a single test may result in missed diagnoses. For instance, G-banding does not detect chromosomal microdeletions, maternal uniparental disomy, or defects in the imprinting center, while chromosomal microarray fails to identify maternal uniparental disomy or point mutations [33,35,46]. Antenatal diagnosis of PWS can be achieved using specific molecular genetic tests, such as methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA). Understanding the prenatal features of PWS allows for a heightened suspicion of the disorder, which can facilitate early diagnosis through targeted genetic testing. Notably, fetal growth restriction—a key prenatal feature of PWS—exemplifies how epigenetic dysregulation (e.g., aberrant methylation at 15q11.2-q13) can alter developmental programming. As highlighted by Salmeri et al. [51], elucidating the role of epigenetics in the developmental origins of health and disease represents a new challenge for the coming years, with the goal of providing early interventions and prevention strategies and, hopefully, new treatment opportunities. In cases without invasive prenatal diagnosis, the fetal condition may be suspected before birth, enabling the family to prepare for a potential diagnosis. Multidisciplinary intervention could be started early, and newborns can receive the attention of serial multidisciplinary team assessment for the earliest diagnosis and management to improve outcome in early and later life.
5. Conclusions
PWS should be listed in differential diagnoses if a fetus has the following features: polyhydramnios, decreased fetal movements, and poor growth of abdominal circumference. Further comprehensive serial ultrasound assessment is needed to see if additional possible features of PWS are present. This report identifies the presence of small kidneys and a single umbilical artery as potential new prenatal sonographic markers for PWS. Understanding more of the prenatal characteristics of PWS helps to select effective diagnostic tests in the prenatal period to avoid missed diagnosis.
Author Contributions
Conceptualization, L.L.; writing—original draft preparation, L.L.; writing—review and editing, M.H.Y.T., S.L., A.S.-Y.K. and K.Y.K.C.; clinical management, C.C., Y.X., L.N. and H.H. genetic analysis, C.K.Y.C., X.D. and T.Z.; data collection, Y.L.; supervision, M.H.Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Shenzhen Medical Research Fund (C2401032), Shenzhen Innovation and Technology Commission Foundation (Grant No. JCYJ20210324114612034), Sanming project of Medicine in Shenzhen (No. SZSM202311022), and Shenzhen Clinical Research Center for Rare Diseases (LCYSSQ20220823091402005), as well as being partially supported by the Hong Kong Metropolitan University Research Grant (No. RD/2023/2.24).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the HKU-SZH Research Ethics Committee (protocol code IRB Ref.No:[2025]021, 7 February 2025).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The authors declare that the data for this research are available from the correspondence authors upon reasonable request.
Acknowledgments
We acknowledge the work and contribution of all the health providers.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PWS | Prader–Willi syndrome |
| MS-MLPA | Methylation-specific multiplex ligation-dependent probe amplification |
| NIPS | Noninvasive prenatal screening |
| EFW | Estimated fetal weight |
| AC | Abdominal circumference |
| SUA | Single umbilical artery |
| AFI | Amniotic fluid index |
| FGR | Fetal growth restriction |
| CTG | Cardiotocography |
| UPD | Uniparental disomy |
References
- Prader, A. Ein syndrom von adipositas, kleinwuchs, kryptorchismus und oligophrenie nach myatonieartigem zustand im neugeborenenalter. Schweiz. Med. Wochenschr. 1956, 86, 1260–1261. [Google Scholar]
- Tsai, J.H.; Scheimann, A.O.; McCandless, S.E.; Strong, T.V.; Bridges, J.F.P. Caregiver priorities for endpoints to evaluate treatments for Prader-Willi syndrome: A best-worst scaling. J. Med. Econ. 2018, 21, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Whitman, B.Y. Prader-Willi Syndrome: The More We Know, the Less We Know. Mo. Med. 2024, 121, 235–241. [Google Scholar] [PubMed]
- Butler, M.G. Prader-Willi Syndrome and Chromosome 15q11.2 BP1-BP2 Region: A Review. Int. J. Mol. Sci. 2023, 24, 4271. [Google Scholar] [CrossRef]
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi Syndrome—Clinical Genetics, Diagnosis and Treatment Approaches: An Update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef]
- Mahmoud, R.; Kimonis, V.; Butler, M.G. Clinical Trials in Prader-Willi Syndrome: A Review. Int. J. Mol. Sci. 2023, 24, 2150. [Google Scholar] [CrossRef]
- Driscoll, D.J.; Miller, J.L.; Cassidy, S.B. Prader-Willi Syndrome. In GeneReviews(®); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Tauber, M.; Hoybye, C. Endocrine disorders in Prader-Willi syndrome: A model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021, 9, 235–246. [Google Scholar] [CrossRef]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef]
- Bravo, J.P.; Pérez, P.D.; Canals Cifuentes, A. Nutritional phases of Prader-Willi syndrome. Andes Pediatr. 2021, 92, 359–366. [Google Scholar] [CrossRef]
- Höybye, C.; Tauber, M. Approach to the Patient With Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, 1698–1705. [Google Scholar] [CrossRef]
- Shubina, J.; Barkov, I.Y.; Stupko, O.K.; Kuznetsova, M.V.; Goltsov, A.Y.; Kochetkova, T.O.; Trofimov, D.Y.; Sukhikh, G.T. Prenatal diagnosis of Prader-Willi syndrome due to uniparental disomy with NIPS: Case report and literature review. Mol. Genet. Genom. Med. 2020, 8, e1448. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.K.; Park, J.E.; Kang, K.M.; Shim, S.H.; Shim, S.H.; Han, Y.J.; Cho, H.Y.; Cha, D.H. Prenatal Diagnosis of Uniparental Disomy in Cases of Rare Autosomal Trisomies Detected Using Noninvasive Prenatal Test: A Case of Prader-Willi Syndrome. Diagnostics 2023, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, H.; Kozuma, S.; Hayashi, N.; Unno, N.; Fujii, T.; Tsutsumi, O.; Okai, T.; Taketani, Y. A fetus with Prader-Willi syndrome showing normal diurnal rhythm and abnormal ultradian rhythm on heart rate monitoring. Fetal Diagn. Ther. 2000, 15, 304–307. [Google Scholar] [CrossRef]
- Fong, B.F.; De Vries, J.I. Obstetric aspects of the Prader-Willi syndrome. Ultrasound Obstet. Gynecol. 2003, 21, 389–392. [Google Scholar] [CrossRef]
- Geysenbergh, B.; De Catte, L.; Vogels, A. Can fetal ultrasound result in prenatal diagnosis of Prader-Willi syndrome? Genet. Couns. 2011, 22, 207–216. [Google Scholar] [PubMed]
- Fermin Gutierrez, M.A.; Daley, S.F.; Mendez, M.D. Prader-Willi Syndrome. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Oto, Y.; Murakami, N.; Imatani, K.; Inoue, T.; Itabashi, H.; Shiraishi, M.; Nitta, A.; Matsubara, K.; Kobayashi, S.; Ihara, H.; et al. Perinatal and neonatal characteristics of Prader-Willi syndrome in Japan. Pediatr. Int. 2023, 65, e15540. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.Q.; Su, Y.Y.; Qiu, X.Y.; Lu, X.Y.; Li, J.X.; Huang, M.; Luo, X.P. Clinical screening and genetic diagnosis for Prader-Willi syndrome. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 1001–1006. [Google Scholar] [CrossRef]
- Chitty, L.S.; Altman, D.G. Charts of fetal size: Kidney and renal pelvis measurements. Prenat. Diagn. 2003, 23, 891–897. [Google Scholar] [CrossRef]
- Koirla, S.; Adhikari, K.; Devkota, K. Ultrasound Measurement of Fetal Kidney Length in Normal Pregnancy and its Correlation with Gestational Age. J. Nepal. Health Res. Counc. 2023, 20, 958–961. [Google Scholar] [CrossRef]
- Choudhary, A.; Sibia, P.; Kaur, S.; Gupta, S.; Gambhir, P.; Kaur, R. Evaluation of fetal kidney length as a marker for fetal biometry. Arch. Gynecol. Obstet. 2024, 310, 1451–1459. [Google Scholar] [CrossRef]
- de Vries, J.I.; Visser, G.H.; Prechtl, H.F. The emergence of fetal behaviour. I. Qualitative aspects. Early Hum. Dev. 1982, 7, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.N.; Pang, M.W.; Daljit, S.S.; Leung, T.Y.; Poon, C.F.; Wong, S.M.; Lau, T.K. Fetal biometry in ethnic Chinese: Biparietal diameter, head circumference, abdominal circumference and femur length. Ultrasound Obstet. Gynecol. 2008, 31, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Srebnik, N.; Gross Even-Zohar, N.; Salama, A.; Sela, H.Y.; Hirsch, H.J.; Gross-Tsur, V.; Eldar-Geva, T. Recognizing the unique prenatal phenotype of Prader-Willi Syndrome (PWS) indicates the need for a diagnostic methylation test. Prenat. Diagn. 2020, 40, 878–884. [Google Scholar] [CrossRef]
- Bar, C.; Diene, G.; Molinas, C.; Bieth, E.; Casper, C.; Tauber, M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet J. Rare Dis. 2017, 12, 118. [Google Scholar] [CrossRef]
- Gross, N.; Rabinowitz, R.; Gross-Tsur, V.; Hirsch, H.J.; Eldar-Geva, T. Prader-Willi syndrome can be diagnosed prenatally. Am. J. Med. Genet. A 2015, 167A, 80–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, M.S.; Li, G.Y.; Zhang, Z.J.; Ding, J.; Xu, Y.W.; Qiu, Z.Q.; Song, H.M. Analysis of the clinical perinatal characteristics of 226 patients with Prader-Willi syndrome in China. Zhonghua Er Ke Za Zhi 2021, 59, 466–470. [Google Scholar] [CrossRef]
- Grootjen, L.N.; Uyl, N.E.M.; van Beijsterveldt, I.; Damen, L.; Kerkhof, G.F.; Hokken-Koelega, A.C.S. Prenatal and Neonatal Characteristics of Children with Prader-Willi Syndrome. J. Clin. Med. 2022, 11, 679. [Google Scholar] [CrossRef]
- Murata, T.; Fukuda, T.; Kanno, A.; Kyozuka, H.; Yamaguchi, A.; Shimizu, H.; Watanabe, T.; Fujimori, K. Polyhydramnios and abnormal foetal heart rate patterns in a foetus with Prader-Willi syndrome: A case report. Case Rep. Women’s Health 2020, 27, e00227. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, S.; Li, J.; Li, J.; Chen, Q.; Luo, J.; Li, C.; Li, H.; Qi, H.; Li, R. Possibility of early diagnosis in a fetus affected by Prader-Willi syndrome with maternal hetero-UPD15: A lesson to be learned. Mol. Med. Rep. 2019, 20, 95–102. [Google Scholar] [CrossRef]
- Traisrisilp, K.; Sirikunalai, P.; Sirilert, S.; Chareonsirisuthigul, T.; Tongsong, T. Cardiac rhabdomyoma as a possible new prenatal sonographic feature of Prader-Willi syndrome. J. Obstet. Gynaecol. Res. 2022, 48, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Bigi, N.; Faure, J.M.; Coubes, C.; Puechberty, J.; Lefort, G.; Sarda, P.; Blanchet, P. Prader-Willi syndrome: Is there a recognizable fetal phenotype? Prenat. Diagn. 2008, 28, 796–799. [Google Scholar] [CrossRef] [PubMed]
- L’Herminé, A.C.; Aboura, A.; Brisset, S.; Cuisset, L.; Castaigne, V.; Labrune, P.; Frydman, R.; Tachdjian, G. Fetal phenotype of Prader-Willi syndrome due to maternal disomy for chromosome 15. Prenat. Diagn. 2003, 23, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.; Eddiry, S.; Lacassagne, E.; Laurier, V.; Molinas, C.; Bieth, É.; Franchitto, N.; Salles, J.P.; Tauber, M. Patients with PWS and related syndromes display differentially methylated regions involved in neurodevelopmental and nutritional trajectory. Clin. Epigenet. 2021, 13, 159. [Google Scholar] [CrossRef]
- Schwartz, L.; Caixàs, A.; Dimitropoulos, A.; Dykens, E.; Duis, J.; Einfeld, S.; Gallagher, L.; Holland, A.; Rice, L.; Roof, E.; et al. Behavioral features in Prader-Willi syndrome (PWS): Consensus paper from the International PWS Clinical Trial Consortium. J. Neurodev. Disord. 2021, 13, 25. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Faggiano, F.; Maiorino, M.I.; Parrillo, M.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Savastano, S.; Colao, A. Obesity in Prader-Willi syndrome: Physiopathological mechanisms, nutritional and pharmacological approaches. J. Endocrinol. Investig. 2021, 44, 2057–2070. [Google Scholar] [CrossRef]
- Amaro, A.S.; Rubin, D.A.; Teixeira, M.; Ferreira, A.J., Jr.; Rodrigues, G.M.; Carreiro, L.R.R. Health Problems in Individuals With PWS Are Associated With Lower Quality of Life for Their Parents: A Snapshot in the Brazilian Population. Front. Pediatr. 2022, 10, 746311. [Google Scholar] [CrossRef]
- Itani, R.; Gillett, E.S.; Perez, I.A. Sleep Consequences of Prader-Willi Syndrome. Curr. Neurol. Neurosci. Rep. 2023, 23, 25–32. [Google Scholar] [CrossRef]
- Duis, J. Prader-Willi syndrome: An update. Curr. Opin. Pulm. Med. 2023, 29, 539–542. [Google Scholar] [CrossRef]
- McQuivey, K.S.; Chung, A.S.; Jones, M.R.; Makovicka, J.L.; Christopher, Z.K.; Brinkman, J.C.; Belthur, M. Hospital outcomes in pediatric patients with Prader-Willi syndrome (PWS) undergoing orthopedic surgery: A 12-year analysis of national trends in surgical management and inpatient hospital outcomes. J. Orthop. Sci. 2022, 27, 1304–1308. [Google Scholar] [CrossRef]
- Kusz, M.J.; Gawlik, A.M. Adrenal insufficiency in patients with Prader-Willi syndrome. Front. Endocrinol. 2022, 13, 1021704. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Palop, O.; Romero, A.; Casamitjana, L.; Pareja, R.; Rigla, M.; Caixàs, A. Effect of semaglutide on weight loss and glycaemic control in patients with Prader-Willi Syndrome and type 2 diabetes. Endocrinol. Diabetes Nutr. 2024, 71, 83–87. [Google Scholar] [CrossRef]
- Grootjen, L.N.; Trueba-Timmermans, D.J.; Damen, L.; Mahabier, E.F.; Kerkhof, G.F.; Hokken-Koelega, A.C.S. Long-Term Growth Hormone Treatment of Children with PWS: The Earlier the Start, the Better the Outcomes? J. Clin. Med. 2022, 11, 2496. [Google Scholar] [CrossRef] [PubMed]
- Salvatoni, A.; Nosetti, L.; Salvatore, S.; Agosti, M. Benefits of multidisciplinary care in Prader-Willi syndrome. Expert Rev. Endocrinol. Metab. 2021, 16, 63–71. [Google Scholar] [CrossRef]
- Ahakoud, M.; Daha Belghiti, H.; Nedbour, A.; Bouramtane, A.; Chaouki, S.; Bouguenouch, L.; Ouldim, K. The Diagnosis and Genetic Mechanisms of Prader-Willi Syndrome: Findings From a Moroccan Population Study. Cureus 2023, 15, e37866. [Google Scholar] [CrossRef]
- Erhardt, É.; Molnár, D. Prader-Willi Syndrome: Possibilities of Weight Gain Prevention and Treatment. Nutrients 2022, 14, 1950. [Google Scholar] [CrossRef]
- Godler, D.E.; Singh, D.; Butler, M.G. Genetics of Prader-Willi and Angelman syndromes: 2024 update. Curr. Opin. Psychiatry 2025, 38, 95–100. [Google Scholar] [CrossRef]
- Ma, V.K.; Mao, R.; Toth, J.N.; Fulmer, M.L.; Egense, A.S.; Shankar, S.P. Prader-Willi and Angelman Syndromes: Mechanisms and Management. Appl. Clin. Genet. 2023, 16, 41–52. [Google Scholar] [CrossRef]
- Salmeri, N.; Carbone, I.F.; Cavoretto, P.I.; Farina, A.; Morano, D. Epigenetics Beyond Fetal Growth Restriction: A Comprehensive Overview. Mol. Diagn. Ther. 2022, 26, 607–626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).