Non-Invasive Diagnostic Imaging in Kaposi Sarcoma Evaluation
Abstract
1. Introduction
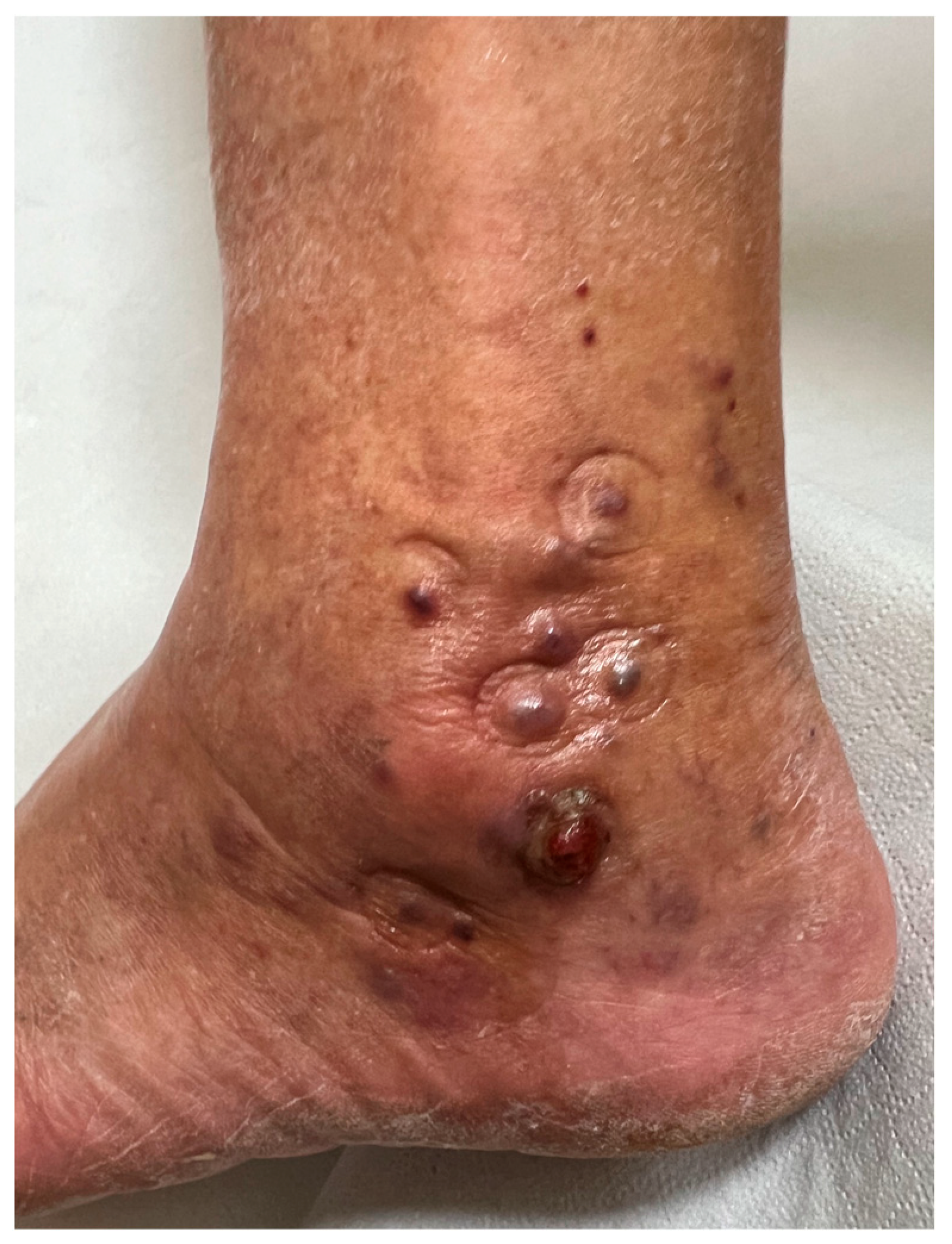
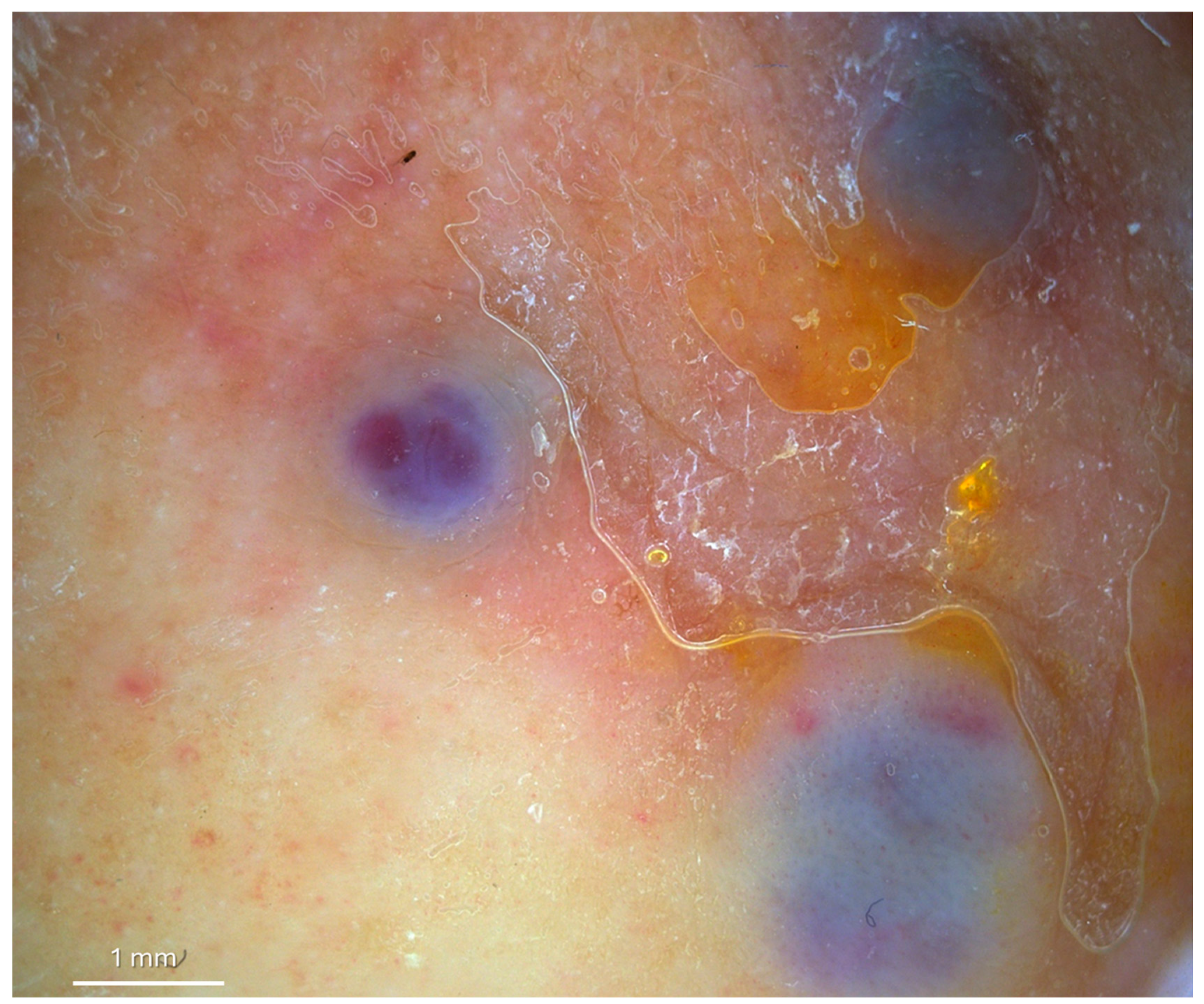
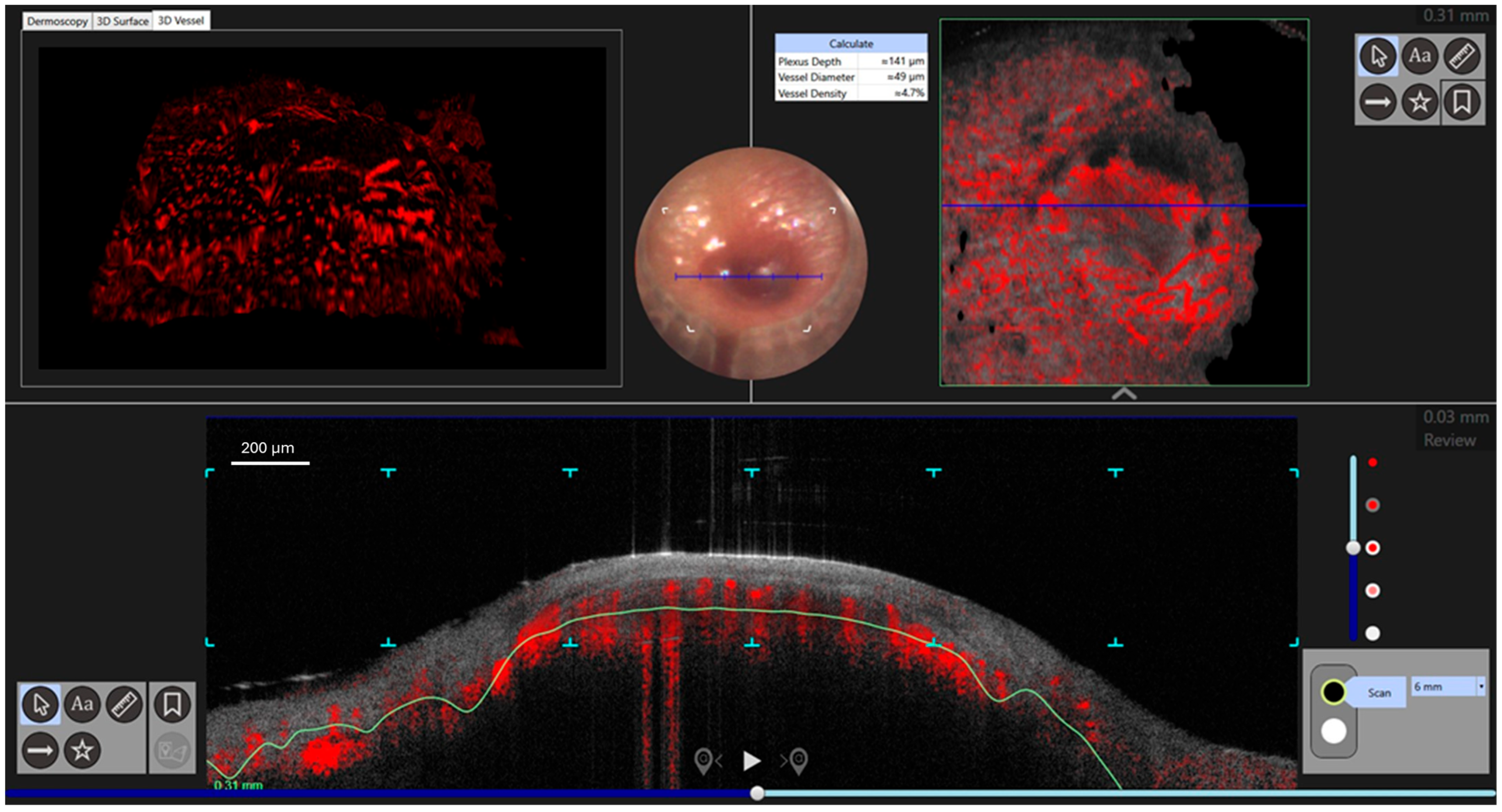
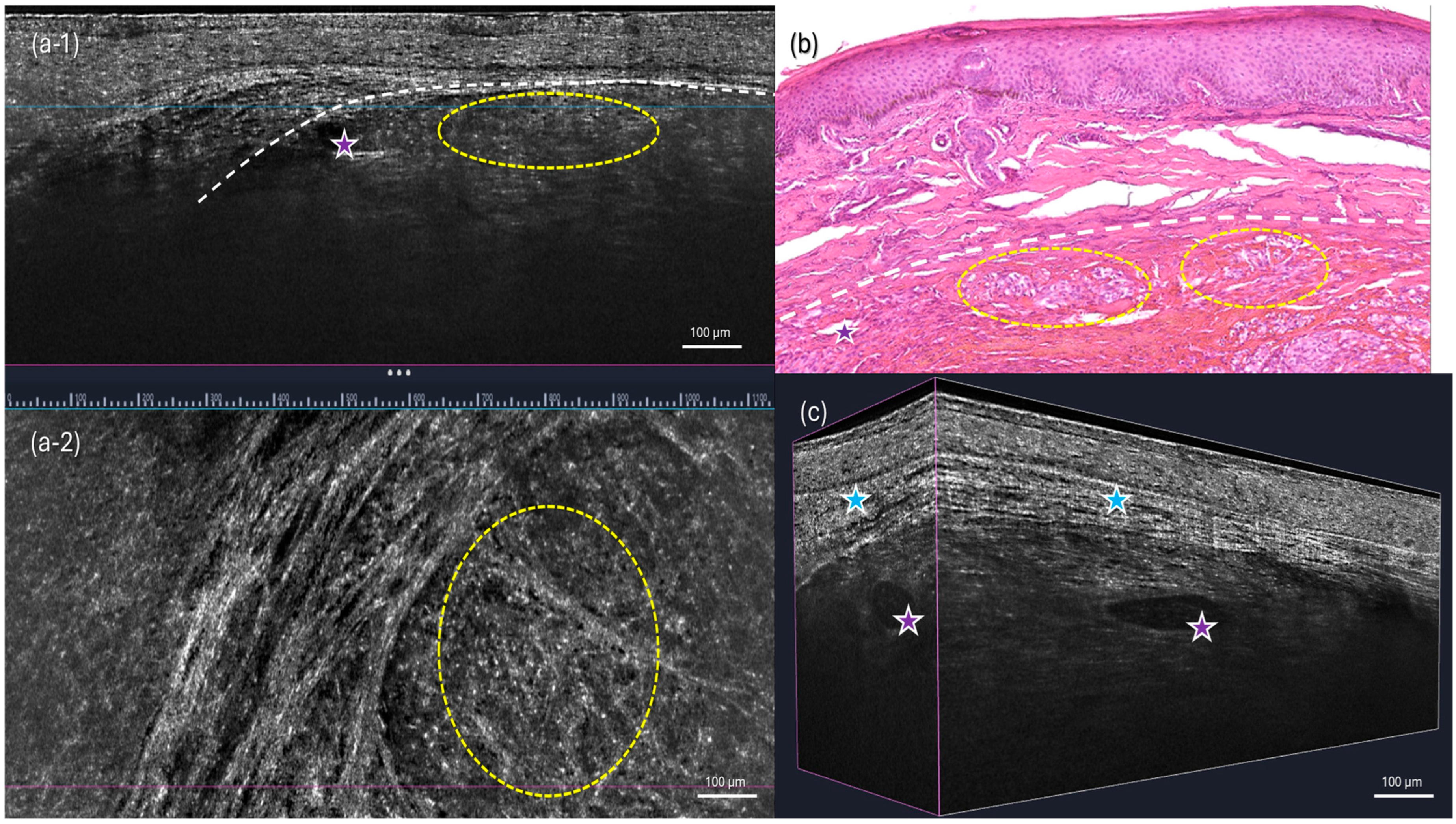
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KS | Kaposi Sarcoma |
| HHV-8 | Herpesvirus 8 |
| d-OCT | Dynamic Optical Coherence Tomography |
| Lc-OCT | Line-field Optical Coherence Tomography |
References
- Kaposi, M. Idiopathisches multiples Pigmentsarkom der Haut. Arch. Dermatol. Syph. 1872, 4, 265–273. [Google Scholar] [CrossRef]
- Stănescu, L.; Foarfă, C.; Georgescu, A.C.; Georgescu, I. Kaposi’s sarcoma associated with AIDS. Rom. J. Morphol. Embryol. 2007, 48, 181–187. [Google Scholar] [PubMed]
- Kemény, L.; Gyulai, R.; Kiss, M.; Nagy, F.; Dobozy, A. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8: A new virus in human pathology. J. Am. Acad. Dermatol. 1997, 37, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, E.; Ruocco, V.; Tornesello, M.L.; Gambardella, A.; Wolf, R.; Buonaguro, F.M. Kaposi’s sarcoma: Etiology and pathogenesis, inducing factors, causal associations, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 413–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giraldo, G.; Beth, E.; Coeur, P.; Vogel, C.L.; Dhru, D.S. Kaposi’s sarcoma: A new model in the search for viruses associated with human malignancies. J. Natl. Cancer Inst. 1972, 49, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, G.; Beth, E.; Haguenau, F. Herpes-type virus particles in tissue culture of Kaposi’s sarcoma from different geographic regions. J. Natl. Cancer Inst. 1972, 49, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, P.; Konrad, A.; Jochmann, R.; Lausen, B.; Holz, P.; Naschberger, E.; Neipel, F.; Britzen-Laurent, N.; Stürzl, M. Structural proteins of Kaposi’s sarcoma-associated herpesvirus antagonize p53-mediated apoptosis. Oncogene 2015, 34, 639–649. [Google Scholar] [CrossRef]
- Juillard, F.; De León Vázquez, E.; Tan, M.; Li, S.; Kaye, K.M. Kaposi’s Sarcoma-Associated Herpesvirus LANA-Adjacent Regions with Distinct Functions in Episome Segregation or Maintenance. J. Virol. 2019, 93, e02158-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marušić, Z.; Billings, S.D. Histopathology of Spindle Cell Vascular Tumors. Surg. Pathol. Clin. 2017, 10, 345–366. [Google Scholar] [CrossRef]
- Bisceglia, M.; Minenna, E.; Altobella, A.; Sanguedolce, F.; Panniello, G.; Bisceglia, S.; Ben-Dor, D.J. Anaplastic Kaposi’s Sarcoma of the Adrenal in an HIV-negative Patient with Literature Review. Adv. Anat. Pathol. 2019, 26, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Mohanna, S.; Maco, V.; Bravo, F.; Gotuzzo, E. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: A critical review of an old disease. Int. J. Infect. Dis. 2005, 9, 239–250. [Google Scholar] [CrossRef]
- Grabar, S.; Costagliola, D. Epidemiology of Kaposi’s Sarcoma. Cancers 2021, 13, 5692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batash, R.; Crimí, A.; Kassem, R.; Asali, M.; Ostfeld, I.; Biz, C.; Ruggieri, P.; Schaffer, M. Classic Kaposi sarcoma: Diagnostics, treatment modalities, and genetic implications—A review of the literature. Acta Oncol. 2024, 63, 783–790. [Google Scholar] [CrossRef]
- Temelkova, I.; Tronnier, M.; Terziev, I.; Wollina, U.; Lozev, I.; Goldust, M.; Tchernev, G. A Series of Patients with Kaposi Sarcoma (Mediterranean/Classical Type): Case Presentations and Short Update on Pathogenesis and Treatment. J. Med. Sci. 2018, 6, 1688–1693. [Google Scholar] [CrossRef]
- Chatlynne, L.G.; Ablashi, D.V. Seroepidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV). Semin. Cancer Biol. 1999, 9, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Tun, A.; Gupta, A.; Berkowitz, L.B.; Anwar, R.; Liu, Y.; Guevara, E. A case of classic Kaposi sarcoma in an immunocompetent human immunodeficiency virus-negative Dominican man. SAGE Open Med. Case Rep. 2020, 8, 2050313X20938249. [Google Scholar] [CrossRef]
- Lebbe, C.; Garbe, C.; Stratigos, A.J.; Harwood, C.; Peris, K.; Marmol, V.D.; Malvehy, J.; Zalaudek, I.; Hoeller, C.; Dummer, R.; et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur. J. Cancer 2019, 114, 117–127. [Google Scholar] [CrossRef]
- Patel, R.; Lurain, K.; Yarchoan, R.; Ramaswami, R. Clinical management of Kaposi sarcoma herpesvirus-associated diseases: An update on disease manifestations and treatment strategies. Expert. Rev. Anti Infect. Ther. 2023, 21, 929–941. [Google Scholar] [CrossRef]
- Elmas, O.F.; Mayisoglu, H.; Celik, M.; Kilitci, A.; Akdeniz, N. Dermoscopic rainbow pattern: A strong clue to malignancy or just a light show? North. Clin. Istanb. 2020, 7, 494–498. [Google Scholar] [CrossRef]
- Ertürk Yılmaz, T.; Akay, B.N.; Okçu Heper, A. Dermoscopic findings of Kaposi sarcoma and dermatopathological correlations. Australas. J. Dermatol. 2020, 61, e46–e53. [Google Scholar] [CrossRef]
- Villani, A.; Fabbrocini, G.; Peduto, T.; Scalvenzi, M. Reflectance confocal microscopy for Kaposi’s sarcoma treatment monitoring after intralesional vincristine chemotherapy. Dermatol. Ther. 2022, 35, e15539. [Google Scholar] [CrossRef]
- Ulrich, M.; Themstrup, L.; de Carvalho, N.; Ciardo, S.; Holmes, J.; Whitehead, R.; Welzel, J.; Jemec, G.B.E.; Pellacani, G. Dynamic optical coherence tomography of skin blood vessels-proposed terminology and practical guidelines. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 152–155. [Google Scholar] [CrossRef]
- Cantisani, C.; Baja, A.V.; Gargano, L.; Rossi, G.; Ardigò, M.; Soda, G.; Boostani, M.; Kiss, N.; Pellacani, G. Optical Coherence Tomography as a Valuable Tool for the Evaluation of Cutaneous Kaposi Sarcoma Treated with Imiquimod 5% Cream. Diagnostics 2023, 13, 2901. [Google Scholar] [CrossRef]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. [Google Scholar] [CrossRef]
- Tognetti, L.; Carraro, A.; Lamberti, A.; Cinotti, E.; Suppa, M.; Luc Perrot, J.; Rubegni, P. Kaposi sarcoma of the glans: New findings by line field confocal optical coherence tomography examination. Skin. Res. Technol. 2021, 27, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Tourlaki, A.; Nazzaro, G.; Wei, Y.; Buffon, S.; Mattioli, M.A.; Marzano, A.V.; Brambilla, L. Clinical, Dermoscopic, Ultrasonographic, and Histopathologic Correlations in Kaposi’s Sarcoma Lesions and Their Differential Diagnoses: A Single-Center Prospective Study. J. Clin. Med. 2023, 12, 278. [Google Scholar] [CrossRef]
- Al-Sukhni, L.; Mui, U.N.; Tarbox, M. A spectrum of diseases with the dermatoscopic rainbow pattern. JAAD Case Rep. 2022, 21, 144–147. [Google Scholar] [CrossRef]
- Cappilli, S.; Suppa, M.; Ricci, C.; Del Marmol, V.; Peris, K.; Di Stefani, A. Line-field confocal optical coherence tomography of cutaneous vascular lesions: Morphological assessment and histopathological correlations. J. Eur. Acad. Dermatol. Venereol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L.; Satriano, R.A.; Ruocco, E.; Castello, G.; Ruocco, V. Kaposi’s sarcoma: Aetiopathogenesis, histology and clinical features. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 138–154. [Google Scholar] [CrossRef]
- Mandel, V.D.; Ardigò, M. Non-Invasive Diagnostic Techniques in Dermatology. J. Clin. Med. 2023, 12, 1081. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantisani, C.; Di Guardo, A.; Ardigò, M.; Suppa, M.; Gonzalez, S.; Longo, C.; Taliano, A.; Rovaldi, E.; Cinotti, E.; Pellacani, G. Non-Invasive Diagnostic Imaging in Kaposi Sarcoma Evaluation. Diagnostics 2025, 15, 1665. https://doi.org/10.3390/diagnostics15131665
Cantisani C, Di Guardo A, Ardigò M, Suppa M, Gonzalez S, Longo C, Taliano A, Rovaldi E, Cinotti E, Pellacani G. Non-Invasive Diagnostic Imaging in Kaposi Sarcoma Evaluation. Diagnostics. 2025; 15(13):1665. https://doi.org/10.3390/diagnostics15131665
Chicago/Turabian StyleCantisani, Carmen, Antonio Di Guardo, Marco Ardigò, Mariano Suppa, Salvador Gonzalez, Caterina Longo, Alberto Taliano, Emanuele Rovaldi, Elisa Cinotti, and Giovanni Pellacani. 2025. "Non-Invasive Diagnostic Imaging in Kaposi Sarcoma Evaluation" Diagnostics 15, no. 13: 1665. https://doi.org/10.3390/diagnostics15131665
APA StyleCantisani, C., Di Guardo, A., Ardigò, M., Suppa, M., Gonzalez, S., Longo, C., Taliano, A., Rovaldi, E., Cinotti, E., & Pellacani, G. (2025). Non-Invasive Diagnostic Imaging in Kaposi Sarcoma Evaluation. Diagnostics, 15(13), 1665. https://doi.org/10.3390/diagnostics15131665







