Endo-Periodontal Lesions in Endodontically Treated Teeth with Periapical Pathology
Abstract
1. Introduction
- -
- EPLs with Necrotic Pulp—lesions originating from endodontic infection (e.g., pulp necrosis) that extends to the periodontal tissues. These lesions are characterized by the presence of a deep periodontal pocket, purulent exudate, swelling, sensitivity to percussion, and potential fistula formation.
- -
- EPLs with Vital Pulp—lesions develop when periodontal disease affects the apical portion of the root leading to secondary pulpal inflammation, with vital pulp tissue. These lesions are characterized by the presence of a periodontal pocket and clinical signs of periodontal involvement without signs of endodontic infection.
- -
- Combined EPLs—lesions resulting from concurrent endodontic and periodontal in-fections that unite over time. Pulp and periodontal tissues are independently affected, developing simultaneously extensive tissue destruction. Combined EPLs are responsible for more than 50% of general tooth loss [4].
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S219–S229. [Google Scholar] [CrossRef] [PubMed]
- Kuoch, P.; Bonte, E. Endoperiodontal Lesions and Chicago’s New Classification of Periodontal and Peri-implant Diseases and Conditions. J. Contemp. Dent. Pract. 2020, 21, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.D.; Luepke, P.G.; Ibrahim, M.S.; Guentsch, A. Treatment of a periodontic-endodontic lesion in a patient with aggressive periodontitis. Case Rep. Dent. 2016, 7080781. [Google Scholar] [CrossRef]
- Ruetters, M.; Gehrig, H.; Kronsteiner, D.; Kim, T.S.; Schuessler, D.L. Prevalence of endo-perio lesions according to the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Disease in a university hospital. Quintessence Int. 2022, 53, 134–142. [Google Scholar] [CrossRef]
- Rotstein, I. Interaction between endodontics and periodontics. Periodontology 2000 2017, 74, 11–39. [Google Scholar] [CrossRef]
- Zaharescu, A.; Mârțu, I.; Luchian, A.I.; Mârțu, M.A.; Șufaru, I.G.; Mârțu, C.; Solomon, S.M. Role of adjunctive therapy with subantimicrobial doses of doxycycline in glycemic control (HbA1c) in patients with diabetes and endo-periodontal lesions to prevent sinus complications. Exp. Ther. Med. 2021, 21, 277. [Google Scholar] [CrossRef]
- Antohi, C.; Salceanu, M.; Aminov, L.; Mârțu, M.-A.; Dascălu, C.G.; Dodi, G.; Stoica, G.; Bandol, G.; Iancu, D.; Dobrovat, B.; et al. Assessment of Systemic and Maxillary Bone Loss in Cancer Patients with Endo-Periodontal Lesions Using Dkk-1 Biomarker and Dental Radiological Examinations. Appl. Sci. 2022, 12, 5235. [Google Scholar] [CrossRef]
- Zaharescu, A.; Solomon, S.M.; Luca, M.G.; Toma, V.; Luchian, I.; Șufaru, I.G.; Mârțu, S. Quantification of proinflammatory molecules (IL1-α, IL1-β, IL2, IL12, IFN-γ, TNF-α) in crevicular fluid and serum in patients with endo-periodontal lesions. Rev. Chim. 2019, 70, 2252–2255. [Google Scholar] [CrossRef]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S.J. Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Rai, A.K.; Sharma, M.; Tripathi, M.; Prasad, R. Microbial biofilms: Recent advances and progress in environmental bioremediation. Sci. Total Environ. 2022, 824, 153843. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Røder, H.L.; Madsen, J.S.; Bjarnsholt, T.; Sørensen, S.J.; Burmølle, M. Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization. Front. Microbiol. 2016, 7, 1366. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Li, H.; Guan, R.; Sun, J.; Hou, B. Bacteria community study of combined periodontal-endodontic lesions using denaturing gradient gel electrophoresis and sequencing analysis. J. Periodontol. 2014, 85, 1442–1449. [Google Scholar] [CrossRef]
- Gomes, B.P.; Berber, V.B.; Kokaras, A.S.; Chen, T.; Paster, B.J. Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J. Endod. 2015, 41, 1975–1984. [Google Scholar] [CrossRef]
- Gambin, D.J.; Vitali, F.C.; Casanova, K.A.S.; De Carli, J.P.; Mazzon, R.R.; Gomes, B.P.F.A.; Trentin, M.S.; Duque, T.M. Prevalence of species of yellow, purple and green microbial complexes in endo-perio lesions: A systematic review. Braz. Oral. Res. 2024, 38, e048. [Google Scholar] [CrossRef]
- Lopes, E.M.; Passini, M.R.Z.; Kishi, L.T.; Chen, T.; Paster, B.J.; Gomes, B.P.F.A. Interrelationship between the Microbial Communities of the Root Canals and Periodontal Pockets in Combined Endodontic-Periodontal Diseases. Microorganisms 2021, 9, 1925. [Google Scholar] [CrossRef]
- Didilescu, A.C.; Rusu, D.; Anghel, A.; Nica, L.; Iliescu, A.; Greabu, M.; Bancescu, G.; Stratul, S.I. Investigation of six selected bacterial species in endo-periodontal lesions. Int. Endod. J. 2012, 45, 282–293. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Berber, V.B.; Chiarelli-Neto, V.M.; Aveiro, E.; Chapola, R.C.; Passini, M.R.Z.; Lopes, E.M.; Chen, T.; Paster, B.J. Microbiota present in combined endodontic-periodontal diseases and its risks for endocarditis. Clin. Oral. Investig. 2023, 27, 4757–4771. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Busi, S.; Imchen, M.; Kumavath, R.; Mohan, M.S.; Salim, S.A.; Subhaswaraj, P.; Dyavaiah, M. Polymicrobial Infections and Biofilms: Clinical Significance and Eradication Strategies. Antibiotics 2022, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.-A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.-G.; Martu, M.-A.; Luchian, I.; Stoleriu, S.; Diaconu-Popa, D.; Martu, C.; Teslaru, S.; Pasarin, L.; Solomon, S.M. The Effects of 810 nm Diode Laser and Indocyanine Green on Periodontal Parameters and HbA1c in Patients with Periodontitis and Type II Diabetes Mellitus: A Randomized Controlled Study. Diagnostics 2022, 12, 1614. [Google Scholar] [CrossRef]
- Nicolae, V.D.; Chiscop, I.; Cioranu, V.S.I.; Mârțu, M.-A.; Luchian, A.I.; Mârțu, S.; Solomon, S.M. The use of photoactivated blue-O toluidine for periimplantitis treatment in patients with periodontal disease. Rev. Chim. 2016, 66, 2121–2123. [Google Scholar]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Periodontol. 2018, 89 (Suppl. 1), S85–S102. [Google Scholar] [CrossRef]
- Martu, C.; Martu, M.-A.; Maftei, G.-A.; Diaconu-Popa, D.A.; Radulescu, L. Odontogenic Sinusitis: From Diagnosis to Treatment Possibilities—A Narrative Review of Recent Data. Diagnostics 2022, 12, 1600. [Google Scholar] [CrossRef]
- Fan, X.; Xu, X.; Yu, S.; Liu, P.; Chen, C.; Pan, Y.; Lin, L.; Li, C. Prognostic Factors of Grade 2-3 Endo-Periodontal Lesions Treated Nonsurgically in Patients with Periodontitis: A Retrospective Case-Control Study. Biomed. Res. Int. 2020, 2020, 1592910. [Google Scholar] [CrossRef]
- Buca, B.R.; Mititelu-Tartau, L.; Lupusoru, R.V.; Popa, G.E.; Rezus, C.; Lupusoru, C.E. New nitric oxide donors with therapeutic potential. Med.-Surg. J. 2016, 120, 942–946. [Google Scholar]
- Popa, G.R.; Ochiuz, L.Ă.; Stoleriu, I.U.; Cojocaru, I.L.; Popovici, I. Formulation and preparation of orally disintegrating tablets using innovative binder. Farmacia 2013, 61, 1131–1136. [Google Scholar]
- Solomon, S.M.; Timpu, D.; Forna, D.A.; Stefanache, M.A.; Martu, S.; Stoleriu, S. AFM comparative study of root surface morphology after three methods of scaling. Mater. Plast. 2016, 53, 546–549. [Google Scholar] [CrossRef]
- Solomon, S.M.; Stoleriu, S.; Agop Forna, D.; Timpu, D.; Martu Stefanache, M.A.; Ursarescu, I.G.; Martu, S. The quantitative and qualitative assessment of dental substance loss as consequence of root planing by three different techniques. Mater. Plast. 2016, 53, 305–307. [Google Scholar]
- Martu, M.-A.; Luchian, I.; Mares, M.; Solomon, S.; Ciurcanu, O.; Danila, V.; Rezus, E.; Foia, L. The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-Rheumatic Drugs. Bioengineering 2023, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Y.; Lin, X.; Yang, X.; Shi, W.; Lu, X. Prognostic Factors of Combined Periodontal and Endodontic Lesions: A Retrospective Study. Contrast Media Mol. Imaging 2022, 2022, 5042097. [Google Scholar] [CrossRef]
- Shiggaon, L.B.; Kingaonkar, A.; Kour, T.; Bhavsar, S.; Ayaz, M.; Chaudhary, A.; Gupta, S. Assessment of Risk Factors and Prognostic Predictors for Endo-Perio Lesions in Indian Cohorts: An Observational Study. Cureus 2024, 16, e69598. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, M.; Wang, X.; Wang, S. Endo-Periodontal Lesions—An Overlooked Etiology of Odontogenic Sinusitis. J. Clin. Med. 2023, 12, 6888. [Google Scholar] [CrossRef]
- Tietmann, C.; Tezer, I.; Youssef, E.; Jepsen, S.; Jepsen, K. Management of Teeth with Grade 3 Endo-Periodontal Lesions by Combined Endodontic and Regenerative Periodontal Therapy. J. Clin. Med. 2023, 13, 93. [Google Scholar] [CrossRef]
- AlJasser, R.; Bukhary, S.; AlSarhan, M.; Alotaibi, D.; AlOraini, S.; Habib, S.R. Regenerative Therapy Modality for Treatment of True Combined Endodontic-Periodontal Lesions: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 6220. [Google Scholar] [CrossRef]
- Adam, M. ‘Combined endo-perio lesions’—What is the best treatment? Evid. Based Dent. 2021, 22, 158–159. [Google Scholar] [CrossRef]
- Oh, S.; Chung, S.H.; Han, J.Y. Periodontal regenerative therapy in endo-periodontal lesions: A retrospective study over 5 years. J. Periodontal Implant. Sci. 2019, 49, 90–104. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Y.; Lin, M.; Zhang, Y.; Li, Y.; Ouyang, X.; Ge, S.; Lin, J.; Pan, Y.; Xu, Y.; et al. Expert consensus on the diagnosis and therapy of endo-periodontal lesions. Int. J. Oral Sci. 2024, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Evans, M. The endodontic-periodontal juncture: Where two worlds meet. An overview of endo-perio lesions. Aust. Dent. J. 2023, 68 (Suppl. 1), S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Ochiuz, L.; Popa, G.; Stoleriu, I.; Tomoiagă, A.M.; Popa, M. Microencapsulation of metoprolol tartrate into chitosan for improved oral administration and patient compliance. Ind. Eng. Chem. Res. 2013, 52, 17432–17441. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamazaki, K.; Yamazaki, M.; Kato, Y.; Baba, Y. Personalized Medicine Based on the Pathogenesis and Risk Assessment of Endodontic-Periodontal Lesions. J. Pers. Med. 2022, 12, 1688. [Google Scholar] [CrossRef]
- Orstavik, D.; Kerekes, K.; Eriksen, H.M. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod. Dent. Traumatol. 1986, 2, 20–34. [Google Scholar] [CrossRef]
- Nur, B.G.; Ok, E.; Altunsoy, M.; Ağlarci, O.S.; Çolak, M.; Güngör, E. Evaluation of technical quality and periapical health of root-filled teeth by using cone-beam CT. J. Appl. Oral. Sci. 2014, 22, 502–508. [Google Scholar] [CrossRef]
- El Ouarti, I.; Chala, S.; Sakout, M.; Abdallaoui, F. Prevalence and risk factors of Apical periodontitis in endodontically treated teeth: Cross-sectional study in an Adult Moroccan subpopulation. BMC Oral Health 2021, 21, 124. [Google Scholar] [CrossRef]
- Travassos, R.M.C.; de Almeida, A.C.; de Albuquerque, D.S.; Pereira, M.A.L.; Rangel, L.S.; da Silva, M.C.F.C.; Lopes, D.S.; Prosini, P. Primary endodontic lesion summarizing an endo-perio lesion. LUMEN ET VIRTUS 2024, 15, 374–382. [Google Scholar] [CrossRef]
- Holtfreter, B.; Albandar, J.M.; Dietrich, T.; Dye, B.A.; Eaton, K.A.; Eke, P.I.; Papapanou, P.N.; Kocher, T.; Joint EU/USA Periodontal Epidemiology Working Group. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: Proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J. Clin. Periodontol. 2015, 42, 407–412. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Thornton-Evans, G. Public Health Aspects of Periodontitis: Recent Advances and Contributions by Dr. Robert J. Genco. Curr. Oral. Health Rep. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sălceanu, M.; Dascălu, C.; Melian, A.; Giuroiu, C.; Antohi, C.; Concita, C.; Hamburda, T.; Topoliceanu, C.; Mârţu, M.A. Assessment of Periodontitis Risk Factors in Endodontically Treated Teeth: A Cross-Sectional Study. Diagnostics 2024, 14, 1972. [Google Scholar] [CrossRef] [PubMed]
- Kurgan, S.; Terzioglu, H.; Yılmaz, B. Stress distribution in reduced periodontal supporting tissues surrounding splinted teeth. Int. J. Periodontics Restor. Dent. 2014, 34, e93–e101. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B.; Chen, F.M.; Milward, M.R.; Ling, M.R.; Chapple, I.L. Neutrophil superoxide production in the presence of cigarette smoke extract, nicotine and cotinine. J. Clin. Periodontol. 2012, 39, 626–634. [Google Scholar] [CrossRef]
- Silva, H. Tobacco Use and Periodontal Disease-The Role of Microvascular Dysfunction. Biology 2021, 10, 441. [Google Scholar] [CrossRef]
- Li, Y.; Jin, X.; Mao, L. Protective effect of catalpol on nicotine-induced injury of alveolar bone and associated underlying mechanisms. Mol. Med. Rep. 2017, 16, 8345–8350. [Google Scholar] [CrossRef]
- Jaff, L.B.; Dizayee, W.M.; Rostum, I.D.; Ibrahim, M.M. A Destructive Connection: A Review of Cigarette Smoking Impact on Periodontal Health. Saudi J. Oral Dent. Res. 2025, 10, 221–232. [Google Scholar] [CrossRef]
- Vasques, A.M.V.; da Silva, A.C.R.; Bueno, C.R.E.; Duarte, M.A.H.; Ervolino, E.; Cintra, L.T.A.; Junior, E.D. Bone resorption in apical periodontitis enhanced by cigarette smoke inhalation: Histometric, immunohistochemical, and microtomographic analysis in rats. J. Endod. 2024, 50, 493–498. [Google Scholar] [CrossRef]
- Pinto, K.P.; Ferreira, C.M.; Maia, L.C.; Sassone, L.M.; Fidalgo, T.K.S.; Silva, E.J.N.L. Does tobacco smoking predispose to apical periodontitis and endodontic treatment need? A systematic review and meta-analysis. Int. Endod. J. 2020, 53, 1068–1083. [Google Scholar] [CrossRef]
- Rodriguez, F.R.; Taner, B.; Weiger, R.; Walter, C. Is smoking a predictor of apical periodontitis? Clin. Oral. Investig. 2013, 17, 1947–1955. [Google Scholar] [CrossRef][Green Version]
- Tiron, B.; Forna, N.; Topoliceanu, C.; Ghiorghe, A.; Stoleriu, S.; Pancu, G.; Nica, I.; Georgescu, A.; Brânzan, R.; Iovan, G. Assessment of factors influencing the esthetic, functional and biological status of posterior composite resins restorations. Rom. J. Oral Rehabil. 2023, 15, 29–41. [Google Scholar]
- Pancu, G.; Georgescu, A.; Moldovanu, A.; Ghiorghe, A.; Stoleriu, S.; Nica, I.; Tărăboanţă, I.; Iovan, A.; Andrian, S.; Topoliceanu, C. Non-intervention versus repair/replacement decisions in posterior composite restorations aged 3-5 years: A retrospective study. Rom. J. Oral Rehabil. 2024, 16, 186–195. [Google Scholar] [CrossRef]
- Keratiotis, G.; Spineli, L.; De Bruyne, M.A.A.; De Moor, R.J.G.; Meire, M.A. A 22-year follow-up cross-sectional study on periapical health in relation to the quality of root canal treatment in a Belgian population. Int. Endod. J. 2024, 57, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.H.L.; Gaêta-Araujo, H.; Andrade, M.F.S.; Freitas, D.Q. Prevalence of technical errors and periapical lesions in a sample of endodontically treated teeth: A CBCT analysis. Clin. Oral Investig. 2018, 22, 2495–2503. [Google Scholar] [CrossRef]
- Haji-Hassani, N.; Bakhshi, M.; Shahabi, S. Frequency of iatrogenic errors through root canal treatment procedure in 1335 charts of dental patients. J. Int. Oral Health 2015, 7, 14–17. [Google Scholar]
- Litvinov, E.; Litvinov, A. The relationship between periodontitis, gingivitis, smoking, missing teeth, endodontic infections, aortic aneurysm, and coronary artery disease: The 10-year results of 25 patients. Cureus 2024, 16, e73584. [Google Scholar] [CrossRef]
- Ferraz, D.C.; Moura, C.C.G.; Signorelli, N.S.M.; Rosa, R.C.; Pereira, S.A.D.L.; Borges, A.L.S.; Bittar, V.P.; Duarte, R.M.F.; Teixeira, R.R.; Bertolini, M.; et al. The Interaction of Apical Periodontitis, Cigarette Smoke, and Alcohol Consumption on Liver Antioxidant Status in Rats. Int. J. Mol. Sci. 2024, 25, 12011. [Google Scholar] [CrossRef]
- Friedrich, F.; Scalabrin, S.A.; Weissheimer, T.; Rösing, C.K.; Só, G.B.; da Rosa, R.A.; Só, M.V.R. Influence of the timing of periodontal intervention on periapical/periodontal repair in endodontic-periodontal lesions: A systematic review. Clin. Oral Investig. 2023, 27, 933–942. [Google Scholar]
- Anton, D.-M.; Martu, M.-A.; Maris, M.; Maftei, G.-A.; Sufaru, I.-G.; Tatarciuc, D.; Luchian, I.; Ioanid, N.; Martu, S. Study on the Effects of Melatonin on Glycemic Control and Periodontal Parameters in Patients with Type II Diabetes Mellitus and Periodontal Disease. Medicina 2021, 57, 140. [Google Scholar] [CrossRef]
- Nara, Y.; Tavelli, L.; Maekawa, S. Surgical treatment for severe endodontic-periodontal lesion: A case report with 2-year follow-up. Clin. Adv. Periodontics 2025, 1. [Google Scholar] [CrossRef]
- Sandini-Trentin, M.; Gomes-Dallepiane, F.; Serraglio-Figueiredo, P.; Becker, A.L.; Leocovick, S.; Duque, T.M.; De Carli, J.P.; Vanni, J.R. Management of Endo-Perio Lesion in a Tooth with an Unfavorable Prognosis: A Clinical Case Report with an 18-Month Follow-Up. Odovtos Int. J. Dent. Sci. 2024, 26, 46–54. [Google Scholar] [CrossRef]
- Ardila, C.M.; Vivares-Builes, A.M. Clinical Efficacy of Treatment of Endodontic-Periodontal Lesions: A Systematic Scoping Review of Experimental Studies. Int. J. Environ. Res. Public Health 2022, 19, 13649. [Google Scholar] [CrossRef] [PubMed]
- Sy, K.; Chevalier, C.; Maton, M.; Mokbel, I.; Mahieux, S.; Houcke, I.; Neut, C.; Grosgogeat, B.; Deveaux, E.; Gritsch, K.; et al. Therapeutic Potential of Chlorhexidine-Loaded Calcium Hydroxide-Based Intracanal Medications in Endo-Periodontal Lesions: An Ex Vivo and In Vitro Study. Antibiotics 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, X.; Lin, F.; Yu, Q.; Hou, B.; Chen, Z.; Wei, X.; Qiu, L.; Wenxia, C.; Li, J.; et al. Expert consensus on endodontic therapy for patients with systemic conditions. Int. J. Oral. Sci. 2024, 16, 45. [Google Scholar] [CrossRef]
- Colonia-Garcia, A.; Gutierrez-Velez, M.; Duque-Duque, A.; de Andrade, C.R. Possible association of periodontal disease with oral cancer and oral potentially malignant disorders: A systematic review. Acta Odontol. Scand. 2020, 78, 553–559. [Google Scholar] [CrossRef]
- Karmakar, S.; Modak, B.; Solomon, M.C. Exploring the causal relationship between chronic periodontitis and oral cancer: An insight. Oral Oncol. Rep. 2024, 11, 100468. [Google Scholar] [CrossRef]
- Evangelista, K.; de Faria Vasconcelos, K.; Teodoro, A.B.; Cavalcanti, M.G.P.; de Mendonça, E.F.; Watanabe, S.; Silva, M.A.G. Malignant tumours mimicking periapical lesions: A report of three cases and literature review. Aust. Endod. J. 2022, 48, 515–521. [Google Scholar] [CrossRef]
- Levi, P.A., Jr.; Kim, D.M.; Harsfield, S.L.; Jacobson, E.R. Squamous cell carcinoma presenting as an endodontic-periodontic lesion. J. Periodontol. 2005, 76, 1798–1804. [Google Scholar] [CrossRef]
- Zheng, F.Y.; Qiu, J.Y.; Liao, K.H.; Lin, N.C. Oral rhabdomyosarcoma, a rare malignant tumor mimicking an endodontic-periodontal lesion in an adult patient: A case report. BMC Oral Health 2024, 24, 92. [Google Scholar] [CrossRef]
- Cristea, I.; Agop-Forna, D.; Martu, M.-A.; Dascălu, C.; Topoliceanu, C.; Török, R.; Török, B.; Bardis, D.; Bardi, P.M.; Forna, N. Oral and Periodontal Risk Factors of Prosthetic Success for 3-Unit Natural Tooth-Supported Bridges versus Implant-Supported Fixed Dental Prostheses. Diagnostics 2023, 13, 852. [Google Scholar] [CrossRef]
- Rosen, E.; Tsesis, I.; Kavalerchik, E.; Salem, R.; Kahn, A.; Del Fabbro, M.; Taschieri, S.; Corbella, S. Effect of guided tissue regeneration on the success of surgical endodontic treatment of teeth with endodontic-periodontal lesions: A systematic review. Int. Endod. J. 2023, 56, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.H.D.S.D.; Ferreira, C.L.; Andere, N.M.R.; Araujo, C.F. Evaluation of different clinical periodontal protocols for the treatment of combined endo-periodontal lesions: A clinical and CBCT-based 6-month follow up study. J. Int. Acad. Periodontol. 2022, 24, 189–199. [Google Scholar]
- Di Murro, B.; Papi, P.; Di Murro, C.; Pompa, G.; Gambarini, G. Correlation between endodontic pulpal/periapical disease and retrograde peri-implantitis: A case series. Aust. Endod. J. 2021, 47, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Tabiyar, K.; Balachandran, R.; Priya, H.; Agarwal, D.; Sharma, S.; Kumar, V.; Chawla, A.; Logani, A. Influence of intracanal medicaments on the periodontal and periapical healing in concurrent endodontic-periodontal lesions with/without communication: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 6371–6382. [Google Scholar] [CrossRef]

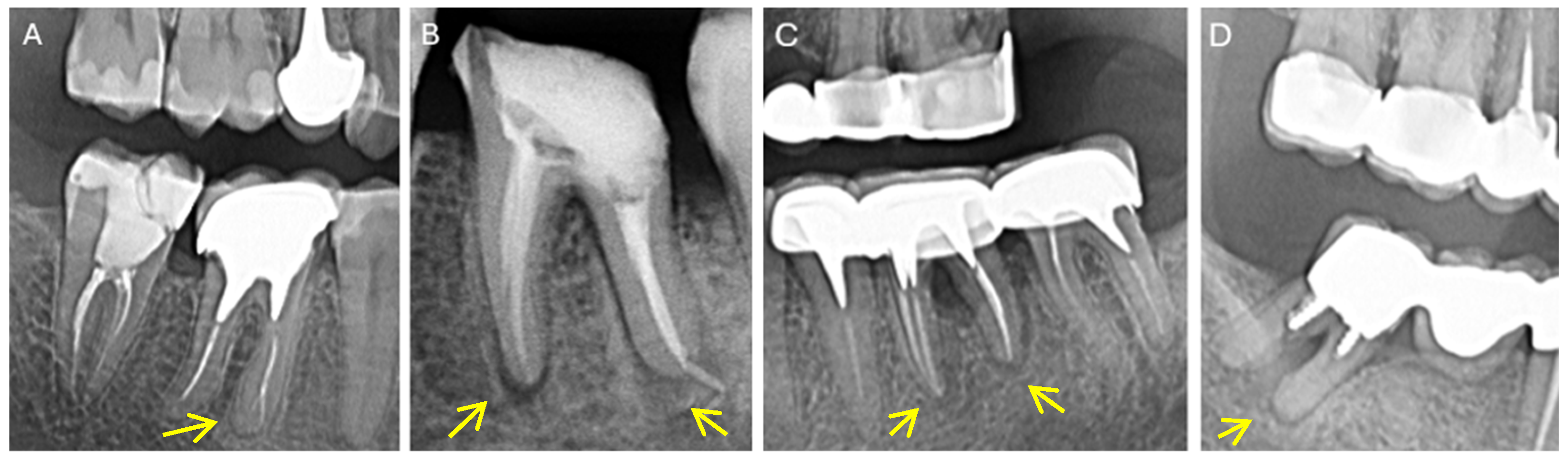

| Parameters | Criteria | Description |
|---|---|---|
| Parameters of root canal filling quality | ||
| Density of root canal filling | Adequate | Absence of gaps in the root filling or between root filling and root canal walls |
| Poor | Presence of gaps in the root filling or between root filling and root canal walls | |
| Length of root canal filling | Adequate | Root filling ending ≤ 2 mm from radiographic apex |
| Poor | Root filling over the radiographic apex or root filling > 2 mm from radiographic apex | |
| Conicity of root canal filling | Adequate | Continuously tapering funneled root canal preparation from the canal entrance to the apex, and cross-sectional diameter narrower at every point apically |
| Poor | Inconsistent taper from the canal entrance to the apical part of the filling, or root filling deviated from the original canal | |
| Parameters of coronal restoration quality | ||
| Adequate | Any permanent coronal restoration with intact radiographic aspect | |
| Poor | Any permanent coronal restoration with detectable signs of recurrent caries or open margins or presence of temporary coronal restoration | |
| Missing | Absence of permanent or temporary coronal restoration |
| Parameter | Absent E-P (Control) | E-P (Test) | Total | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Global | 40 | 100.0 | 50 | 100.0 | 90 | 100.0 | |
| Gender | 0.809 | ||||||
| M | 15 | 37.5 | 20 | 40.0 | 35 | 38.9 | |
| F | 25 | 62.5 | 30 | 60.0 | 55 | 61.1 | |
| Age (years) (mean, SD) | Globally: 44.50 ± 12.393 | Globally: 50.72 ± 13.820 | Globally: 47.96 ± 13.495 | 0.029 * | |||
| M: 45.13 ± 13.309 | M: 48.70 ± 14.357 | M: 47.17 ± 13.832 | |||||
| F: 44.12 ± 12.077 | F: 52.07 ± 13.820 | F: 48.45 ± 13.380 | |||||
| p = 0.806 | p = 0.404 | p = 0.663 | |||||
| Age group (years) | 0.280 | ||||||
| 20–39 | 13 | 32.5 | 12 | 24.0 | 25 | 27.8 | |
| 40–59 | 23 | 57.5 | 27 | 54.0 | 50 | 55.6 | |
| ≥60 | 4 | 10.0 | 11 | 22.0 | 15 | 16.7 | |
| Absent EPLs (Control) | EPLs (Test) | Total | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Parameter | n | % | n | % | n | % | |
| Global | 62 | 100.0 | 64 | 100.0 | 126 | 100.0 | |
| Gender | 0.67 | ||||||
| M | 21 | 33.9 | 24 | 37.5 | 45 | 35.7 | |
| F | 41 | 66.1 | 40 | 62.5 | 81 | 64.3 | |
| Age group (years) | 0.02 * | ||||||
| 20–39 | 21 | 33.9 | 14 | 21.9 | 35 | 27.8 | |
| 40–59 | 33 | 53.2 | 29 | 45.3 | 62 | 49.2 | |
| ≥60 | 8 | 12.9 | 21 | 32.8 | 29 | 23.0 | |
| Smoking | 19 | 30.6 | 14 | 21.9 | 33 | 26.2 | 0.26 |
| OHI | 0.12 | ||||||
| 1 | 6 | 9.7 | 2 | 3.1 | 8 | 6.3 | |
| 2 | 33 | 53.2 | 44 | 68.8 | 77 | 61.1 | |
| 3 | 23 | 37.1 | 18 | 28.1 | 41 | 32.5 | |
| Location (MX/MD) | 0.42 | ||||||
| MX | 20 | 32.3 | 25 | 39.1 | 45 | 35.7 | |
| MD | 42 | 67.7 | 39 | 60.9 | 81 | 64.3 | |
| Coronal restoration type | |||||||
| Composite resin filing | 25 | 40.3 | 25 | 39.1 | 50 | 39.7 | 0.88 |
| Class I | 6 | 24.0 | 6 | 24.0 | 12 | 24.0 | 1.00 |
| Class II | 19 | 76.0 | 19 | 76.0 | 38 | 76.0 | |
| Full-coverage crown | 37 | 59.7 | 39 | 60.9 | 76 | 60.3 | 0.88 |
| Intracanal posts | 11 | 17.7 | 13 | 20.3 | 24 | 19.0 | 0.71 |
| Coronal restoration quality | 0.79 | ||||||
| Adequate | 5 | 8.1 | 6 | 9.4 | 11 | 8.7 | |
| Poor | 57 | 91.9 | 58 | 90.6 | 115 | 91.3 | |
| Root canal filling quality | 0.68 | ||||||
| Adequate | 2 | 3.2 | 4 | 6.3 | 6 | 4.8 | |
| Poor—reasons: | 60 | 96.8 | 60 | 93.8 | 120 | 95.2 | |
| Under-filling | 53 | 85.5 | 47 | 73.4 | 100 | 79.4 | 0.09 |
| Over-filling | 6 | 9.7 | 7 | 10.9 | 13 | 10.3 | 0.81 |
| Inadequate homogeneity | 55 | 88.7 | 54 | 84.4 | 109 | 86.5 | 0.47 |
| Inadequate taper (conicity) | 58 | 93.5 | 57 | 89.1 | 115 | 91.3 | 0.37 |
| Opposing tooth | 0.008 ** | ||||||
| YES | 42 | 67.7 | 56 | 87.5 | 98 | 77.8 | |
| NO | 20 | 32.3 | 8 | 12.5 | 28 | 22.2 | |
| Recurrent caries | 0.86 | ||||||
| YES | 53 | 85.5 | 54 | 84.4 | 107 | 84.9 | |
| NO | 9 | 14.5 | 10 | 15.6 | 19 | 15.1 | |
| PPD ≥ 4 mm | <0.001 ** | ||||||
| YES | 19 | 30.6 | 55 | 85.9 | 74 | 58.7 | |
| NO | 43 | 69.4 | 9 | 14.1 | 52 | 41.3 | |
| Periodontal status (stage) | 0.10 | ||||||
| I | 18 | 29.0 | 9 | 14.1 | 27 | 21.4 | |
| II | 27 | 43.5 | 26 | 40.6 | 53 | 42.1 | |
| III | 13 | 21.0 | 23 | 35.9 | 36 | 28.6 | |
| IV | 4 | 6.5 | 6 | 9.4 | 10 | 7.9 | |
| Periodontal status (grade) | 0.014 * | ||||||
| A | 22 | 35.5 | 6 | 9.4 | 28 | 22.2 | |
| B | 24 | 38.7 | 36 | 56.3 | 60 | 47.6 | |
| C | 16 | 25.8 | 22 | 34.4 | 38 | 30.2 | |
| n | % | |
|---|---|---|
| Grade 1 | 17 | 26.57% |
| Grade 2 | 22 | 34.37% |
| Grade 3 | 25 | 39.06% |
| Absent E-P (Control) | E-P (Test) | Total | p-Value | |
|---|---|---|---|---|
| PPD (mean, SD) | 3.81 ± 0.78 | 4.70 ± 1.04 | 4.26 ± 1.02 | <0.001 ** |
| MBL-D (mean, SD) | 2.19 ± 0.88 | 3.28 ± 1.54 | 2.75 ± 1.37 | <0.001 ** |
| MBL-M (mean, SD) | 2.73 ± 1.13 | 4.04 ± 1.85 | 3.40 ± 1.66 | <0.001 ** |
| AUC (95% CI) | p | Cut-off Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| PPD | 0.73 (0.64 ÷ 0.82) | 0.000 ** | 3.62 | 0.92 | 0.51 |
| MBL-D | 0.74 (0.66 ÷ 0.83) | 0.000 ** | 2.25 | 0.75 | 0.67 |
| MBL-M | 0.71 (0.62 ÷ 0.80) | 0.000 ** | 2.75 | 0.78 | 0.54 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age ≥ 60 years | 3.29 (1.33 ÷ 8.16) | 0.008 ** | 5.39 (1.398 ÷ 20.79) | 0.014 * |
| Opposing tooth | 3.33 (1.33 ÷ 8.30) | 0.008 ** | 1.68 (0.535 ÷ 5.32) | 0.372 |
| PPD ≥ 4 mm | 13.83 (5.69 ÷ 33.60) | <0.001 ** | 6.58 (1.511 ÷ 28.70) | 0.012 * |
| Periodontal status II, III, or IV | 2.50 (1.02 ÷ 6.10) | 0.041 * | 0.42 (0.107 ÷ 1.67) | 0.221 |
| Cut-off PPD ≥ 3.625 | 12.58 (4.44 ÷ 35.60) | <0.001 ** | 6.16 (0.974 ÷ 38.99) | 0.053 * |
| MBL-D ≥ 2.250 | 6.30 (2.89 ÷ 13.70) | <0.001 ** | 1.39 (0.504 ÷ 3.87) | 0.520 |
| MBL-M ≥ 2.750 | 4.33 (1.99 ÷ 9.41) | <0.001 ** | 0.45 (0.121 ÷ 1.69) | 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sălceanu, M.; Melian, A.; Dascălu, C.; Giuroiu, C.; Concita, C.; Topoliceanu, C.; Melian, D.; Frumuzache, A.; Solomon, S.M.; Mârţu, M.-A. Endo-Periodontal Lesions in Endodontically Treated Teeth with Periapical Pathology. Diagnostics 2025, 15, 1663. https://doi.org/10.3390/diagnostics15131663
Sălceanu M, Melian A, Dascălu C, Giuroiu C, Concita C, Topoliceanu C, Melian D, Frumuzache A, Solomon SM, Mârţu M-A. Endo-Periodontal Lesions in Endodontically Treated Teeth with Periapical Pathology. Diagnostics. 2025; 15(13):1663. https://doi.org/10.3390/diagnostics15131663
Chicago/Turabian StyleSălceanu, Mihaela, Anca Melian, Cristina Dascălu, Cristian Giuroiu, Corina Concita, Claudiu Topoliceanu, Diana Melian, Andreea Frumuzache, Sorina Mihaela Solomon, and Maria-Alexandra Mârţu. 2025. "Endo-Periodontal Lesions in Endodontically Treated Teeth with Periapical Pathology" Diagnostics 15, no. 13: 1663. https://doi.org/10.3390/diagnostics15131663
APA StyleSălceanu, M., Melian, A., Dascălu, C., Giuroiu, C., Concita, C., Topoliceanu, C., Melian, D., Frumuzache, A., Solomon, S. M., & Mârţu, M.-A. (2025). Endo-Periodontal Lesions in Endodontically Treated Teeth with Periapical Pathology. Diagnostics, 15(13), 1663. https://doi.org/10.3390/diagnostics15131663








