Potential Protective Effect of Hepatitis B Immunity Against Diabetes Mellitus: A Retrospective Propensity-Matched Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
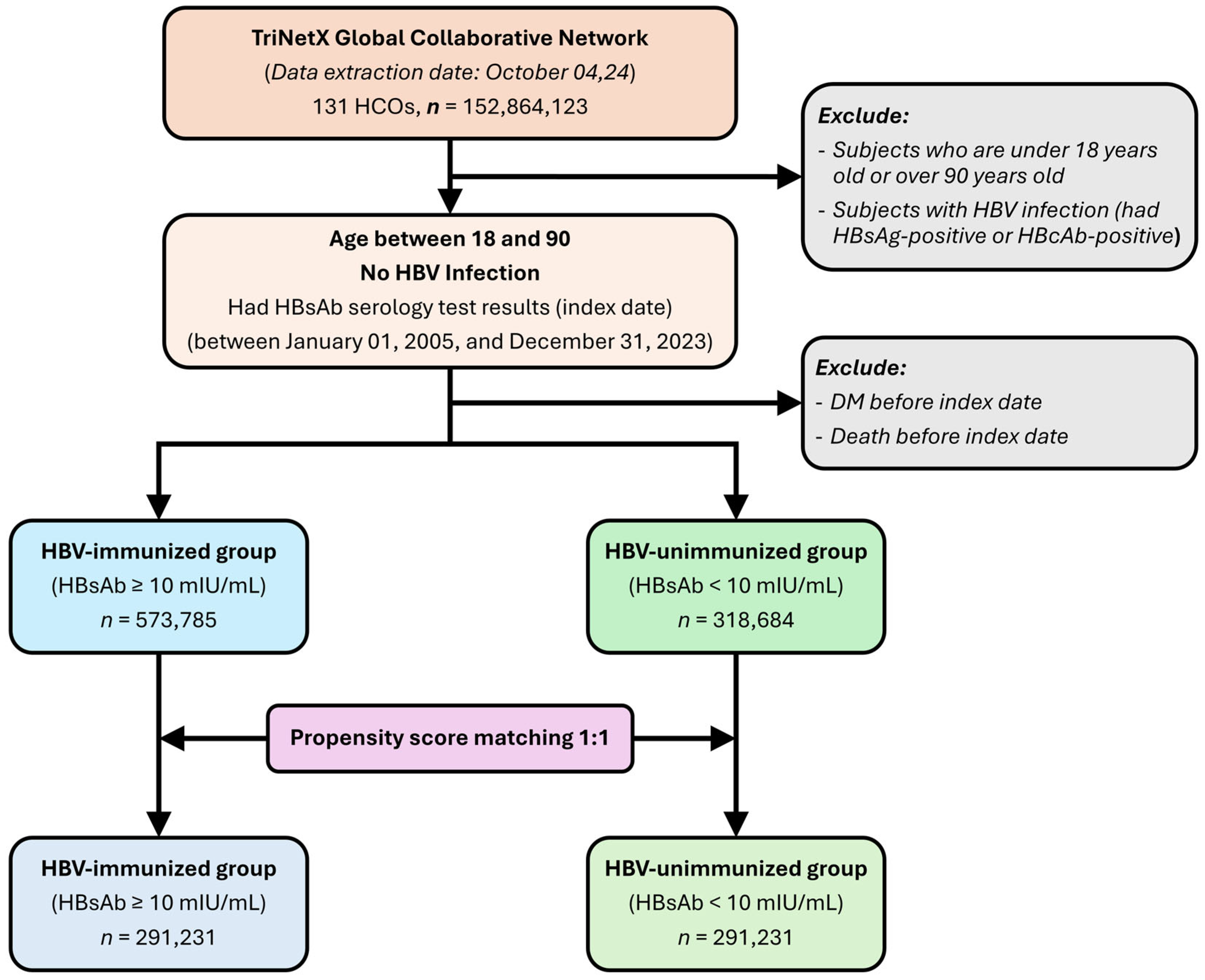
2.2. Study Participants and Cohorts
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
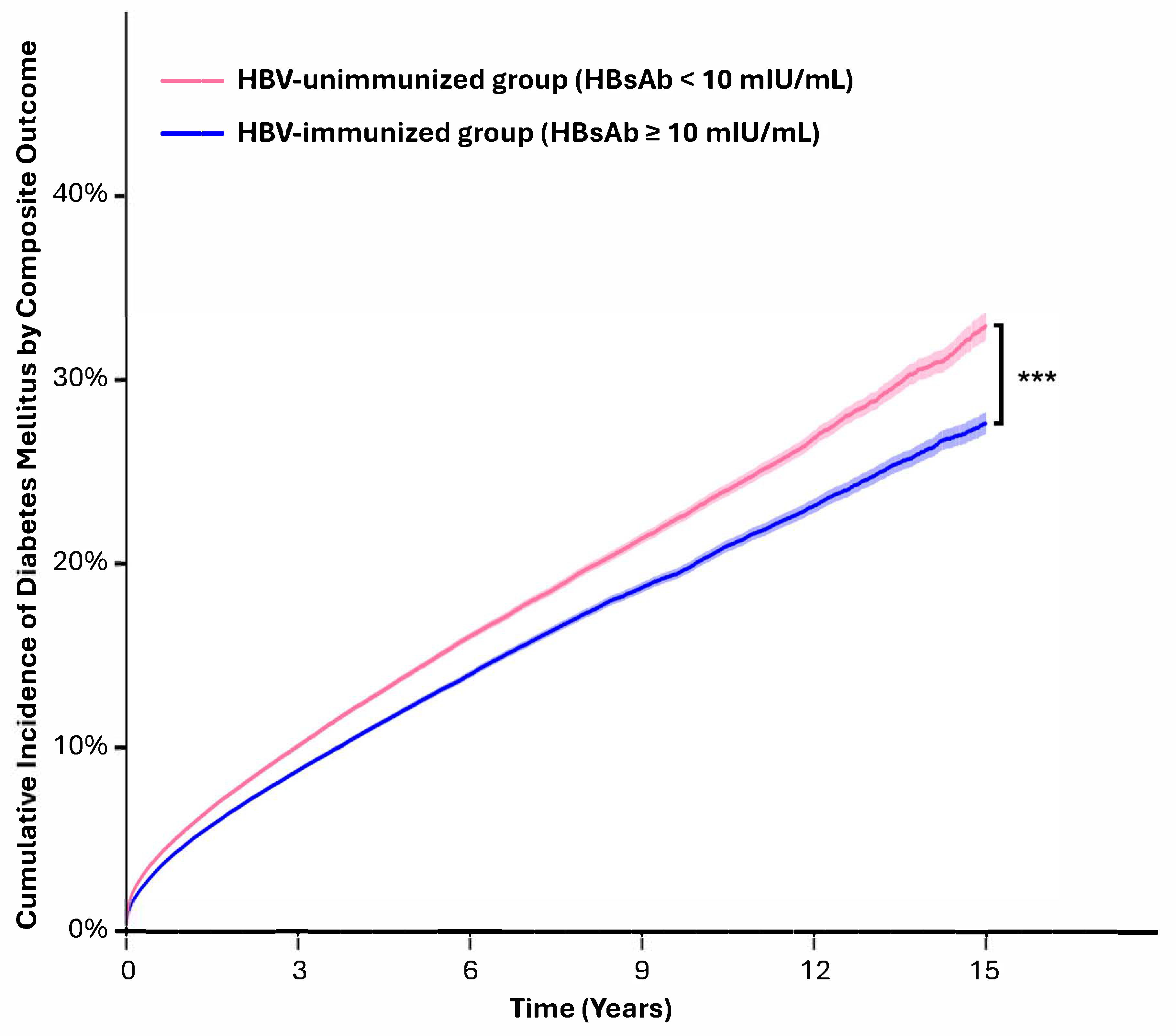
3.2. The HBV-Immunized Group Had a Lower Risk of New-Onset Diabetes
3.3. Dose–Response Analysis
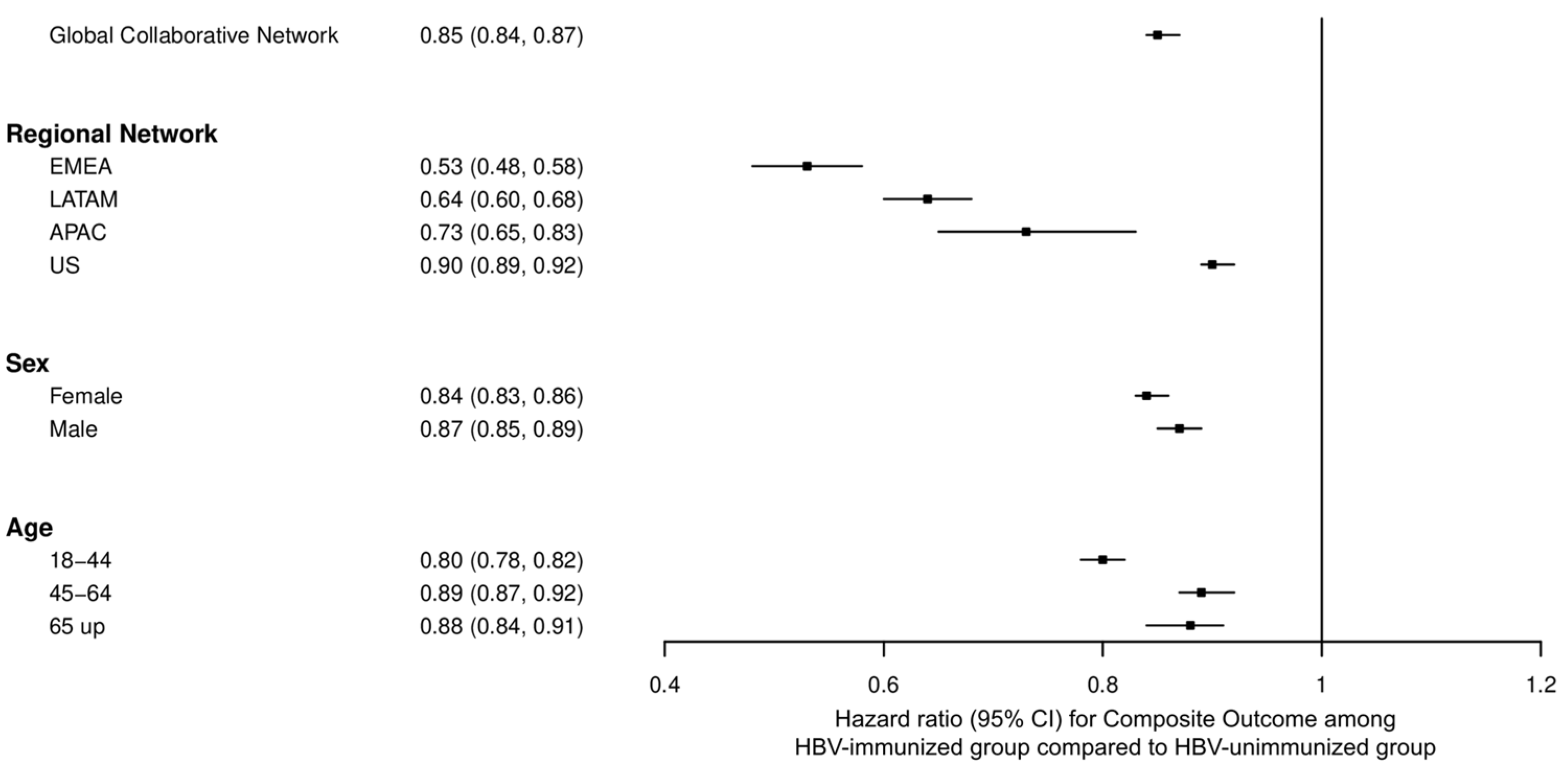
3.4. Stratified Analysis
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| HBV | Hepatitis B virus |
| HBsAb | Hepatitis B surface antibody |
| HBsAg | Hepatitis B surface antigen |
| HBcAb | Hepatitis B core antibody |
| HR | Hazard ratio |
| HCOs | healthcare organizations |
| EMRs | electronic medical records |
| ICD-10-CM | International Classification of Diseases, Tenth Revision, Clinical Modification |
| HbA1c | Glycated hemoglobin |
| CI | Confidence interval |
| PSM | Propensity score matching |
| OR | Odds ratio |
References
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Federation, I.D. IDF Diabetes Atlas, 11th ed.; IDF: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org (accessed on 10 June 2025).
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-X.; Huang, C.-J.; Yang, Z.-G. Impact of hepatitis B virus infection on hepatic metabolic signaling pathway. World J. Gastroenterol. 2016, 22, 8161–8167. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Park, Y.-H.; Kim, S.-U.; Moon, H.-B.; Park, D.S.; Han, Y.-H.; Lee, C.-H.; Lee, D.-S.; Song, I.-S.; Lee, D.H.; et al. Hepatitis B virus X protein regulates hepatic glucose homeostasis via activation of inducible nitric oxide synthase. J. Biol. Chem. 2011, 286, 29872–29881. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zeng, J.; Wu, H.; Shi, R.; Wei, M.; Gao, Y.; Ma, W. Association between hepatitis B virus infection and diabetes mellitus: A meta-analysis. Exp. Ther. Med. 2015, 10, 693–698. [Google Scholar] [CrossRef]
- Khalili, M.; Lombardero, M.; Chung, R.T.; Terrault, N.A.; Ghany, M.G.; Kim, W.R.; Lau, D.; Lisker-Melman, M.; Sanyal, A.; Lok, A.S.; et al. Diabetes and prediabetes in patients with hepatitis B residing in North America. Hepatology 2015, 62, 1364–1374. [Google Scholar] [CrossRef]
- Hong, Y.S.; Chang, Y.; Ryu, S.; Cainzos-Achirica, M.; Kwon, M.-J.; Zhang, Y.; Choi, Y.; Ahn, J.; Rampal, S.; Zhao, D.; et al. Hepatitis B and C virus infection and diabetes mellitus: A cohort study. Sci. Rep. 2017, 7, 4606. [Google Scholar] [CrossRef]
- Locarnini, S.; Hatzakis, A.; Chen, D.-S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef]
- Conners, E.E.; Panagiotakopoulos, L.; Hofmeister, M.G.; Spradling, P.R.; Hagan, L.M.; Harris, A.M.; Rogers-Brown, J.S.; Wester, C.; Nelson, N.P. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations—United States 2023. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef]
- Huang, J.; Ou, H.-Y.; Lin, J.; Karnchanasorn, R.; Feng, W.; Samoa, R.; Chuang, L.-M.; Chiu, K.C. The Impact of Hepatitis B Vaccination Status on the Risk of Diabetes, Implicating Diabetes Risk Reduction by Successful Vaccination. PLoS ONE 2015, 10, e0139730. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Cai, H.; Shao, J.-G.; Zhang, Y.-Y.; Liu, Y.-M.; Qin, G.; Qin, Y. Identifying patients with chronic hepatitis B at high risk of type 2 diabetes mellitus: A cross-sectional study with pair-matched controls. BMC Gastroenterol. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, Y.; Cai, H.; Liu, Y.; Qin, G. Hepatitis B virus infection status and risk of type 2 diabetes mellitus: A meta-analysis. Hepatol. Res. 2015, 45, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Goeijenbier, M.; van Sloten, T.; Slobbe, L.; Mathieu, C.; van Genderen, P.; Beyer, W.E.; Osterhaus, A.D. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine 2017, 35, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Laupèze, B.; Del Giudice, G.; Doherty, M.T.; Van der Most, R. Vaccination as a preventative measure contributing to immune fitness. NPJ Vaccines 2021, 6, 93. [Google Scholar] [CrossRef]
- Palchuk, M.B.; London, J.W.; Perez-Rey, D.; Drebert, Z.J.; Winer-Jones, J.P.; Thompson, C.N.; Esposito, J.; Claerhout, B. A global federated real-world data and analytics platform for research. JAMIA Open 2023, 6, ooad035. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gonzalez, M.D.; Harrington, A.; Jerris, R.C.; Kehl, S.C.; Leal, S.M.; et al. Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2024 Update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin. Infect. Dis. 2024, ciae104. [Google Scholar]
- HIV, PoGftPaToOIiAaAW. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection (accessed on 10 June 2025).
- Komatsu, H.; Klenerman, P.; Thimme, R. Discordance of hepatitis B vaccination policies for healthcare workers between the USA, the UK, and Germany. Hepatol. Res. 2020, 50, 272–282. [Google Scholar] [CrossRef]
- Davila, S.; Froeling, F.E.M.; Tan, A.; Bonnard, C.; Boland, G.J.; Snippe, H.; Hibberd, M.L.; Seielstad, M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010, 11, 232–238. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Guan, Y.; Peng, H.; Wang, S.; Zhang, P.; Su, B. Positive hepatitis B surface antibody is associated with reduced risk of diabetes mellitus in retired female Chinese workers. J. Diabetes 2016, 8, 158–161. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, C.; Hao, Y.; Xu, L.; Zhang, W.; Jin, Y.L.; Zhu, T.; Lam, T.H. Association of hepatitis B surface antigen seropositivity and hepatitis B surface antibody seropositivity with diabetes: A cross-sectional study based on two Chinese populations in Guangdong, China. BMJ Open 2020, 10, e028968. [Google Scholar] [CrossRef]
- Lei, S.; Chen, S.; Zhao, X.; Zhang, Y.; Cheng, K.; Zhang, X.; Wang, Z.; Sun, Y.; Wu, S.; Wang, L. Hepatitis B virus infection and diabetes mellitus: The Kailuan prospective cohort study in China. Hepatol. Int. 2020, 14, 743–753. [Google Scholar] [CrossRef]
- Punthakee, Z.; Miller, M.E.; Simmons, D.L.; Riddle, M.C.; Ismail-Beigi, F.; Brillon, D.J.; Bergenstal, R.M.; Savage, P.J.; Hramiak, I.; Largay, J.F.; et al. Durable change in glycaemic control following intensive management of type 2 diabetes in the ACCORD clinical trial. Diabetologia 2014, 57, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S.; Vellozzi, C.; Reingold, A.; Harris, A.; Haber, P.; Ward, J.W.; Nelson, N.P. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2018, 67, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Di Lello, F.A.; Martínez, A.P.; Flichman, D.M. Insights into induction of the immune response by the hepatitis B vaccine. World J. Gastroenterol. 2022, 28, 4249–4262. [Google Scholar] [CrossRef] [PubMed]
- Meeren, O.V.D.; Crasta, P.; Cheuvart, B.; Ridder, M.D. Characterization of an age-response relationship to GSK’s recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum. Vaccin. Immunother. 2015, 11, 1726–1729. [Google Scholar]
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Zanis, C.; Thompson, G.; Rea, L.; Toomey, M.; Townshend-Bulson, L.; Rudolph, K.; Bulkow, L.; et al. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J. Infect. Dis. 2016, 214, 16–22. [Google Scholar] [CrossRef]
- Ginzberg, D.; Wong, R.J.; Gish, R. Global HBV burden: Guesstimates and facts. Hepatol. Int. 2018, 12, 315–329. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Anastasiou, O.E.; Widera, M.; Verheyen, J.; Korth, J.; Gerken, G.; Helfritz, F.A.; Canbay, A.; Wedemeyer, H.; Ciesek, S. Clinical course and core variability in HBV infected patients without detectable anti-HBc antibodies. J. Clin. Virol. 2017, 93, 46–52. [Google Scholar] [CrossRef]
- Avettand-Fenoel, V.; Thabut, D.; Katlama, C.; Poynard, T.; Thibault, V. Immune suppression as the etiology of failure to detect anti-HBc antibodies in patients with chronic hepatitis B virus infection. J. Clin. Microbiol. 2006, 44, 2250–2253. [Google Scholar] [CrossRef]

| Outcome | Patients with Outcome | Hazard Ratio † (95% CI) | |
|---|---|---|---|
| HBV-Immunized Group (HBsAb ≥ 10 mIU/mL) | HBV-Unimmunized Group (HBsAb < 10 mIU/mL) | ||
| Composite outcome ‡ | 27,653 | 31,209 | 0.85 (0.84, 0.87) |
| Diabetes mellitus diagnosis | 12,618 | 13,731 | 0.89 (0.87, 0.91) |
| Any anti-hyperglycemic drugs | 21,026 | 24,192 | 0.84 (0.83, 0.86) |
| HbA1c ≥ 6.5% | 7107 | 9178 | 0.75 (0.72, 0.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, N.Q.; Lin, S.-J.; Le, N.H.; Nguyen, V.T.; Mai, T.H.; Chen, J.-H.; Hsu, M.-H.; Hoang, D.K.; Thang, P.M.; Huang, Y.-L.; et al. Potential Protective Effect of Hepatitis B Immunity Against Diabetes Mellitus: A Retrospective Propensity-Matched Cohort Study. Diagnostics 2025, 15, 1610. https://doi.org/10.3390/diagnostics15131610
Phan NQ, Lin S-J, Le NH, Nguyen VT, Mai TH, Chen J-H, Hsu M-H, Hoang DK, Thang PM, Huang Y-L, et al. Potential Protective Effect of Hepatitis B Immunity Against Diabetes Mellitus: A Retrospective Propensity-Matched Cohort Study. Diagnostics. 2025; 15(13):1610. https://doi.org/10.3390/diagnostics15131610
Chicago/Turabian StylePhan, Nhu Quynh, Shih-Jung Lin, Ngoc Hoang Le, Van Thuan Nguyen, Tan Ha Mai, Jin-Hua Chen, Min-Huei Hsu, Dinh Khanh Hoang, Phung Manh Thang, Ya-Li Huang, and et al. 2025. "Potential Protective Effect of Hepatitis B Immunity Against Diabetes Mellitus: A Retrospective Propensity-Matched Cohort Study" Diagnostics 15, no. 13: 1610. https://doi.org/10.3390/diagnostics15131610
APA StylePhan, N. Q., Lin, S.-J., Le, N. H., Nguyen, V. T., Mai, T. H., Chen, J.-H., Hsu, M.-H., Hoang, D. K., Thang, P. M., Huang, Y.-L., & Chen, C. (2025). Potential Protective Effect of Hepatitis B Immunity Against Diabetes Mellitus: A Retrospective Propensity-Matched Cohort Study. Diagnostics, 15(13), 1610. https://doi.org/10.3390/diagnostics15131610







