Periodontal Disease Elevates IL-6 Levels During Initial Symptoms of COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Participant Recruitment and Data Collection
Eligibility for RT–qPCR Testing for SARS-CoV-2
2.3. Sample Collection Procedure
2.4. Self-Reported Periodontal Disease
- Q1: Do you have periodontal or gum disease?
- Q2: Have you ever been told by a dentist that you have periodontal/gum disease with bone loss?
- Q3: Do you notice any areas that appear redder than usual?
- Q4: Do you experience tooth mobility?
- Q5: Do you have food impaction between your teeth?
- Q6: Do you notice that your teeth appear to be getting longer?
2.5. Saliva Collection and IL-6 Quantification
2.6. Sample Collection and RT–qPCR Detection of SARS-CoV-2
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics and COVID-19 Symptomatology Among Groups
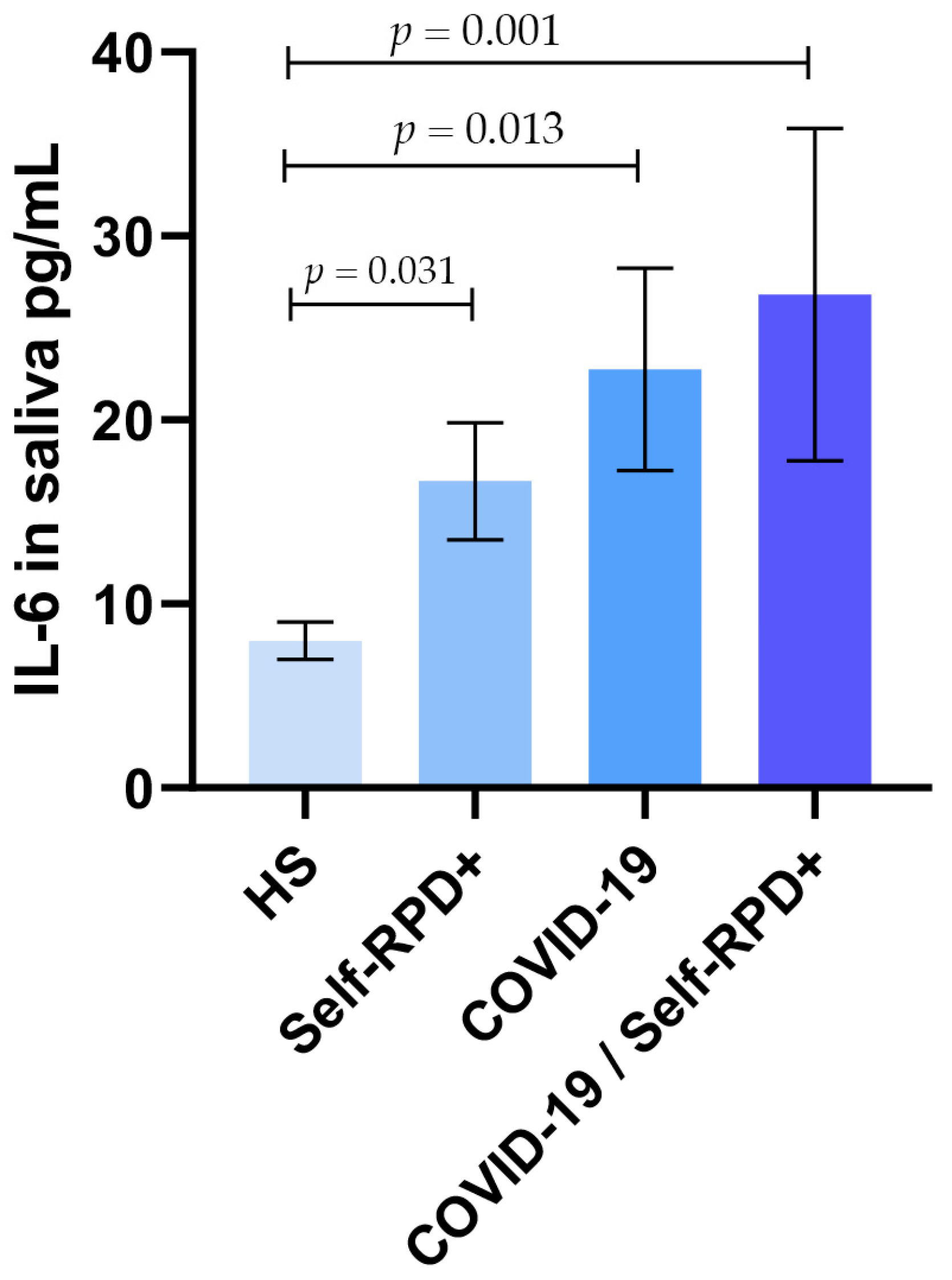
3.2. Salivary IL-6 Levels According to COVID-19 and Self-RPD Status Groups
3.3. Salivary IL-6 Levels According to Symptoms
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohrabi, C.; Alsafi, Z.; O´Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, Transmission, Diagnosis and Management of Coronavirus Disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Banakar, M.; Bagheri Lankarani, K.; Jafarpour, D.; Moayedi, S.; Banakar, M.H.; MohammadSadeghi, A. COVID-19 transmission risk and protective protocols in dentistry: A systematic review. BMC Oral Health 2020, 20, 275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Mootha, A. Is There a Similarity in Serum Cytokine Profile Between Patients with Periodontitis or 2019-Novel Coronavirus Infection?—A Scoping Review. Biology 2023, 12, 550. [Google Scholar] [CrossRef]
- Pitones-Rubio, V.; Chávez-Cortez, E.G.; Hurtado-Camarena, A.; González-Rascón, A.; Serafín-Higuera, N. Is Periodontal Disease a Risk Factor for Severe COVID-19 Illness? Med. Hypotheses 2020, 144, 109969. [Google Scholar] [CrossRef]
- Brock, M.; Bahammam, S.; Sima, C. The Relationships Among Periodontitis, Pneumonia and COVID-19. Front. Oral Health 2021, 2, 801815. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- Nor Rashid, N.; Amrani, L.; Alwan, A.; Mohamed, Z.; Yusof, R.; Rothan, H. Angiotensin-Converting Enzyme-2 (ACE2) Downregulation During Coronavirus Infection. Mol. Biotechnol. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and Periodontal Disease. J. Int. Med. Res. 2021, 49, 3000605211002695. [Google Scholar] [CrossRef]
- Potere, N.; Batticciotto, A.; Vecchié, A.; Porreca, E.; Cappelli, A.; Abbate, A.; Dentali, F.; Bonaventura, A. The Role of IL-6 and IL-6 Blockade in COVID-19. Expert Rev. Clin. Immunol. 2021, 17, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Apolinário Vieira, G.H.; Aparecida Rivas, A.C.; Figueiredo Costa, K.; Ferreira Oliveira, L.F.; Tanaka Suzuki, K.; Reis Messora, M.; Sprone Ricoldi, M.; Gonçalves de Almeida, A.L.; Taba, M. Specific Inhibition of IL-6 Receptor Attenuates Inflammatory Bone Loss in Experimental Periodontitis. J. Periodontol. 2021, 92, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sharma, A.; Sharma, A.; Singh, G.; Khan, S.; Ahmad, S.; Alrashidi, A.A.M.; Sherwani, S.; Mishra, H.; Alsulimani, A. The Effect of Non-Surgical Periodontal Therapy on Salivary IL-6 Levels in Patients with Moderate to Severe Generalized Chronic Periodontitis. Ir. J. Med. Sci. 2023, 192, 2981–2986. [Google Scholar] [CrossRef]
- Boroumand, M.; Olianas, A.; Cabras, T.; Manconi, B.; Fanni, D.; Faa, G.; Desiderio, C.; Messana, I.; Castagnola, M. Saliva, a body fluid with recognized and potential diagnostic applications. J. Sep. Sci. 2021, 44, 3677–3690. [Google Scholar] [CrossRef]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 Infection of the Oral Cavity and Saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef]
- Santos, C.F.; Morandini, A.C.; Dionísio, T.J.; Faria, F.A.; Lima, M.C.; Figueiredo, C.M.; Colombini-Ishikiriama, B.L.; Sipert, C.R.; Maciel, R.P.; Akashi, A.P.; et al. Functional Local Renin-Angiotensin System in Human and Rat Periodontal Tissue. PLoS ONE 2015, 10, e0134601. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Colnaghi, A.; Morittu, S.; Barbieri, S.; Ricci, M.; Guerrisi, G.; Piloni, D.; et al. Assessment of Oral Microbiome Changes in Healthy and COVID-19-Affected Pregnant Women: A Narrative Review. Microorganisms 2021, 9, 2385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.F.; Zhang, Y.J.; Yao, Y.L.; Chen, M.X.; Wang, L.L.; Wang, M.D.; Hu, X.Y.; Tang, X.J.; Zhong, Z.H.; Fu, L.J.; et al. The association of post-embryo transfer SARS-CoV-2 infection with early pregnancy outcomes in in vitro fertilization: A prospective cohort study. Am. J. Obstet. Gynecol. 2024, 230, 436.e1–436.e12. [Google Scholar] [CrossRef]
- Bemquerer, L.M.; Oliveira, S.R.; de Arruda, J.A.A.; Costa, F.P.D.; Miguita, L.; Bemquerer, A.L.M.; de Sena, A.C.V.P.; de Souza, A.F.; Mendes, D.F.; Schneider, A.H.; et al. Clinical, Immunological, and Microbiological Analysis of the Association Between Periodontitis and COVID-19: A Case-control Study. Odontology 2023, 112, 208–220. [Google Scholar] [CrossRef]
- Bachtiar, B.M.; Haerani, N.; Soeroso, Y.; Ismah, N.; Bachtiar, E.W. The presence of ACE2 and regulatory miRNAs (miR-200c-3p and miR-421-5p) in the saliva of periodontitis patients post-COVID-19 vaccination. Front. Dent. Med. 2024, 5, 1438139. [Google Scholar] [CrossRef] [PubMed]
- Moradi Haghgoo, J.; Torkzaban, P.; Farhadian, M.; Moosavi Sedeh, S.A. Association between the severity of periodontitis, COVID-19, C-reactive protein and interleukin-6 levels in hospitalized patients: A case-control study. BMC Oral Health 2023, 23, 556. [Google Scholar] [CrossRef] [PubMed]
- Abbood, H.M.; Hinz, J.; Cherukara, G.; Macfarlane, T.V. Validity of Self-Reported Periodontal Disease: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Symptoms of COVID-19; CDC: Atlanta, GA, USA, 2025. Available online: https://www.cdc.gov/covid/signs-symptoms/index.html (accessed on 23 May 2025).
- Khader, Y.; Alhabashneh, R.; Alhersh, F. Development and Validation of a Self-Reported Periodontal Disease Measure among Jordanians. Int. Dent. J. 2015, 65, 203–210. [Google Scholar] [CrossRef]
- Coden, E.; Russo, F.; Arosio, A.D.; Castelnuovo, P.; Karligkiotis, A.; Volpi, L. Optimum Naso-oropharyngeal Swab Procedure for COVID-19: Step-by-Step Preparation and Technical Hints. Laryngoscope 2020, 130, 2564–2567. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Natoli, V.; Bruni, A.; Coscione, C.; Magliano, G.; Giacobbo, G.; Morelli, A.; Moressa, S.; Scribante, A. Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. J. Clin. Med. 2020, 9, 3914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatia, V. Can Improved Periodontal Health Be a Key Factor in Preventing Severe COVID-19 Complications: An Evidence-Based Review. Adv. Dent. 2021, 2, 1–4. [Google Scholar] [CrossRef]
- Siddharthan, S.; Naing, N.N.; Wan-Arfah, N. Periodontal Disease and COVID 19. J. Pharm. Res. Int. 2020, 32, 88–91. [Google Scholar] [CrossRef]
- Pragya, G.; Saraniya, D.R.; Sathish, R.; Logaranjini, A.; Prashanthi, P. Covid-19 and Periodontal Disease—An Overview. Int. J. Sci. Res. 2020, 9, 2277–8179. [Google Scholar] [CrossRef]
- Ghosh, A.; Joseph, B.; Anil, S. Does Periodontitis Influence the Risk of COVID-19? A Scoping Review. Clin. Exp. Dent. Res. 2022, 8, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Aulia Azhar, S. Molecular Docking Study of Hyaluronic Acid Against Interleukin-6 (7DC8 Protein) in COVID-19 Patients With Periodontitis. Iium J. Orofac. Health Sci. 2023, 4, 140–144. [Google Scholar] [CrossRef]
- Anand, P.S.; Jadhav, P.; Kamath, K.P.; Kumar, S.R.; Vijayalaxmi, S.; Anil, S. A Case-control Study on the Association Between Periodontitis and Coronavirus Disease (COVID-19). J. Periodontol. 2021, 93, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.C.S.; Costa, C.A.; Perazzo, M.F.; Leles, C.R.; Costa, N.L. Severe Generalized Periodontitis as a Risk Factor for Serious Outcomes of COVID-19: A Structural Equation Modelling Approach. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Said, K.N.; Al-Momani, A.M.; Almaseeh, J.A.; Marouf, N.; Shatta, A.; Al-Abdulla, J.; Alaji, S.; Daas, H.; Tharupeedikayil, S.S.; Chinta, V.R.; et al. Association of Periodontal Therapy, with Inflammatory Biomarkers and Complications in COVID-19 Patients: A Case Control Study. Clin. Oral Investig. 2022, 26, 6721–6732. [Google Scholar] [CrossRef]
- Al-Nomay, N.S.; Alolayan, L.; Aljohani, R.; Almashouf, R.; Alharbi, G.M. Association Between Periodontitis and COVID-19 Severity in a Tertiary Hospital: A Retrospective Cohort Study. Saudi Dent. J. 2022, 34, 623–628. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, H.; Pan, Y.; Jin, L.; Hu, R.; Lu, Y.; Deng, W.; Sun, W.; Chen, C.; Shen, X.; et al. Periodontal Disease Increases the Host Susceptibility to COVID-19 and Its Severity: A Mendelian Randomization Study. J. Transl. Med. 2021, 19, 528. [Google Scholar] [CrossRef]
- Basso, L.; Chacun, D.; Sy, K.; Grosgogeat, B.; Gritsch, K. Periodontal Diseases and COVID-19: A Scoping Review. Eur. J. Dent. 2021, 15, 768–775. [Google Scholar] [CrossRef]
- Campisi, G.; Bizzoca, M.E.; Lo Muzio, L. COVID-19 and Periodontitis: Reflecting on a Possible Association. Head Face Med. 2021, 17, 1–6. [Google Scholar] [CrossRef]
- Baima, G.; Marruganti, C.; Sanz, M.; Aimetti, M.; Romandini, M. Periodontitis and COVID-19: Biological Mechanisms and Meta-Analyses of Epidemiological Evidence. J. Dent. Res. 2022, 101, 1430–1440. [Google Scholar] [CrossRef]
- Javed, F.; Al-Askar, M.; Al-Hezaimi, K. Cytokine Profile in the Gingival Crevicular Fluid of Periodontitis Patients With and Without Type 2 Diabetes: A Literature Review. J. Periodontol. 2012, 83, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Taso, E.; Rakić, M.; Stefanovic, V.; Petković-Ćurčin, A.; Stanojević, I.; Djukić, M.; Struillou, X.; Vojvodić, D.; Banovic, T.; Kanjevac, T. Variation of the Cytokine Profiles in Gingival Crevicular Fluid Between Different Groups of Periodontally Healthy Teeth. Serbian J. Exp. Clin. Res. 2020, 21, 333–341. [Google Scholar] [CrossRef]
- Noh, M.K.; Jung, M.H.; Kim, S.H.; Lee, S.R.; Park, K.H.; Kim, D.H.; Kim, H.H.; Park, Y.-G. Assessment of IL-6, IL-8 and TNF-α Levels in the Gingival Tissue of Patients With Periodontitis. Exp. Ther. Med. 2013, 6, 847–851. [Google Scholar] [CrossRef]
- Hao, C.-P.; Cao, N.; Zhu, Y.; Wang, W. The Impact of Smoking on Periodontitis Patients’ GCF/Serum Cytokine Profile Both Before and After Periodontal Therapy: A Meta-Analysis. BMC Oral Health 2023, 23, 60. [Google Scholar] [CrossRef]
- Mancini, L.; Americo, L.M.; Pizzolante, T.; Donati, R.; Marchetti, E. Impact of COVID-19 on Periodontitis and Peri-Implantitis: A Narrative Review. Front. Oral Health 2022, 3, 822824. [Google Scholar] [CrossRef]
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Yokoe, S.; Suzuki, R.; Sato, S.; Iinuma, T.; Imai, K. Expression of the SARS-CoV-2 Receptor ACE2 and Proinflammatory Cytokines Induced by the Periodontopathic Bacterium Fusobacterium Nucleatum in Human Respiratory Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1352. [Google Scholar] [CrossRef]
- Jafrin, S.; Aziz, A.; Islam, M.S. Elevated Levels of Pleiotropic Interleukin-6 (IL-6) and Interleukin-10 (IL-10) Are Critically Involved With the Severity and Mortality of COVID-19: An Updated Longitudinal Meta-Analysis and Systematic Review on 147 Studies. Biomark. Insights 2022, 17, 117727192211066. [Google Scholar] [CrossRef]
- Oktavia, N.; Efrida, E.; Rofinda, Z.D. Comparison in Levels of Interleukin 6, Ferritine and Neutrophil-Lymphocyte Ratio in COVID-19 Patiens Treated in ICU and Non-Icu. J. Profesi Med. J. Kedokt. Dan. Kesehat. 2022, 16, 120–127. [Google Scholar] [CrossRef]
- Larvin, H.; Wilmott, S.; Wu, J.; Kang, J. The Impact of Periodontal Disease on Hospital Admission and Mortality During COVID-19 Pandemic. Front. Med. 2020, 7, 604980. [Google Scholar] [CrossRef]
- Quartuccio, L.; Sonaglia, A.; Pecori, D.; Peghin, M.; Fabris, M.; Tascini, C.; Vita, S. De Higher Levels of IL-6 Early After Tocilizumab Distinguish Survivors From Nonsurvivors in COVID-19 Pneumonia: A Possible Indication for Deeper Targeting of IL-6. J. Med. Virol. 2020, 92, 2852–2856. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison With Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Li, X.; Zou, X.; Bing, P.; Qi, M.; He, B. Vitamin D supplementation for managing COVID-19 in patients with vitamin D deficiency: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2025, 15, e091903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, J.; Xie, M.; Yang, J.; Chao, S.-W.; Xu, E. Clinical Efficacy of Tocilizumab Treatment in Severe and Critical COVID-19 Patients. World J. Clin. Cases 2020, 8, 3763–3773. [Google Scholar] [CrossRef] [PubMed]

| COVID 19/Self-RPD+ (n = 28) | COVID-19 (n = 32) | Self-RPD+ (n = 25) | Healthy Subjects (n = 17) | p | ||
|---|---|---|---|---|---|---|
| Qs1 | Yes | 18 (64.3) | 4 (12.5) | 23 (92) | 0 (0) | 0.000 |
| No | 10 (35.7) | 28 (87.5) | 2 (8 | 17 (100) | ||
| Qs2 | Yes | 8 (28.6) | 0 (0) | 6 (24) | 0 (0) | 0.001 |
| No | 20 (71.4) | 32 (100) | 19 (76) | 17 (100) | ||
| Qs3 | Yes | 24 (85.7) | 21 (65.6) | 21 (84) | 12 (70.6) | 0.213 |
| No | 4 (14.3) | 11 (34.4) | 4 (16) | 5 (29.4) | ||
| Qs4 | Yes | 7 (25) | 4 (12.5) | 7 (28) | 1 (5.9) | 0.191 |
| No | 21 (75) | 28 (87.5) | 18 (72) | 16 (94.1) | ||
| Qs5 | Yes | 19 (67.9) | 2 (6.3) | 19 (76) | 1 (5.9) | 0.000 |
| No | 9 (32.1) | 30 (93.8) | 6 (24) | 16 (94.1) | ||
| Qs6 | Yes | 16 (57.1) | 1 (3.1) | 17 (68) | 0 (0) | 0.000 |
| No | 12 (42.9) | 31 (96.9) | 8 (32) | 17 (100) |
| COVID-19/SELF-RPD+ (n = 28) | COVID-19 (n = 32) | Periodontitis (n = 25) | Healthy Subjects (n = 17) | p | ||
|---|---|---|---|---|---|---|
| Age (years) | 37.3 ± 16.6 | 36.2 ± 17.5 | 38.3 ± 15.3 | 41.4 ± 9.7 | 0.563 | |
| Gender | Female | 15 (53.6) | 18 (56.3) | 12 (48) | 10 (588 | 0.892 |
| Male | 13 (46.4) | 14 (43.8) | 13 (529 | 7 (41.2) | ||
| Smoking | Yes | 6 (10.4) | 6 (18.8)) | 11 (44) | 3 (17.6) | 0.114 |
| No | 22 (78.6) | 26 (81.3) | 14 (56) | 14 (82.4) | ||
| Diseases | Yes | 7 (25) | 9 (28.1) | 6 (24) | 2 (12.8) | 0.243 |
| No | 21 (75) | 23 (71.9) | 19 (76) | 15 (87.2) | ||
| Drugs | Yes | 17 (60.7) | 10 (31.3) | 12 (52) | 5 (29.4) | 0.075 |
| No | 11 (39.3) | 22 (68.8) | 13 (48) | 12 (70.6) | ||
| Symptoms | ||||||
| Fever | Yes | 18 (64.3) | 11 (34.4) | 9 (36) | 13 (76.5) | 0.006 |
| No | 10 (35.7) | 21 (65.6) | 16 (64) | 4 (23.5) | ||
| Dry cough | Yes | 20 (71.4) | 18 (56.3) | 18 (72) | 11 (64.7) | 0.558 |
| No | 8 (28.6) | 14 (43.8) | 7 (28) | 6 (35.3) | ||
| Nasal congestion | Yes | 18 (64.3) | 18 (56.3) | 7 (28) | 7 (41.2) | 0.045 |
| No | 10 (35.7) | 14 (43.8) | 18 (72) | 10 (58.8) | ||
| Tiredness | Yes | 16 (57.1) | 10 (31.3) | 13 (52) | 9 (52.9) | 0.189 |
| No | 12 (42.9) | 22 (68.8) | 12 (48) | 8 (47.1) | ||
| Cough with phlegm | Yes | 7 (25) | 16 (46.9) | 4 (16) | 2 (11.8) | 0.018 |
| No | 21 (75) | 17 (53.1) | 21 (84) | 15 (88.2) | ||
| Shortness of breath | Yes | 4 (14.3) | 3 (9.4) | 2 (8) | 1 (5.9) | 0.808 |
| No | 2 (85.7) | 29 (90.6) | 23 (92) | 16 (94.1) | ||
| Body aches | Yes | 11 (39.3) | 2 (6.3) | 8 (32) | 7 (41.2) | 0.011 |
| No | 17 (60.7) | 30 (93.8) | 17 (68) | 10 (58.8) | ||
| Headache | Yes | 18 (64.3) | 17 (53.1) | 13 (52) | 8 (47.1) | 0.694 |
| No | 10 (35.7) | 15 (46.9) | 12 (48) | 9 (52.9) | ||
| Chill | Yes | 7 (25) | 6 (18.8) | 5 (20) | 6 (35.3) | 0.586 |
| No | 21 (75) | 26 (81.3) | 20 (80) | 11 (64.7) | ||
| Muscle pain | Yes | 9 (32.1) | 9 (28.1) | 8 (32) | 9 (52.9) | 0.360 |
| No | 19 (67.9) | 23 (79.1) | 17(68) | 8 (47.1) | ||
| Joint Pain | Yes | 5 (17.9) | 10 (31.3) | 8 (32) | 5 (29.4) | 0.613 |
| No | 23 (82.1) | 22 (68.8) | 18 (68) | 12 (70.6) | ||
| Runny mucus | Yes | 9 (32.1) | 11 (34.4) | 9 (36) | 4 (23.2) | 0.866 |
| No | 19 (67.9) | 21 (65.6) | 16 (64) | 13 (76.5) | ||
| Burning throat | Yes | 14 (50) | 14 (43.8) | 12 (48) | 6 (35.3) | 0.794 |
| No | 14 (50) | 18 (56.3) | 13 (52) | 11 (64.7) | ||
| Flu | Yes | 8 (28.6) | 13 (40.6) | 6 (24) | 2 (11.8) | 0.180 |
| No | 20 (71.4) | 19 (59.4) | 19 (76) | 15 (88.2) | ||
| Conjunctivitis | Yes | 6 (21.4) | 9 (28.1) | 6 (24) | 2 (11.8) | 0.651 |
| No | 22 (78.6) | 23 (71.9) | 19 (76) | 15 (88.2) | ||
| Diarrhea | Yes | 1 (3.6) | 9 (28.1) | 4 (16) | 2 (11.8) | 0.067 |
| No | 27 (96.4) | 23 (71.9) | 21 (84) | 15 (88.2) | ||
| Vomit | Yes | 1 (3.9) | 6 (18.8) | 3 (12) | 2 (11.8) | 0.350 |
| No | 27 (96.4) | 26 (81.3) | 22 (88) | 15 (88.2) | ||
| Stomachache | Yes | 3 (10.7) | 2 (6.3) | 6 (24) | 1 (5.9) | 0.160 |
| No | 25 (89.3) | 30 (93.8) | 19 (76) | 16 (94.1) | ||
| Fast breathing | Yes | 2 (7.1) | 1 (3.1) | 3 (12) | 2 (11.8) | 0.603 |
| No | 26 (92.6) | 31 (96.9) | 22 (88) | 15 (88.2) | ||
| Convulsions | Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| No | 28 (100) | 32 (100) | 25 (100) | 11 (100) | ||
| Olfactory disturbance | Yes | 17 (60.7) | 4 (12.5) | 8 (32) | 6 (35.3) | 0.001 |
| No | 11 (39.3) | 28 (87.5) | 17 (68) | 11 (64.7) | ||
| Gustatory disturbance | Yes | 15 (53.6) | 16 (50) | 7 (28) | 7 (41.2) | 0.247 |
| No | 13 (46.4) | 16 (50) | 18 (72) | 10 (58.8) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Montaño, R.; Baltazar-Díaz, T.A.; Hernández-Mora, O.; Isiordia-Espinoza, M.A.; Del Muro-Casas, F.; González-González, R.; Bologna-Molina, R.; López-Verdín, S. Periodontal Disease Elevates IL-6 Levels During Initial Symptoms of COVID-19. Diagnostics 2025, 15, 1650. https://doi.org/10.3390/diagnostics15131650
Rodríguez-Montaño R, Baltazar-Díaz TA, Hernández-Mora O, Isiordia-Espinoza MA, Del Muro-Casas F, González-González R, Bologna-Molina R, López-Verdín S. Periodontal Disease Elevates IL-6 Levels During Initial Symptoms of COVID-19. Diagnostics. 2025; 15(13):1650. https://doi.org/10.3390/diagnostics15131650
Chicago/Turabian StyleRodríguez-Montaño, Ruth, Tonatiuh Abimael Baltazar-Díaz, Oscar Hernández-Mora, Mario Alberto Isiordia-Espinoza, Fatima Del Muro-Casas, Rogelio González-González, Ronell Bologna-Molina, and Sandra López-Verdín. 2025. "Periodontal Disease Elevates IL-6 Levels During Initial Symptoms of COVID-19" Diagnostics 15, no. 13: 1650. https://doi.org/10.3390/diagnostics15131650
APA StyleRodríguez-Montaño, R., Baltazar-Díaz, T. A., Hernández-Mora, O., Isiordia-Espinoza, M. A., Del Muro-Casas, F., González-González, R., Bologna-Molina, R., & López-Verdín, S. (2025). Periodontal Disease Elevates IL-6 Levels During Initial Symptoms of COVID-19. Diagnostics, 15(13), 1650. https://doi.org/10.3390/diagnostics15131650










