MR Defecography Improves Diagnosis of Postoperative Pelvic Floor Dysfunction After Gynecological Surgery
Abstract
1. Introduction
2. Anatomical Structure of the Pelvic Floor
3. Importance of Early Diagnosis and Evaluation of PFD
3.1. Role of Surgery
3.2. Imaging Techniques for Diagnosing PFD
3.3. Role of MRI in Diagnosing Postoperative Complications
4. MR Imaging Protocol
4.1. Patient Preparation
4.2. Equipment and Positioning
4.3. Contrast and Opacification
4.4. Imaging Sequences
- Static Imaging:
- High-resolution T2-weighted images in three orthogonal planes: axial, sagittal, and coronal.
- Dynamic Imaging:
- Steady-state or balanced steady-state free precession (SSFP) sequences.
- Acquired in the sagittal plane during various maneuvers: straining, squeezing, and evacuation.
- Optional dynamic sequences in axial and coronal planes during straining may be considered to further evaluate complex pelvic floor dysfunctions.
4.5. Key Maneuvers
4.6. Imaging Analysis of the Pelvic Floor
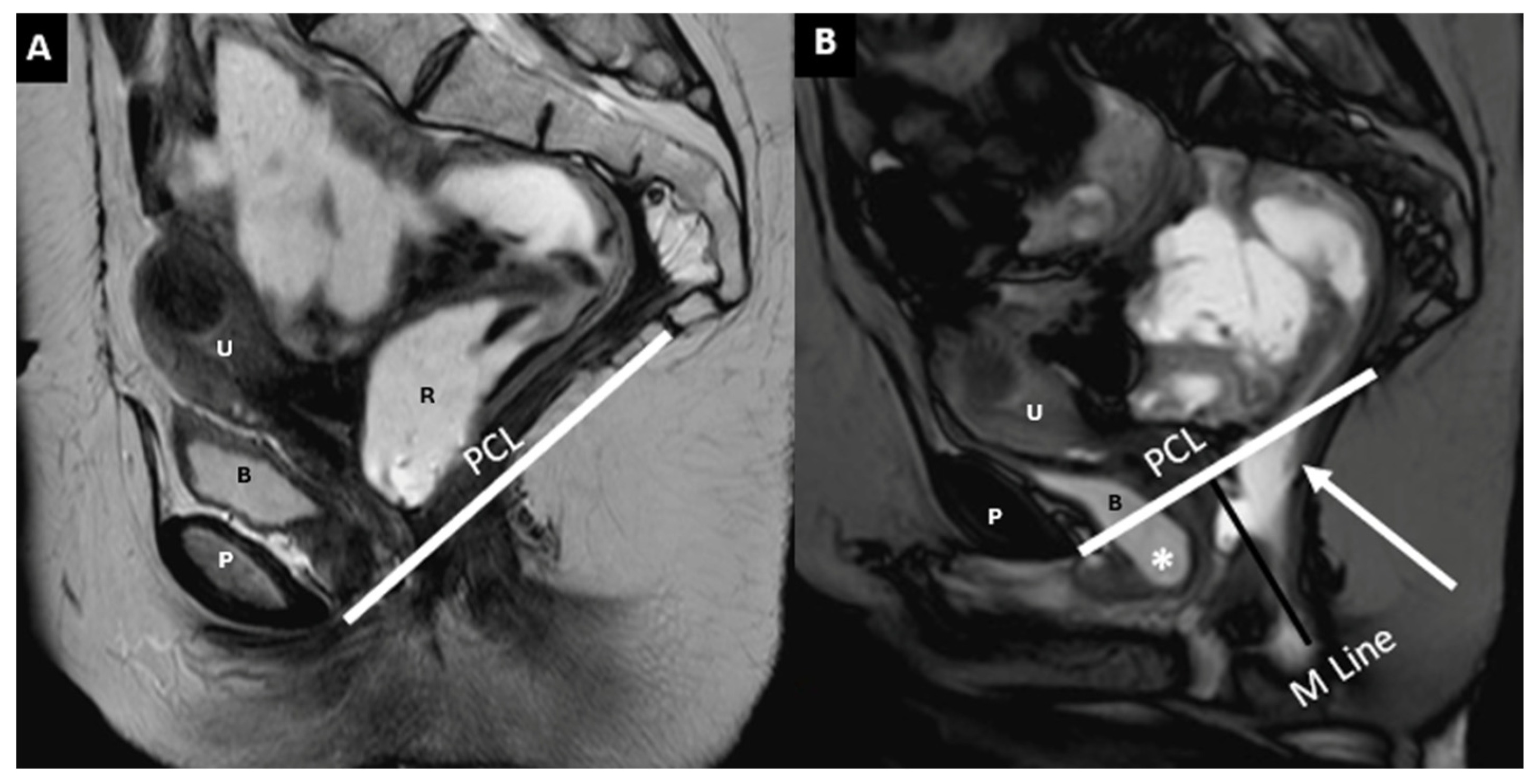
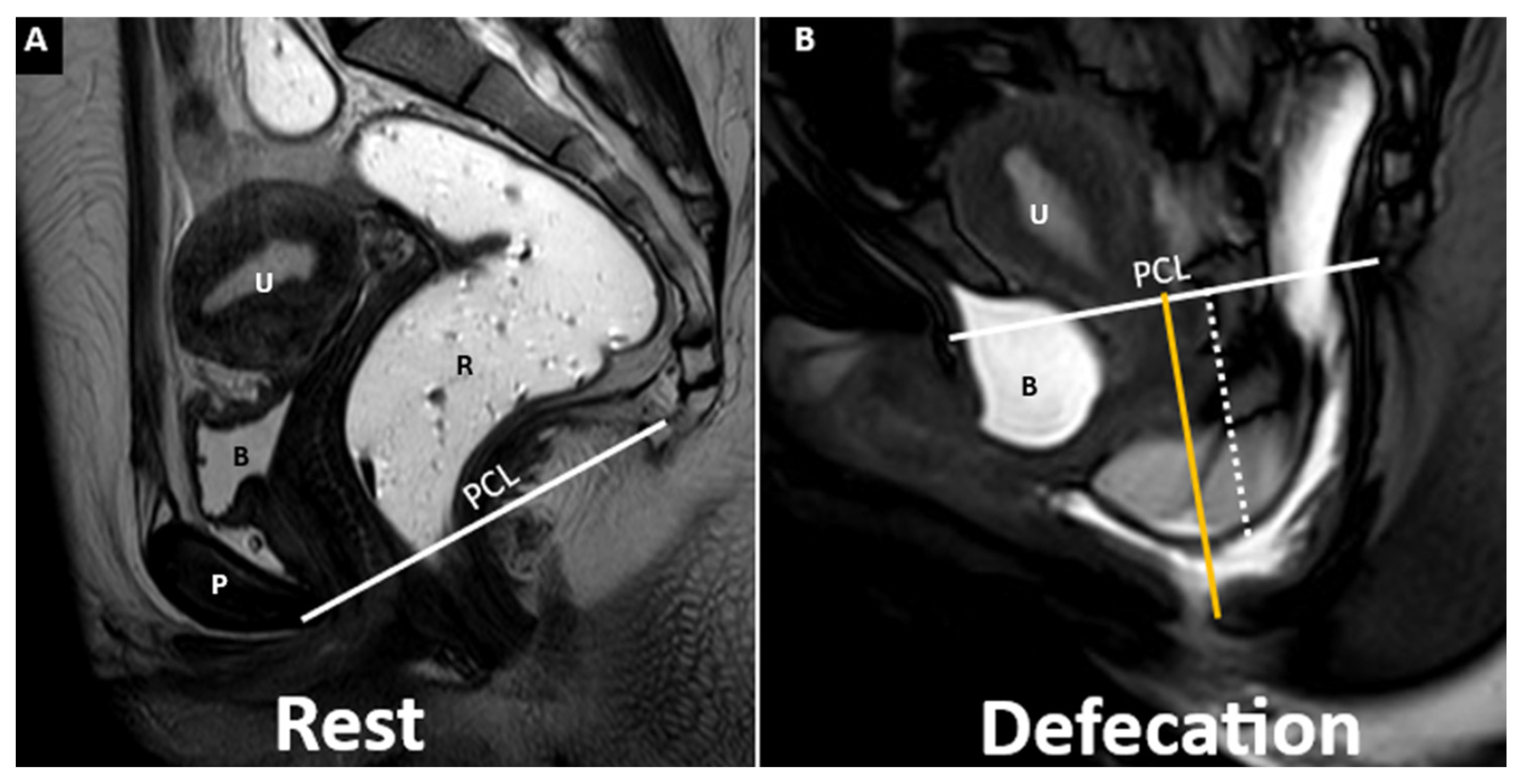
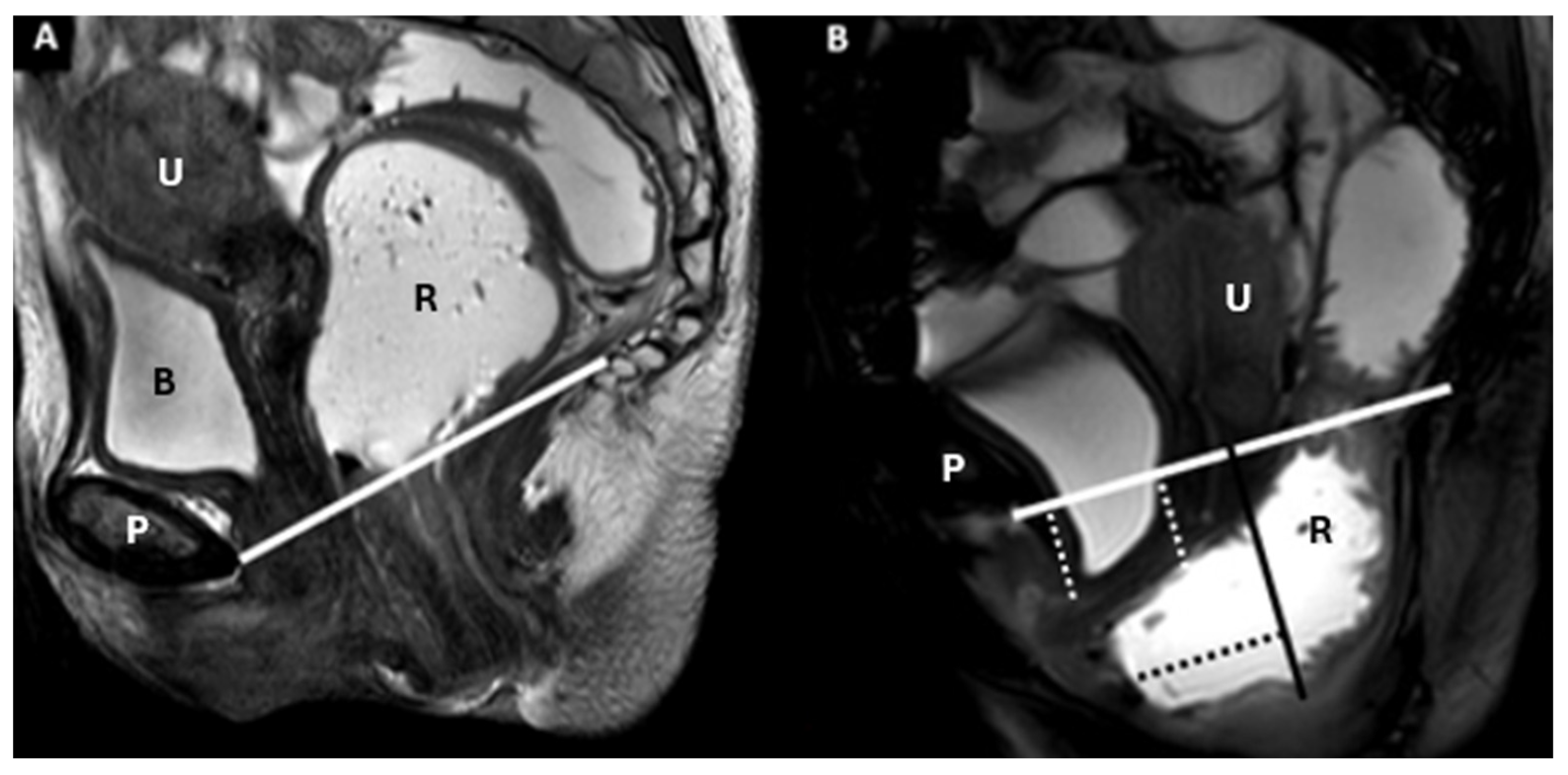
5. The HMO System
- Point A: The inferior margin of the symphysis pubis
- Point B: The convex posterior margin of the puborectalis muscle sling
- Point C: The junction between the first and second coccygeal segments
6. Evaluating Pelvic Floor Disorders with MR Defecography
6.1. Anterior Compartment
Cystoceles
6.2. Middle Compartment
6.2.1. Uterine Prolapse
6.2.2. Vaginal Prolapse
6.3. Posterior Compartment
6.3.1. Enterocele
6.3.2. Sigmoidocele
6.3.3. Peritoneocele
6.3.4. Rectocele
6.3.5. Anterior Rectocele
6.3.6. Rectal Intussusception
6.3.7. Dyssynergia
7. Current Challenges of MR Defecography
8. Needs for Future Research
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peinado-Molina, R.A.; Hernández-Martínez, A.; Martínez-Vázquez, S.; Rodríguez-Almagro, J.; Martínez-Galiano, J.M. Pelvic floor dysfunction: Prevalence and associated factors. BMC Public Health 2023, 23, 2005. [Google Scholar] [CrossRef] [PubMed]
- Tim, S.; Mazur-Bialy, A.I. The most common functional disorders and factors affecting female pelvic floor. Life 2021, 11, 1397. [Google Scholar] [CrossRef]
- Al-Badr, A.; Saleem, Z.; Kaddour, O.; Almosaieed, B.; Dawood, A.; Al-Tannir, M.; AlTurki, F.; Alharbi, R.; Alsanea, N. Prevalence of pelvic floor dysfunction: A Saudi national survey. BMC Womens Health 2022, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Benti Terefe, A.; Gemeda Gudeta, T.; Teferi Mengistu, G.; Abebe, S.S. Determinants of pelvic floor disorders among women visiting the gynecology outpatient department in wolkite university specialized center, wolkite. Ethiop. Obs. Gynecol. Int. 2022, 13, 6949700. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Gala, T.; Igualada-Martinez, P.; Brown, H.W.; Weinstein, M.; Hainsworth, A. Multidisciplinary team (MDT) approach to pelvic floor disorders. Continence 2023, 7, 100716. [Google Scholar] [CrossRef]
- La Rosa, V.L.; Garzon, S.; Gullo, G.; Fichera, M.; Sisti, G.; Gallo, P.; Riemma, G.; Schiattarella, A. Fertility preservation in women affected by gynaecological cancer: The importance of an integrated gynaecological and psychological approach. Ecancermedi-calscience 2020, 14, 1035. [Google Scholar] [CrossRef]
- Raju, R.; Linder, B.J. Evaluation and Management of Pelvic Organ Prolapse. Mayo Clin. Proc. 2021, 96, 3122–3129. [Google Scholar] [CrossRef]
- Mbaye, M.; Autumn Edenfield, L.; Woll, A.; Swift, S.E. Factors affecting patient choice for continued observation versus intervention for pelvic organ prolapse. Int. Urogynecol. J. 2021, 32, 273–278. [Google Scholar] [CrossRef]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef]
- Barbier, H.; Carberry, C.L.; Karjalainen, P.K.; Mahoney, C.K.; Galán, V.M.; Rosamilia, A.; Ruess, E.; Shaker, D.; Thariani, K. International Urogynecology consultation chapter 2 committee 3: The clinical evaluation of pelvic organ prolapse including investigations into associated morbidity/pelvic floor dysfunction. Int. Urogynecol. J. 2023, 34, 2657–2688. [Google Scholar] [CrossRef]
- HageFransen, M.A.H.; Wiezer, M.; Otto, A.; Wieffer Platvoet, M.S.; Slotman, M.H.; van der Nijhuis Sanden, M.W.G.; Pool-Goudzwaard, A.L. Pregnancy- and obstetric-related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 373–382. [Google Scholar] [CrossRef]
- Gilyadova, A.; Ishchenko, A.; Puchkova, E.; Mershina, E.; Petrovichev, V.; Reshetov, I. Diagnostic Value of Dynamic Magnetic Resonance Imaging (dMRI) of the Pelvic Floor in Genital Prolapses. Biomedicines 2023, 11, 2849. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, D.G.; Abdelatif, M.; Thabet, W.M.; Elshafei, A.M.; Shady, M.M. Role of static and dynamic MRI in evaluation of pelvic posterior compartment pathologies: Prospective case series. Egypt. J. Radiol. Nucl. Med. 2020, 51, 72. [Google Scholar] [CrossRef]
- Jha, A.A.; Sandhu, A.S.; Dash, S.C.; Talwar, R.; Govindaiah, M.; Singh, G.; Handa, A.; Solanki, N. Comparison of Surgical and Functional Outcome of Laparoscopic Pyeloplasty and Robot-assisted Pyeloplasty for Congenital Uretero Pelvic Junction Obstruction. J. Urol. Surg. 2022, 9, 20–24. [Google Scholar] [CrossRef]
- Bordoni, B.; Sugumar, K.; Leslie, S.W. Anatomy, Abdomen and Pelvis, Pelvic Floor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482200/ (accessed on 7 June 2025).
- Lipetskaia, L.; Gupta, A.; Cheung, R.Y.K.; Khullar, V.; Ismail, S.; Bradley, M.; Karmakar, R.; Clifton, S.; Doo, J.; Quiroz, L. International Urogynecological Consultation Chapter 2.2: Imaging in the Diagnosis of Pelvic Organ Prolapse. Int. Urogynecol. J. 2025, 36, 759–781. [Google Scholar] [CrossRef]
- Siccardi, M.A.; Valle, C. Anatomy, Bony Pelvis and Lower Limb: Pelvic Fascia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK518984/ (accessed on 7 June 2025).
- Gowda, S.N.; Bordoni, B. Anatomy, Abdomen and Pelvis: Levator Ani Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556078/ (accessed on 7 June 2025).
- Clark, K.M.; Pandya, A.M. Anatomy, Abdomen and Pelvis: Cardinal Ligaments (Mackenrodts, Transverse Cervical, or Lateral Cervical Ligaments). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557384/ (accessed on 7 June 2025).
- Bolla, S.R.; Hoare, B.S.; Varacallo, M.A. Anatomy, Abdomen and Pelvis: Deep Perineal Space. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538272/ (accessed on 7 June 2025).
- Cichowski, S.; Grzybowska, M.E.; Halder, G.E.; Jansen, S.; Gold, D.; Espuña, M.; Jha, S.; Al-Badr, A.; Abdelrahman, A.; Rogers, R.G. International Urogynecology Consultation: Patient Reported Outcome Measures (PROs) use in the evaluation of patients with pelvic organ prolapse. Int. Urogynecol. J. 2022, 33, 2603–2631. [Google Scholar] [CrossRef]
- Knez, J.; Al Mahdawi, L.; Takač, I.; Sobočan, M. The Perspectives of Fertility Preservation in Women with Endometrial Cancer. Cancers 2021, 13, 602. [Google Scholar] [CrossRef]
- Gullo, G.; Cucinella, G.; Chiantera, V.; Dellino, M.; Cascardi, E.; Török, P.; Herman, T.; Garzon, S.; Uccella, S.; Laganà, A.S. Fertility-sparing strategies for early-stage endometrial cancer: Stepping towards precision medicine based on the molecular fingerprint. Int. J. Mol. Sci. 2023, 24, 811. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Zhu, Y.; Lu, A.W.; Zhou, L.; Wang, J.S.; Zhang, Y.; Wang, J.T. The oncological and obstetric results of radical trachelectomy as a fertility-sparing therapy in early-stage cervical cancer patients. BMC Womens Health 2022, 22, 424. [Google Scholar] [CrossRef]
- Dieter, A.A.; Halder, G.E.; Pennycuff, J.F.; Singh, R.; El-Nashar, S.A.; Lipetskaia, L.; Orejuela, F.J.; Jeppson, P.C.; Sleemi, A.; Raman, S.V.; et al. Society of Gynecologic Surgeons Systematic Review Group. Patient-Reported Outcome Measures for Use in Women With Pelvic Organ Prolapse: A Systematic Review. Obs. Gynecol. 2023, 141, 1098–1114. [Google Scholar] [CrossRef]
- Harvey, M.A.; Chih, H.J.; Geoffrion, R.; Amir, B.; Bhide, A.; Miotla, P.; Rosier, P.F.W.M.; Offiah, I.; Pal, M.; Alas, A.N. International Urogynecology Consultation Chapter 1 Committee 5: Relationship of pelvic organ prolapse to associated pelvic floor dysfunction symptoms: Lower urinary tract, bowel, sexual dysfunction and abdominopelvic pain. Int. Urogynecol. J. 2021, 32, 2575–2594. [Google Scholar] [CrossRef] [PubMed]
- Bordeianou, L.G.; Anger, J.T.; Boutros, M.; Birnbaum, E.; Carmichael, J.C.; Connell, K.A.; De, E.J.B.; Mellgren, A.; Staller, K.; Vogler, S.A.; et al. Members of the Pelvic Floor Disorders Consortium Working Groups on Patient-Reported Outcomes. Measuring Pelvic Floor Disorder Symptoms Using Patient-Reported Instruments: Proceedings of the Consensus Meeting of the Pelvic Floor Consortium of the American Society of Colon and Rectal Surgeons, the International Continence Society, the American Urogynecologic Society, and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Female Pelvic Med. Reconstr. Surg. 2020, 26, 5–22. [Google Scholar] [CrossRef]
- Good, M.M.; Solomon, E.R. Pelvic Floor Disorders. Obs. Gynecol. Clin. N. Am. 2019, 46, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Billone, V.; Gullo, G.; Perino, G.; Catania, E.; Cucinella, G.; Ganduscio, S.; Vassiliadis, A.; Zaami, S. Robotic versus mini-laparoscopic colposacropexy to treat pelvic organ prolapse: A retrospective observational cohort study and a medicolegal perspective. J. Clin. Med. 2024, 13, 4802. [Google Scholar] [CrossRef]
- ASTEC Study Group; Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B. Efficacy of Systematic Pelvic Lymphadenectomy in Endometrial Cancer (MRC ASTEC Trial): A Randomised Study. Lancet 2009, 373, 125–136. [Google Scholar]
- Li, J.; Tian, R.; Liu, H.; Chen, H.; Zheng, Z. Short-term outcomes of open versus laparoscopic surgery in patients with metachronous colorectal cancer. BMC Surg. 2025, 25, 37. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; Eggins, R.; Brown, K.; Gebski, V.J.; Brewer, K.; Lai, L.; Bailey, L.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; et al. Patient-Reported Bowel, Urinary, and Sexual Outcomes After Laparoscopic-Assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial (ALaCart). Ann. Surg. 2023, 277, 449–455. [Google Scholar] [CrossRef]
- Paraiso, M.F.R.; Jelovsek, J.E.; Frick, A.; Chen, C.C.G.; Barber, M.D. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: A randomized controlled trial. Obs. Gynecol. 2011, 118, 1005–1013. [Google Scholar] [CrossRef]
- Grimes, W.R.; Stratton, M. Pelvic Floor Dysfunction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559246/ (accessed on 7 June 2025).
- Culligan, P.J.; Heit, M. Urinary incontinence in women: Evaluation and management. Am. Fam. Physician 2000, 62, 2433–2444. Available online: https://www.aafp.org/pubs/afp/issues/2000/1201/p2433.html (accessed on 7 June 2025).
- Harris, S.; Leslie, S.W.; Riggs, J. Mixed Urinary Incontinence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534234/ (accessed on 7 June 2025).
- International Urogynecological Association. (n.d.) Urodynamics. Your Pelvic Floor. Available online: https://www.yourpelvicfloor.org/conditions/urodynamics/#What%20are%20urodynamics (accessed on 7 June 2025).
- Scurr, K.; Gray, T.G.; Jones, G.L.; Radley, S.C. Development and initial psychometric testing of a body-image domain within an electronic pelvic floor questionnaire (ePAQ-pelvic floor). Int. Urogynecol. J. 2020, 31, 1245–1253. [Google Scholar] [CrossRef]
- Munno, G.M.; La Verde, M.; Lettieri, D.; Nicoletti, R.; Nunziata, M.; Fasulo, D.D.; Vastarella, M.G.; Pennacchio, M.; Scalzone, G.; Pieretti, G.; et al. Pelvic Organ Prolapse Syndrome and Lower Urinary Tract Symptom Update: What’s New? Healthcare 2023, 11, 1513. [Google Scholar] [CrossRef]
- Tawfeek, A.M.; Osman, T.; Gad, H.H.; Elmoazen, M.; Osman, D.; Emam, A. Clinical and Urodynamic Findings Before and After Surgical Repair of Pelvic Organ Prolapse in Women with Lower Urinary Tract Symptoms. A Prospective Observational Study. Urology 2022, 167, 90–95. [Google Scholar] [CrossRef]
- Daneshpajooh, A.; Mirzaei, M.; Dehesh, T. Role of Urodynamic Study in the Management of Pelvic Organ Prolapse in Women. Urol. J. 2021, 18, 209–213. [Google Scholar] [CrossRef]
- Glass, D.; Lin, F.C.; Khan, A.A.; Van Kuiken, M.; Drain, A.; Siev, M.; Peyronett, B.; Rosenblum, N.; Brucker, B.M.; Nitti, V.W. Impact of preoperative urodynamics on women undergoing pelvic organ prolapse surgery. Int. Urogynecol. J. 2020, 31, 1663–1668. [Google Scholar] [CrossRef]
- Reid, S.; Brocksom, J.; Hamid, R.; Ali, A.; Thiruchelvam, N.; Sahai, A.; Harding, C.; Biers, S.; Belal, M.; Barrett, R.; et al. British Association of Urological Surgeons (BAUS) and Nurses (BAUN) consensus document: Management of the complications of long-term indwelling catheters. BJU Int. 2021, 127, 410–420. [Google Scholar] [CrossRef]
- Li, S.; Tan, C.; Yang, X. The effects of vaginal surgery and pelvic floor disorders on female sexual function. J. Sex. Med. 2023, 20, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Rosati, A.; Rumolo, V.; Ferrari, F.; Gullo, G.; Karaman, E.; Karaaslan, O.; HacioĞlu, L. Novelties of ultrasound imaging for endometrial cancer preoperative workup. Minerva Med. 2021, 112, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; High, R.A.; Dziadek, O.; Ocon, A.; Muir, T.W.; Xu, J.; Antosh, D.D. Overactive bladder symptoms after pelvic organ prolapse repair. Female Pelvic Med. Reconstr. Surg. 2020, 26, 742–745. [Google Scholar] [CrossRef]
- Tenajas, R.; Miraut, D.; Illana, C.I.; Alonso-Gonzalez, R.; Arias-Valcayo, F.; Herraiz, J.L. Recent Advances in Artificial Intelligence-Assisted Ultrasound Scanning. Appl. Sci. 2023, 13, 3693. [Google Scholar] [CrossRef]
- Kido, A.; Himoto, Y.; Moribata, Y.; Kurata, Y.; Nakamoto, Y. MRI in the Diagnosis of Endometriosis and Related Diseases. Korean J. Radiol. 2022, 23, 426–445. [Google Scholar] [CrossRef]
- Law, Y.M.; Fielding, J.R. MRI of pelvic floor dysfunction: Review. Am. J. Roentgenol. 2012, 191, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Hodler, J.; Kubik-Huch, R.A.; von Schulthess, G.K. (Eds.) Diseases of the Abdomen and Pelvis 2018–2021: Diagnostic Imaging—IDKD Book; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- U.S. Food & Drug Administration. Urogynecologic Surgical Mesh Implants. U.S. Food & Drug Administration. 13 October 2021. Available online: https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants (accessed on 7 June 2025).
- Rechi-Sierra, K.; Sánchez-Ballester, F.; García-Ibáñez, J.; Pardo-Duarte, P.; Flores-DelaTorre, M.; Monzó-Cataluña, A.; López-Alcina, E. Magnetic resonance imaging to evaluate anterior pelvic prolapse: H line is the key. Neurourol. Urodyn. 2021, 40, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, C.; Hindle, A.; Rajashanker, B.; Kearney, R. MR scan evaluation of pelvic organ prolapse mesh complications and agreement with intra-operative findings. Int. Urogynecol. J. 2020, 31, 1559–1566. [Google Scholar] [CrossRef]
- Kinjo, M.; Tanba, M.; Masuda, K.; Nakamura, Y.; Tanbo, M.; Fukuhara, H. Comparison of effectiveness between modified transvaginal mesh surgery and vaginal pessary treatment in patients with symptomatic pelvic organ prolapse. Low. Urin. Tract. Symptoms 2022, 14, 64–71. [Google Scholar] [CrossRef]
- Barinova, E.; Ordiyants, I.M.; Aryutin, D.G.; Ordiyants, E.G.; Zulumyan, T.N.; Damirova, S.F.; Dobrovolskaya, D.A. A modern view of genital prolapse. Bull. Dagestan State Med. Acad. 2020, 3, 49–54. [Google Scholar]
- García del Salto, L.; de Miguel Criado, J.; Aguilera del Hoyo, L.F.; Gutiérrez Velasco, L.; Fraga Rivas, P.; Manzano Paradela, M.; Díez Pérez de las Vacas, M.I.; Marco Sanz, A.G.; Fraile Moreno, E. MR imaging–based assessment of the female pelvic floor. RadioGraphics 2014, 34, 1417–1439. [Google Scholar] [CrossRef] [PubMed]
- Neshatian, L.; Lam, J.P.; Gurland, B.H.; Liang, T.; Becker, L.; Sheth, V.R. MRI biomarker of muscle composition is associated with severity of pelvic organ prolapse. Tech. Coloproctol. 2022, 26, 725–733. [Google Scholar] [CrossRef]
- Etrusco, A.; Fabio, M.; Cucinella, G.; de Tommasi, O.; Guastella, E.; Buzzaccarini, G.; Gullo, G. Utero-cutaneous fistula after caesarean section delivery: Diagnosis and management of a rare complication. Prz Menopauzalny. 2022, 21, 214–217. [Google Scholar] [CrossRef]
- Hill, N.E.; Giampetro, D.M. Fluoroscopy Contrast Materials. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572082/ (accessed on 7 June 2025).
- Sun, M.J.; Chuang, Y.L.; Lau, H.H.; Lo, T.S.; Su, T.H. The efficacy and complications of using transvaginal mesh to treat pelvic organ prolapse in Taiwan: A 10-year review. Taiwan. J. Obs. Gynecol. 2021, 60, 187–192. [Google Scholar] [CrossRef]
- Lorenz, J.; Multidisciplinary Group Publishes Recommendations for Magnetic Resonance Defecography. Diagnostic Imaging. 22 September 2021. Available online: https://www.diagnosticimaging.com/view/multidisciplinary-group-publishes-recommendations-for-magnetic-resonance-defecography (accessed on 7 June 2025).
- Davra, S.V.; Jain, A.; Kumar, A. Static & dynamic pelvic floor assessment using MR defecography: A radiological prospective observational study with ideal reporting template. Egypt. J. Radiol. Nucl. Med. 2025, 56, 34. [Google Scholar] [CrossRef]
- On behalf of the ESUR and ESGAR Pelvic Floor Working Group; El Sayed, R.F.; Alt, C.D.; Maccioni, F.; Meissnitzer, M.; Masselli, G.; Manganaro, L.; Vinci, V.; Weishaupt, D. Magnetic resonance imaging of pelvic floor dysfunction—Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur. Radiol. 2017, 27, 2067–2085. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; Sharma, M.; Feuerhak, K.; Bailey, K.R.; Bharucha, A.E. A comparison of rectoanal pressures during Valsalva maneuver and evacuation uncovers rectoanal discoordination in defecatory disorders. Neurogastroenterol. Motil. 2021, 33, e14126. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, G.T.; dos Santos, H.T.; Virtuoso, J.F. Pelvic floor distress inventory (PFDI)—Systematic review of measurement properties. Int. Urogynecol. J. 2021, 32, 2657–2669. [Google Scholar] [CrossRef]
- Guo, M.; Zbar, A.P.; Wu, Y. Imaging the levator ani and the puborectalis muscle: Implications in understanding regional anatomy, physiology and pathology. Scand. J. Gastroenterol. 2023, 58, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e3. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.; Mastrovito, S.; Masteling, M.; Horner, W.; Ashton-Miller, J.A.; Chen, L. A unified pelvic floor conceptual model for studying morphological changes with prolapse, age, and parity. Am. J. Obs. Gynecol. 2024, 230, 476–484.e2. [Google Scholar] [CrossRef]
- Ghafoor, S.; Beintner-Skawran, S.; Betschart, C.; Winklehner, T.; Reiner, C.S. Assessment of pelvic organ prolapse with the Pelvic Inclination Correction System: Defining the normal range and threshold to pathology. Abdom. Radiol. 2024, 49, 1996–2007. [Google Scholar] [CrossRef]
- Salvador, J.C.; Coutinho, M.P.; Venâncio, J.M.; Viamonte, B. Dynamic magnetic resonance imaging of the female pelvic floor-a pictorial review. Insights Imaging 2019, 10, 4. [Google Scholar] [CrossRef]
- Barbaric, Z.L.; Marumoto, A.K.; Raz, S. Magnetic resonance imaging of the perineum and pelvic floor. Top. Magn. Reson. Imaging 2001, 12, 83–92. [Google Scholar] [CrossRef]
- Pannu, H.K.; Kaufman, H.S.; Cundiff, G.W.; Genadry, R.; Bluemke, D.A.; Fishman, E.K. Dynamic MR imag-ing of pelvic organ prolapse: Spectrum of abnor-malities. Radiographics 2000, 20, 1567–1582. [Google Scholar] [CrossRef]
- Ghafoor, S.; Beintner-Skawran, S.M.; Stöckli, G.; Betschart, C.; Reiner, C.S. Pelvic organ movements in asymptomatic nulliparous and symptomatic premenopausal women with pelvic organ prolapse in dynamic MRI: A feasibility study comparing midsagittal single-slice with multi-slice sequences. Abdom. Radiol. 2023, 48, 2658–2671. [Google Scholar] [CrossRef]
- Palmer, S.L.; Lalwani, N.; Bahrami, S.; Scholz, F. Dynamic fluoroscopic defecography: Updates on rationale, technique, and interpretation from the Society of Abdominal Radiology Pelvic Floor Disease Focus Panel. Abdom. Radiol. 2021, 46, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Flusberg, M.; Kobi, M.; Bahrami, S.; Glanc, P.; Palmer, S.; Chernyak, V.; Kanmaniraja, D.; El Sayed, R.F. Multimodality imaging of pelvic floor anatomy. Abdom. Radiol. 2021, 46, 1302–1311. [Google Scholar] [CrossRef]
- Aboseif, C.; Liu, P. Pelvic Organ Prolapse. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563229/ (accessed on 7 June 2025).
- Di Piazza, A.; Costanzo, M.; Vernuccio, F.; Scopelliti, L.; Tudisca, C.; Calafiore, C.; Midiri, F.; Picone, D.; Cocorullo, G.; Albano, D.; et al. utile non solo nella sindrome da defecazione ostruita. Il G. Ital. Di Radiol. Medica 2016, 3, 468–479. [Google Scholar]
- Wallace, S.L.; Kim, Y.; Lai, E.; Mehta, S.; Gaigbe-Togbe, B.; Zhang, C.A.; Von Bargen, E.C.; Sokol, E.R. Postoperative complications and pelvic organ prolapse recurrence following combined pelvic organ prolapse and rectal prolapse surgery compared with pelvic organ prolapse only surgery. Am. J. Obs. Gynecol. 2022, 227, e1–e317. [Google Scholar] [CrossRef]
- Altman, D.; Zetterstrom, J.; Schultz, I.; Nordenstam, J.; Hjern, F.; Lopez, A.; Mellgren, A. Pelvic organ prolapse and urinary incontinence in women with surgically managed rectal prolapse: A population-based case-control study. Dis. Colon. Rectum. 2006, 49, 28–35. [Google Scholar] [CrossRef]
- Pannu, H.K.; Scatarige, J.C.; Eng, J. Comparison of supine magnetic resonance imaging with and without rectal contrast to fluoroscopic cystocolpoproctography for the diagnosis of pelvic organ prolapse. J. Comput. Assist. Tomogr. 2009, 33, 125–130. [Google Scholar] [CrossRef]
- Carter, J.E. Enterocele repair and vaginal vault suspension. Curr. Opin. Obstet. Gynecol. 2000, 12, 321–330, Erratum in Curr. Opin. Obstet. Gynecol. 2000, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Maglinte, D.D.T.; Bartram, C.I.; Hale, D.A.; Park, J.; Kohli, M.D.; Robb, B.W.; Romano, S.; Lappas, J.C. Functional imaging of the pelvic floor. Radiology 2011, 258, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ladd, M.; Tuma, F. Rectocele. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546689/ (accessed on 7 June 2025).
- Bharucha, A.E.; Knowles, C.H. Rectocele: Incidental or important? Observe or operate? Contemporary diagnosis and management in the multidisciplinary era. Neurogastroenterol. Motil. 2022, 34, e14453. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Hussain, M.I.; Zaman, S.; Randle, S.; Tanveer, Y.; Faiz, N.; Sarma, D.R.; Peravali, R. Delorme’s vs. Altemeier’s in the management of rectal procidentia: Systematic review and meta-analysis. Langenbecks Arch Surg. 2023, 408, 454. [Google Scholar] [CrossRef]
- Gurland, B.H.; Khatri, G.; Ram, R.; Hull, T.L.; Kocjancic, E.; Quiroz, L.H.; Sayed, R.F.E.; Jambhekar, K.R.; Chernyak, V.; Paspulati, R.M.; et al. Consensus Definitions and Interpretation Templates for Magnetic Resonance Imaging of Defecatory Pelvic Floor Disorders: Proceedings of the Consensus Meeting of the Pelvic Floor Disorders Consortium of the American Society of Colon and Rectal Surgeons, the Society of Abdominal Radiology, the International Continence Society, the American Urogynecologic Society, the International Urogynecological Association, and the Society of Gynecologic Surgeons. AJR Am. J. Roentgenol. 2021, 217, 800–812. [Google Scholar] [CrossRef]
- Blaker, K.; Anandam, J.L. Functional Disorders: Rectoanal Intussusception. Clin. Colon. Rectal Surg. 2017, 30, 5–11. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Byrne, C.M.; Heywood, N.; Davenport, M.; Klarskov, N.; Sharma, A.; Kiff, E.; Telford, K. Anal sphincter function in rectal intussusception and high and low “take-off” external rectal prolapse-A prospective observational study. Color. Dis. 2024, 26, 2069–2079. [Google Scholar] [CrossRef]
- Sadeghi, A.; Akbarpour, E.; Majidirad, F.; Bor, S.; Forootan, M.; Hadian, M.R.; Adibi, P. Dyssynergic Defecation: A Comprehensive Review on Diagnosis and Management. Turk. J. Gastroenterol. 2023, 34, 182–195. [Google Scholar] [CrossRef]
- Caroli, A. Diffusion-Weighted Magnetic Resonance Imaging: Clinical Potential and Applications. J. Clin. Med. 2022, 11, 3339. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, I.M.; Luijten, S.P.R.; de Zeeuw, C.I.; Holstege, J.C.; Scheepe, J.R.; van der Zwaag, W.; Blok, B.F.M. Whole brain 7T-fMRI during pelvic floor muscle contraction in male subjects. Neurourol. Urodyn. 2020, 39, 382–392. [Google Scholar] [CrossRef]
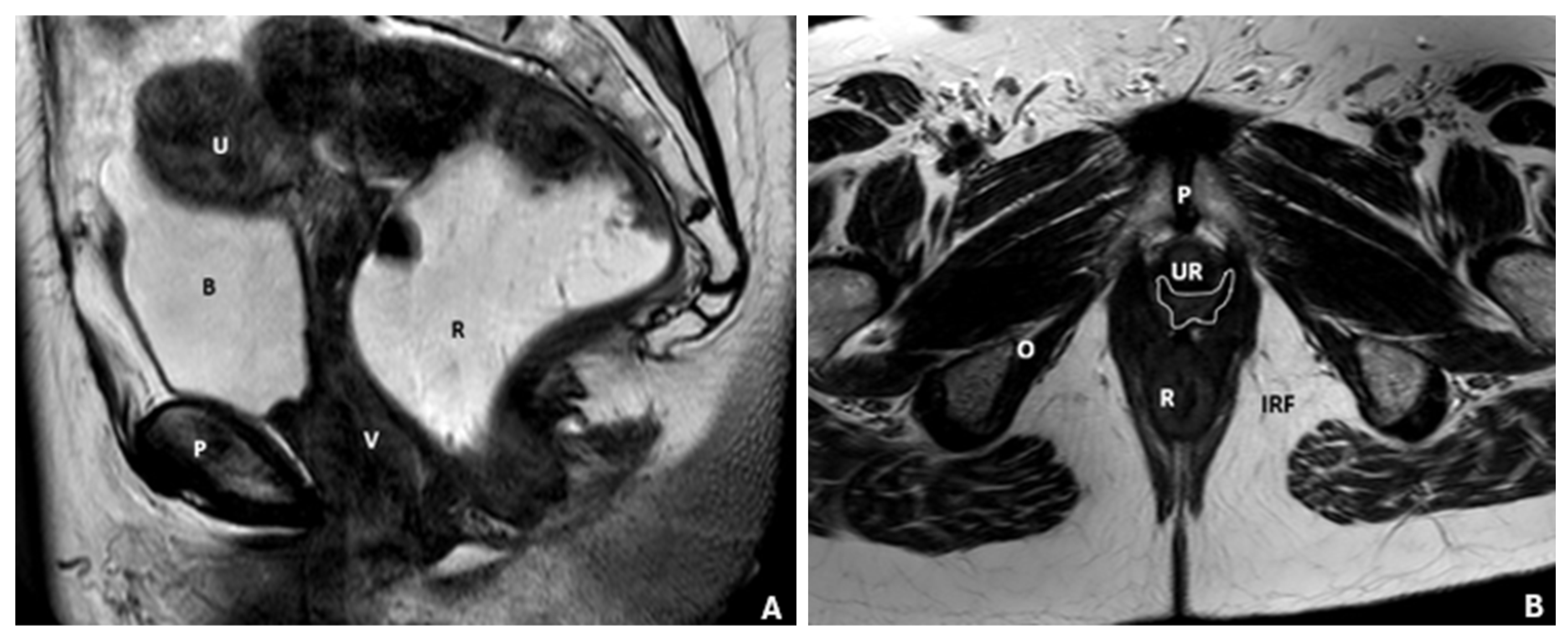
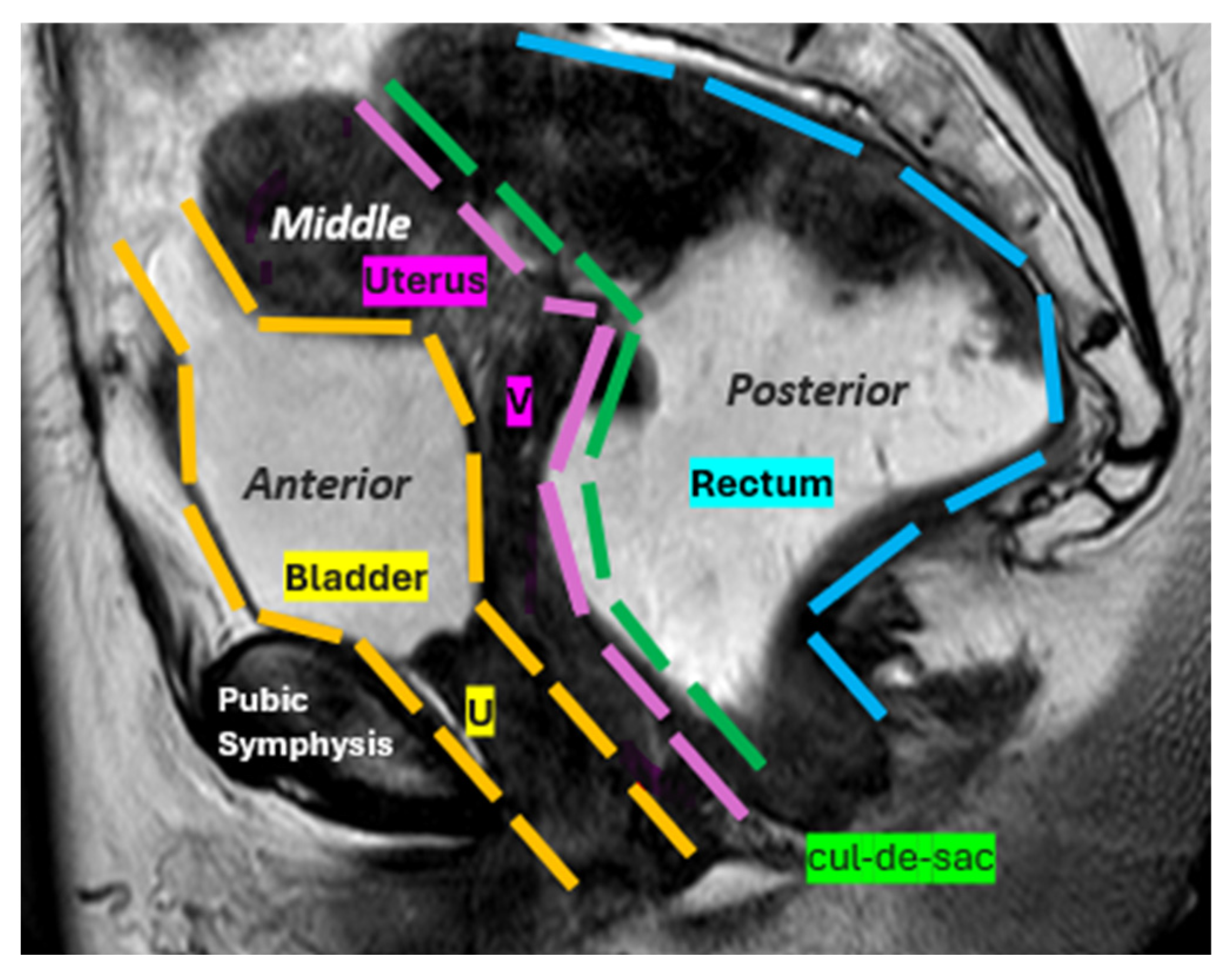
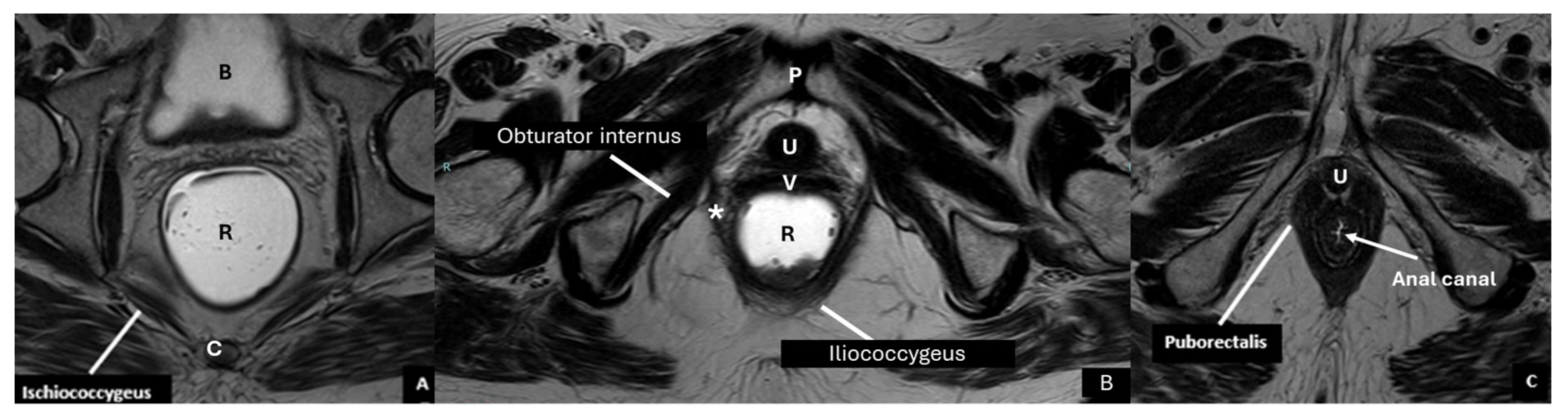
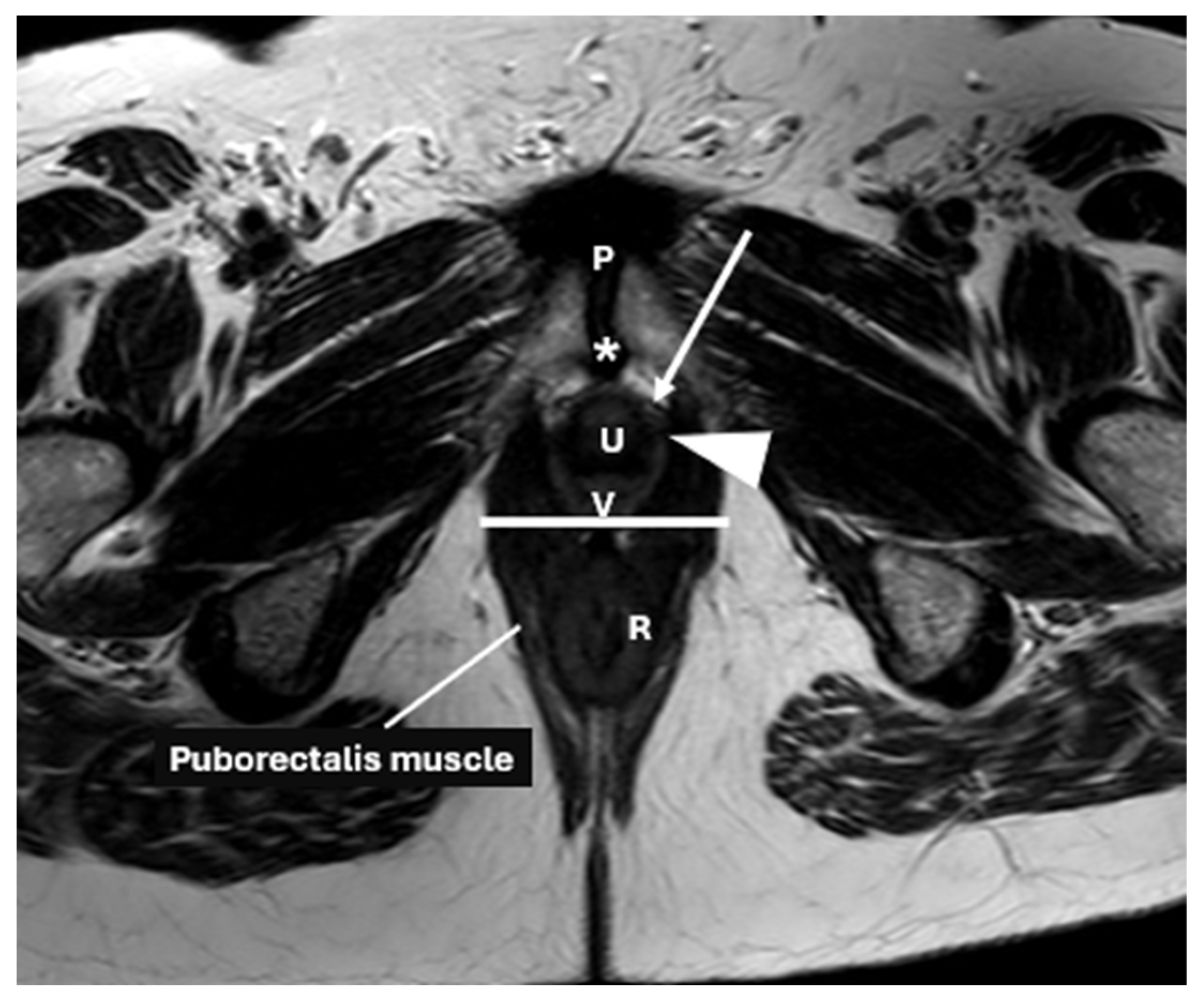
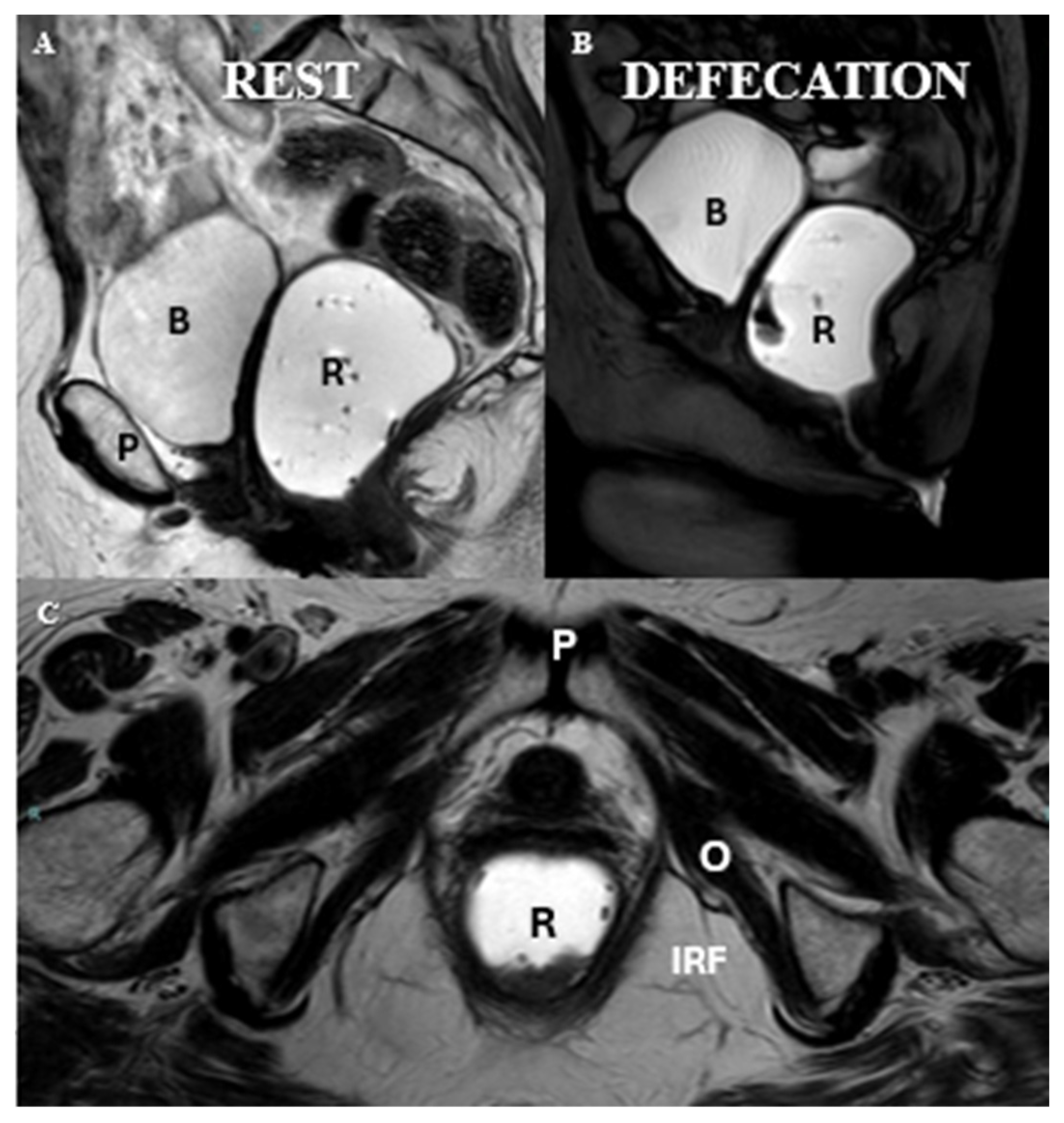
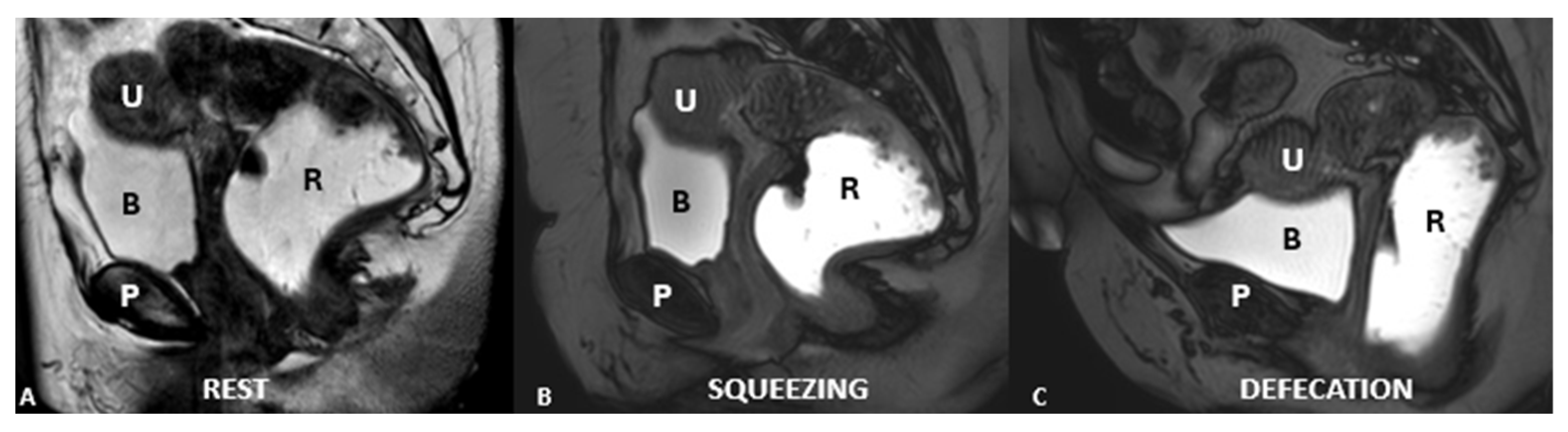
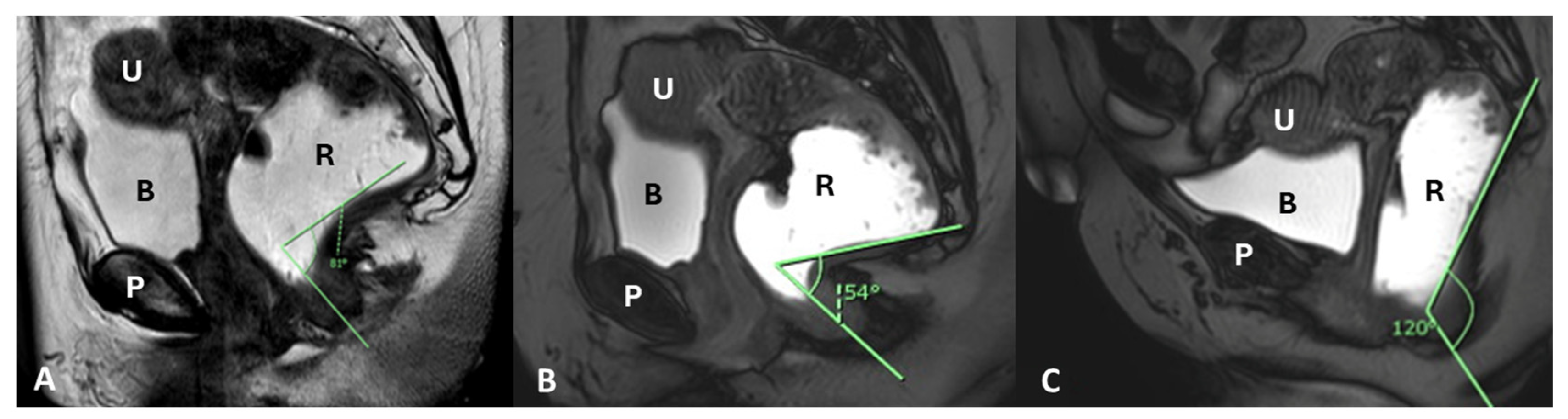
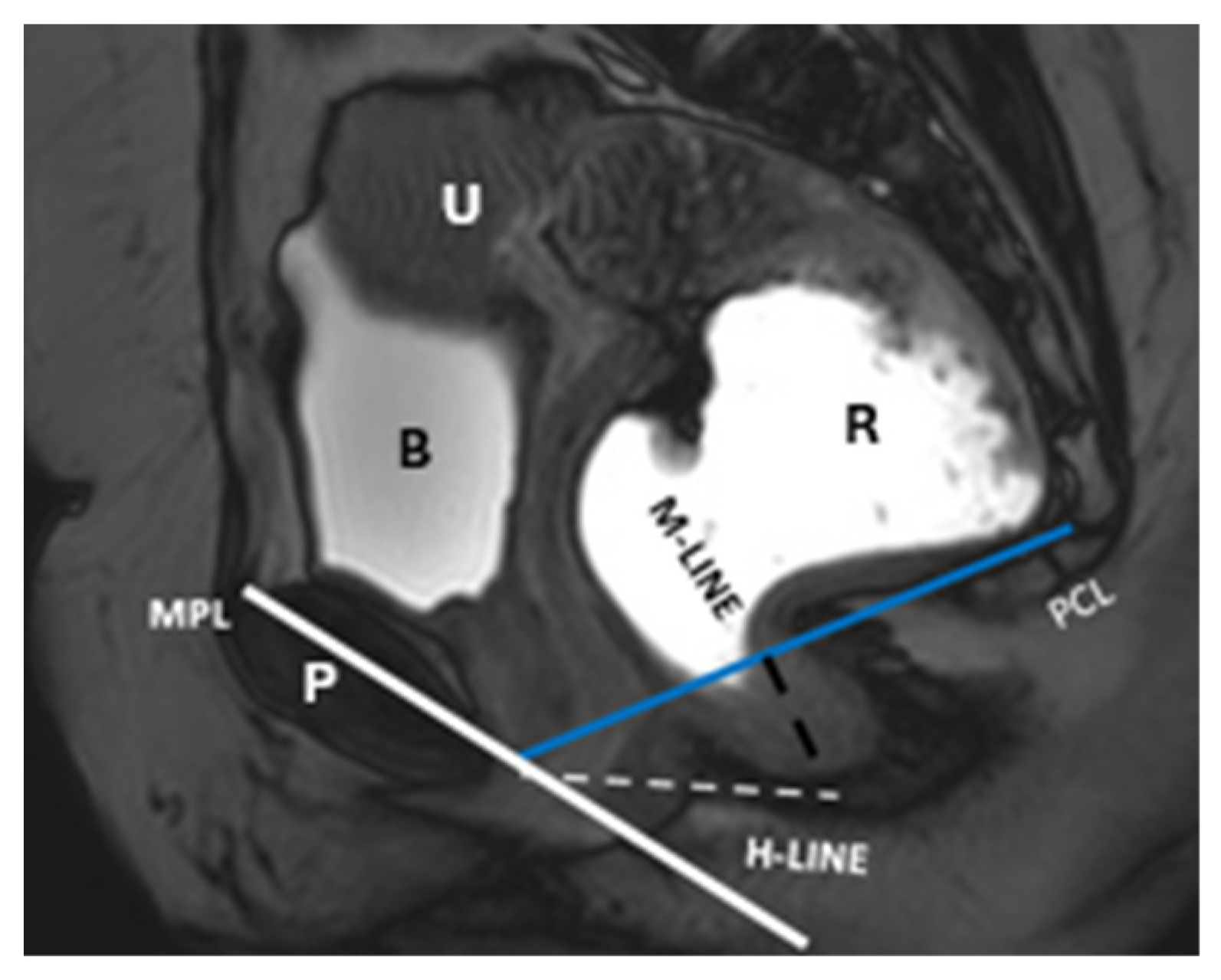
| Layer | Description | Key Muscles or Structures |
|---|---|---|
| Endopelvic fascia | Superior layer enveloping the pelvic diaphragm | Supports pelvic organs |
| Pelvic diaphragm | Composed of ischiococcygeus, iliococcygeus, pubococcygeus, and puborectalis | Forms the levator ani, maintains pelvic support |
| Urogenital diaphragm | Triangular layer, anterior and middle compartments | Encloses the urethral sphincter and the perineal space |
| Imaging Technique | Indications | Limitations | POP Defects | Treatment Guidance |
|---|---|---|---|---|
| Urodynamics | Bladder function, voiding | Invasive, limited anatomy | Stress urinary incontinence | Conservative vs. surgical |
| Video-UDS/Fluoroscopy | Incontinence, hypermobility, prolapse | Radiation, anatomy limits | Cystocele, rectocele, hypermobility | Surgical planning |
| Cystoscopy | Injury, intraoperative check | Invasive, limited scope | Fistulas, erosion | Intraoperative safety, repair guidance |
| Ultrasound | Real-time imaging, mobility | Operator-dependent, limited depth | Anterior/Posterior/Apical defects | Conservative or surgery referral |
| 3D Ultrasound | Levator, implants, mesh | Cost, training, access | Levator avulsion, mesh issues | Surgical planning refinement |
| Dynamic MRI | Multicompartment, planning | Cost, availability | Vault prolapse, complex defects | Advanced surgical planning |
| Imaging Plane | Sequence | Phase | Field of View |
|---|---|---|---|
| Axial | T2 smFOV | Resting | Rectum through the gluteal folds |
| Coronal | T2 smFOV | Resting | Sacrum through pubic symphysis |
| Sagittal | T2 oblique | Resting | Slices through anal canal; rectum distended with ultrasound gel. |
| Sagittal | T2 oblique | Kegel | Slices through anal canal |
| Sagittal | FIESTA or SSFSE dynamic | Defecation | Coccyx through pubic symphysis; start scan and after 5 s, ask the patient to defecate. Ensure the patient fully empties the rectum. Repeat if necessary to capture complete evacuation dynamics. |
| Coronal | T2 breath hold | Valsalva | Sacrum to pubic symphysis |
| Component/Reference Line | Definition | Role in HMO System | Normal Range/Severity/Staging |
|---|---|---|---|
| Pubococcygeal Line (PCL) | Line from the inferior pubic border to the last coccygeal joint | Baseline for measuring organ descent | PCL Compartment Staging Stage 0: Above PCL Stage I: Descent <3 cm below PCL Stage II: Descent 3–6 cm below PCL Stage III: Descent >6 cm below PCL Stage IV: Complete organ prolapse |
| Mid-pubic Line (MPL) | Line drawn through and caudad through the axis of the mid-pubic symphysis on sagittal MRI | Used to assess pelvic organ prolapse (POP); a 90° angle is measured between MPL and the bladder, vaginal vault, and anterior anorectal junction | MPL Compartment Staging Stage 0: >3 cm above MPL or TVL −2 cm Stage I: 1 cm above ≤ X ≤ 1 cm below MPL Stage II: 1 cm above ≤ X ≤ 1 cm below MPL Stage III: ≥1 cm below MPL Stage IV: Complete organ prolapse |
| H Line (Hiatal Line) | Distance between the inferior pubic border and the anorectal junction | Assesses puborectal hiatus (anteroposterior dimension during straining) | POP Grade Hiatal Enlargement Normal: <6 cm Mild: 6–8 cm Moderate: 8–10 cm Severe: >10 cm |
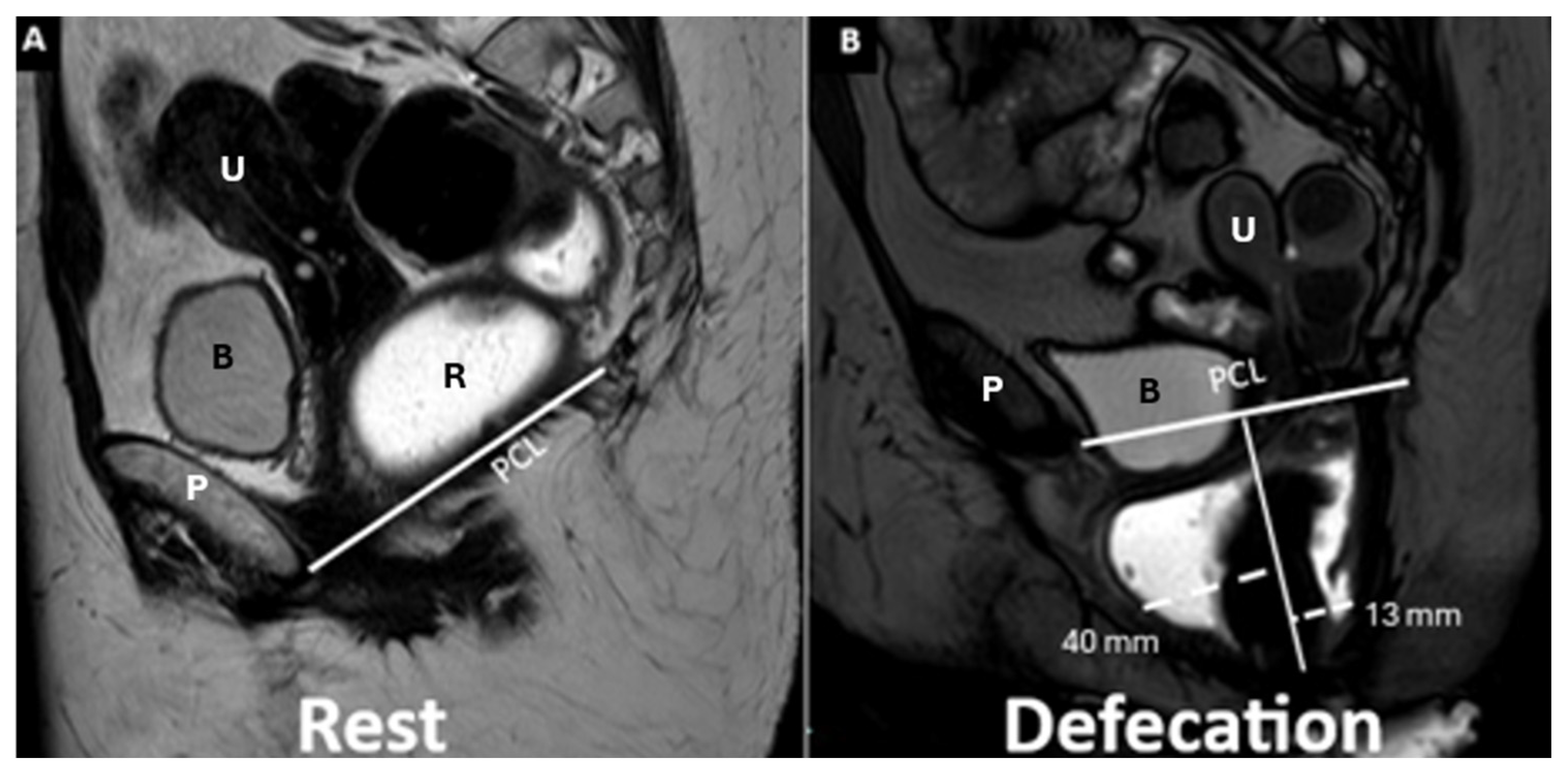
| M Line (Muscle Line) | Perpendicular line from the PCL, measuring organ descent | Evaluates posterior pelvic organ descent | Pelvic Floor Descent Normal: <2 cm Mild: 2–4 cm Moderate: 4–6 cm Severe: >6 cm |
| Anorectal Angle | Angle between the posterior distal rectum and the anal canal’s central axis | Reflects the levator ani muscle function during contraction | 108–127° at rest, decreases by 15–20° during contraction |
| Pelvic Compartment | Contained Organs | Supportive Structures | Condition | Measurement Method | Grading Criteria |
|---|---|---|---|---|---|
| Anterior | Urinary bladder, urethra | Pubocervical fascia (part of endopelvic fascia) | Cystocele (bladder descent) | Bladder neck position relative to PCL | Mild: 1–3 cm, Moderate: 3–6 cm Severe: >6 cm |
| Middle | Vagina, cervix, uterus | Paracolpium, parametrium (endopelvic fascia) | Uterine descent | Uterine fundus position below PCL | Mild: 1–3 cm Moderate: 3–6 cm Severe: >6 cm |
| Vaginal descent | Vaginal fornix position relative to PCL | Mild: 1–3 cm Moderate: 3–6 cm Severe: >6 cm | |||
| Posterior | Colon | Enterocele, sigmoidocele, peritoneocele | Position below the posterior cervicovaginal ligament | Mild: 1–3 cm Moderate: 3–6 cm Severe: >6 cm | |
| Rectum, anal canal | Rectovaginal fascia, perineal body | Rectal intussusception/prolapse | Extent of rectal mucosa relative to rectocele/anal canal | Mild: 1–2 cm Moderate: 2–4 cm Severe: >4 cm |
| Type | Grade | Description |
|---|---|---|
| Internal Prolapse (Mucosal or Full-Thickness Intussusception) | Low-Grade (Grade 1–2) | Mucosal folds are visible or prolapse into the rectal lumen but do not obstruct defecation. |
| High-Grade (Grade 3–4) | Mucosal or full-thickness prolapse causes partial or significant obstruction, leading to defecatory dysfunction. | |
| External Prolapse | Grade 5 | Full-thickness rectal prolapse extends beyond the anal verge, visible externally. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliesi, R.A.; Triscari Barberi, M.; Roccella, G.; Gullo, G.; Billone, V.; Chitoran, E.; Cucinella, G.; Vernuccio, F.; Cannella, R.; Lo Re, G. MR Defecography Improves Diagnosis of Postoperative Pelvic Floor Dysfunction After Gynecological Surgery. Diagnostics 2025, 15, 1625. https://doi.org/10.3390/diagnostics15131625
Pugliesi RA, Triscari Barberi M, Roccella G, Gullo G, Billone V, Chitoran E, Cucinella G, Vernuccio F, Cannella R, Lo Re G. MR Defecography Improves Diagnosis of Postoperative Pelvic Floor Dysfunction After Gynecological Surgery. Diagnostics. 2025; 15(13):1625. https://doi.org/10.3390/diagnostics15131625
Chicago/Turabian StylePugliesi, Rosa Alba, Marika Triscari Barberi, Giovanni Roccella, Giuseppe Gullo, Valentina Billone, Elena Chitoran, Gaspare Cucinella, Federica Vernuccio, Roberto Cannella, and Giuseppe Lo Re. 2025. "MR Defecography Improves Diagnosis of Postoperative Pelvic Floor Dysfunction After Gynecological Surgery" Diagnostics 15, no. 13: 1625. https://doi.org/10.3390/diagnostics15131625
APA StylePugliesi, R. A., Triscari Barberi, M., Roccella, G., Gullo, G., Billone, V., Chitoran, E., Cucinella, G., Vernuccio, F., Cannella, R., & Lo Re, G. (2025). MR Defecography Improves Diagnosis of Postoperative Pelvic Floor Dysfunction After Gynecological Surgery. Diagnostics, 15(13), 1625. https://doi.org/10.3390/diagnostics15131625











