“Could She/He Walk Out of the Hospital?”: Implementing AI Models for Recovery Prediction and Doctor-Patient Communication in Major Trauma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Samples
2.2. Feature and Outcome Variables
- (1)
- Demographics: gender (male or female), age
- (2)
- Arrival Information: mode of arrival (BIBA (Brought in by Ambulance) or PAT (Privately Arranged Transport))
- (3)
- Injury Severity: GCS (Glasgow Coma Scale), ISS (Injury Severity Score)
- (4)
- Clinical Intervention: endotracheal intubation
- (5)
- Vital Signs: SBP (Systolic Blood Pressure), DBP (Diastolic Blood Pressure), RR (Respiratory Rate), HR (Heart Rate)
- (6)
- Hospital Days (Length of Stay)
2.3. Model Building and Performance Evaluation
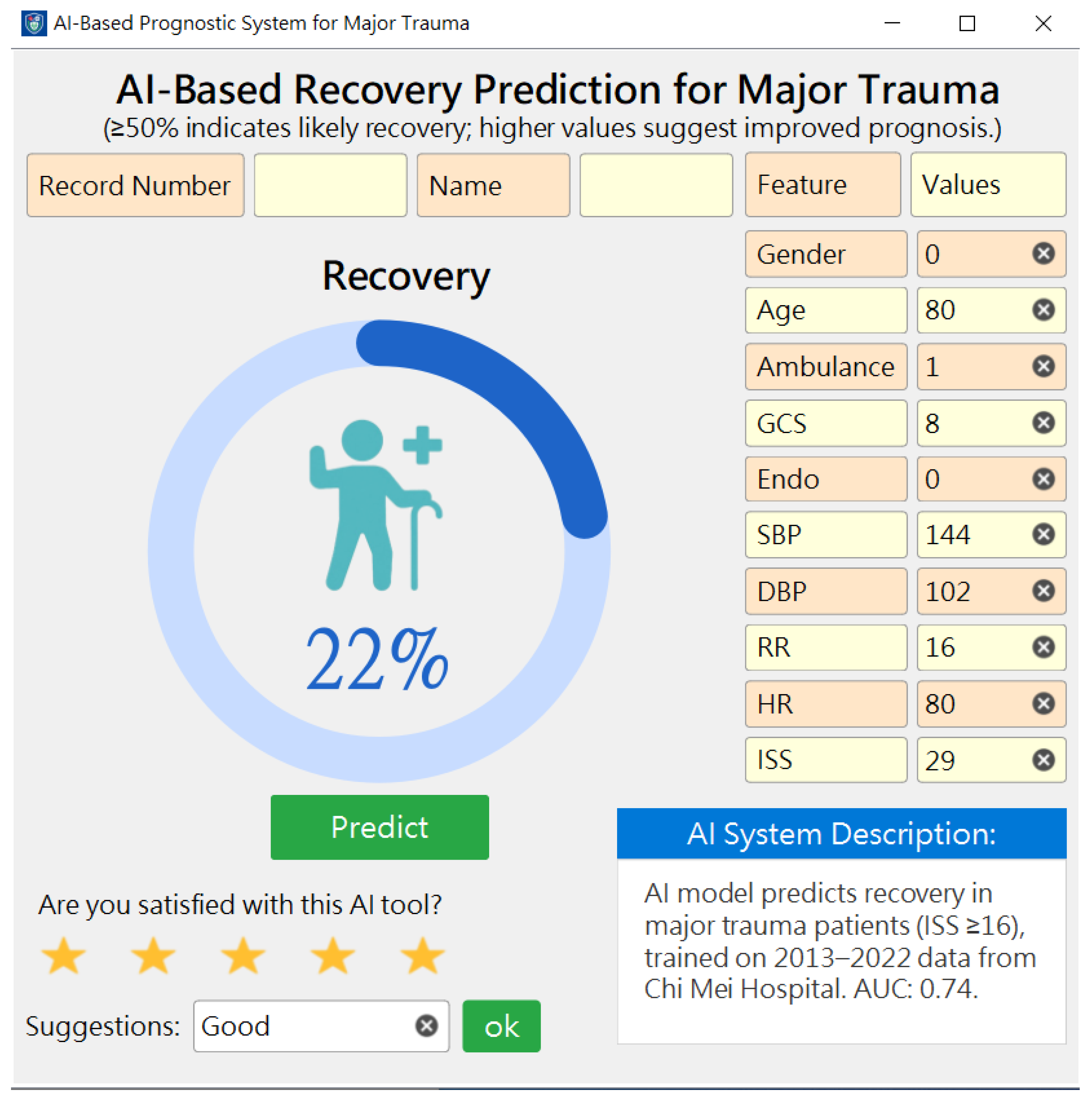
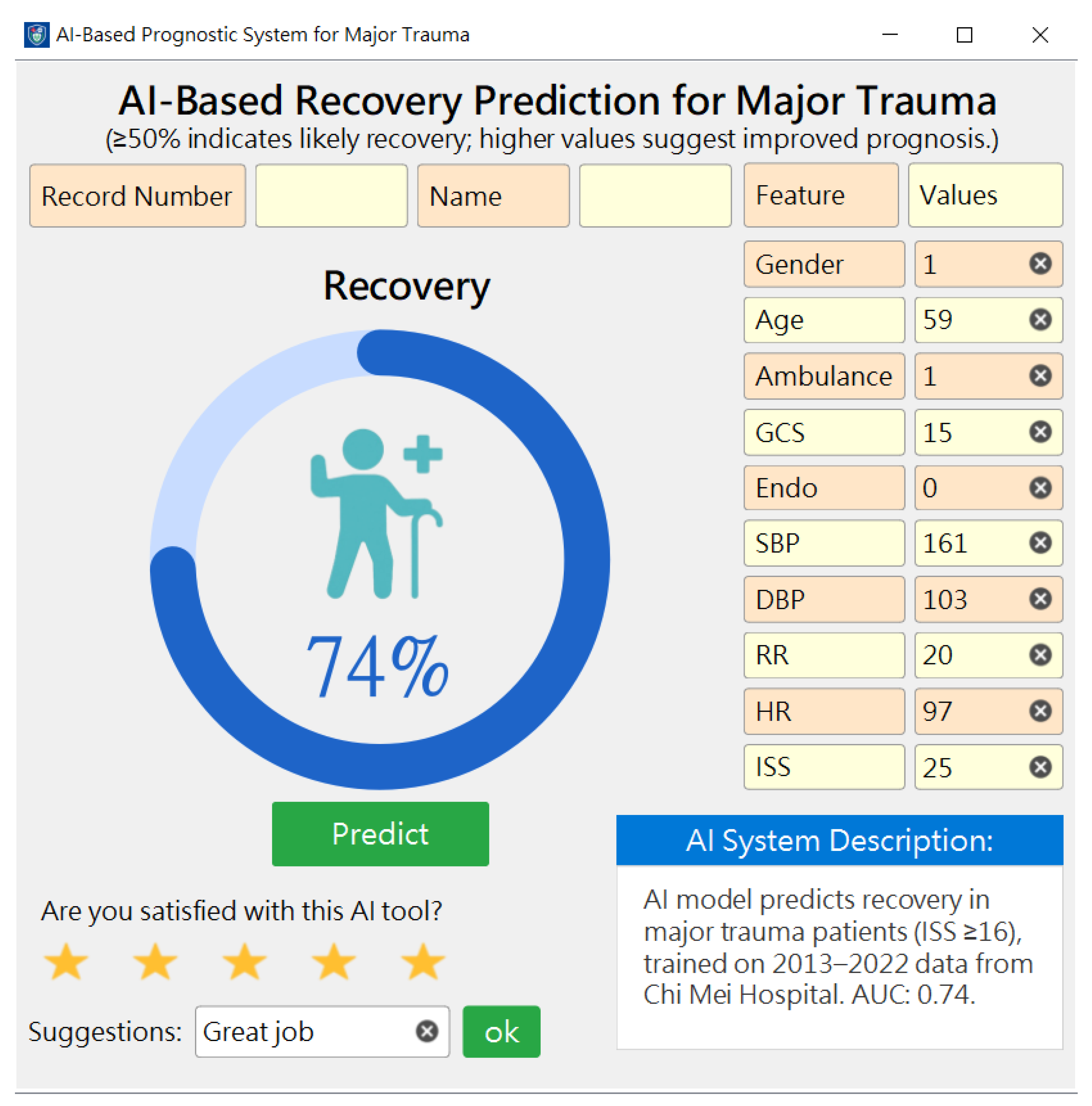
2.4. Implementation and Deployment of the Best Models
3. Results
3.1. Demographics
3.2. Machine-Learning Modeling Results
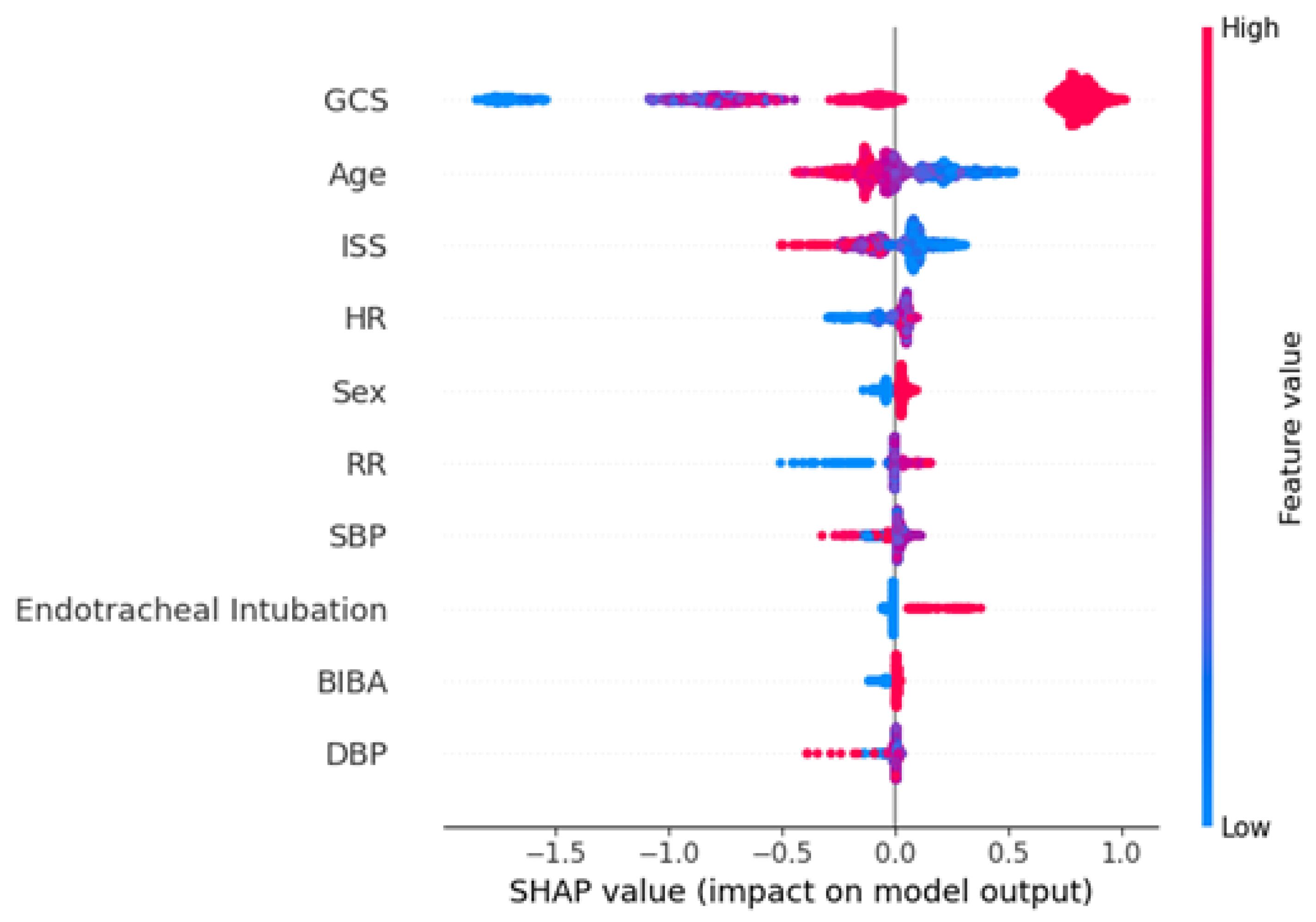
3.3. Interpreting the Feature Importance
3.4. Performance Comparison of AI Models
3.5. Development, Deployment, and Preliminary User Evaluation of the Prediction System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castelao, M.; Lopes, G.; Vieira, M. Epidemiology of major paediatric trauma in a European Country—Trends of a decade. BMC Pediatr. 2023, 23, 194. [Google Scholar] [CrossRef]
- Farrow, L.; Diffley, T.; Gordon, M.W.G.; Khan, A.; Capek, E.; Anand, A.; Paton, M.; Myint, P.K. Epidemiology of major trauma in older adults within Scotland: A national perspective from the Scottish Trauma Audit Group (STAG). Injury 2023, 54, 111065. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.; Kool, B.; Dicker, B.; Davie, G. Epidemiology of major trauma in New Zealand: A systematic review. N. Z. Med. J. 2022, 135, 86–110. [Google Scholar] [PubMed]
- Alam, A.; Gupta, A.; Gupta, N.; Yelamanchi, R.; Bansal, L.; Durga, C. Evaluation of ISS, RTS, CASS and TRISS scoring systems for predicting outcomes of blunt trauma abdomen. Pol. Przegl. Chir. 2021, 93, 9–15. [Google Scholar] [CrossRef]
- Bogner, V.; Brumann, M.; Kusmenkov, T.; Kanz, K.G.; Wierer, M.; Berger, F.; Mutschler, W. [Retrospective computation of the ISS in multiple trauma patients: Potential pitfalls and limitations of findings in full body CT scans]. Unfallchirurg 2016, 119, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hoke, M.H.; Usul, E.; Ozkan, S. Comparison of Trauma Severity Scores (ISS, NISS, RTS, BIG Score, and TRISS) in Multiple Trauma Patients. J. Trauma Nurs. 2021, 28, 100–106. [Google Scholar] [CrossRef]
- Mahajan, C.; Sengupta, D.; Kapoor, I.; Prabhakar, H.; Kumar, V.; Purohit, S.; Priya, V.; Srivastava, S.; Thakur, D.; Karnik, H.; et al. Evaluation of the GCS-Pupils Score for PrOgnosis in trauMatic brAin injury- The COMA Study. Brain Inj. 2023, 37, 1041–1047. [Google Scholar] [CrossRef]
- Moris, D.; Henao, R.; Hensman, H.; Stempora, L.; Chasse, S.; Schobel, S.; Dente, C.J.; Kirk, A.D.; Elster, E. Multidimensional machine learning models predicting outcomes after trauma. Surgery 2022, 172, 1851–1859. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Using trauma registry data to predict prolonged mechanical ventilation in patients with traumatic brain injury: Machine learning approach. PLoS ONE 2020, 15, e0235231. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients with post traumatic brain injury using National Trauma Registry and Machine Learning Approach. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 44. [Google Scholar] [CrossRef]
- Cardosi, J.D.; Shen, H.; Groner, J.I.; Armstrong, M.; Xiang, H. Machine learning for outcome predictions of patients with trauma during emergency department care. BMJ Health Care Inform. 2021, 28, e100407. [Google Scholar] [CrossRef]
- Lee, K.C.; Hsu, C.C.; Lin, T.C.; Chiang, H.F.; Horng, G.J.; Chen, K.T. Prediction of Prognosis in Patients with Trauma by Using Machine Learning. Medicina 2022, 58, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, M.; Li, P.; Lu, H.; Li, A.; Xu, S. Prediction models for the complication incidence and survival rate of dental implants-a systematic review and critical appraisal. Int. J. Implant. Dent. 2025, 11, 5. [Google Scholar] [CrossRef]
- van de Beld, J.J.; Crull, D.; Mikhal, J.; Geerdink, J.; Veldhuis, A.; Poel, M.; Kouwenhoven, E.A. Complication Prediction after Esophagectomy with Machine Learning. Diagnostics 2024, 14, 439. [Google Scholar] [CrossRef] [PubMed]
- Florquin, R.; Florquin, R.; Schmartz, D.; Dony, P.; Briganti, G. Pediatric cardiac surgery: Machine learning models for postoperative complication prediction. J. Anesth. 2024, 38, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Shiren, Y.; Jiangnan, Y.; Xinhua, Y.; Xinye, N. Interpretable prediction model for assessing diabetes complication risks in Chinese sufferers. Diabetes Res. Clin. Pract. 2024, 209, 111560. [Google Scholar] [CrossRef] [PubMed]
- Bosnjic, J. Prediction of pulmonary embolism and its complication in diabetes mellitus type 2: A 5-year retrospective study. Med. Glas. 2024, 21, 36–44. [Google Scholar] [CrossRef]
- O’Connor, S.; Tilston, G.; Jones, O.; Sharma, A.; Ormesher, L.; Quinn, B.; Wilson, A.; Myers, J.; Peek, N.; Palin, V. Acceptability of data linkage to identify women at risk of postnatal complication for the development of digital risk prediction tools and interventions to better optimise postnatal care, a qualitative descriptive study design. BMC Med. 2024, 22, 276. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Maye, H.; Waqar, M.; Colombo, F.; Lekka, E. External validation of the GCS-Pupils Score as an outcome predictor after traumatic brain injury in adults: A single-center experience. Acta Neurochir. 2023, 165, 289–297. [Google Scholar] [CrossRef]
- Bakke, H.K.; Wisborg, T. The trauma chain of survival—Each link is equally important (but some links are more equal than others). Injury 2017, 48, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Soreide, K. Strengthening the trauma chain of survival. Br. J. Surg. 2012, 99 (Suppl. S1), 1–3. [Google Scholar] [CrossRef]
- Haering, S.; Schulze, L.; Geiling, A.; Meyer, C.; Klusmann, H.; Schumacher, S.; Knaevelsrud, C.; Engel, S. Higher risk-less data: A systematic review and meta-analysis on the role of sex and gender in trauma research. J. Psychopathol. Clin. Sci. 2024, 133, 257–272. [Google Scholar] [CrossRef]
- Clarke, J.R.; Cebula, D.P.; Webber, B.L. Artificial intelligence: A computerized decision aid for trauma. J. Trauma 1988, 28, 1250–1254. [Google Scholar] [CrossRef]
- Becalick, D.C.; Coats, T.J. Comparison of artificial intelligence techniques with UKTRISS for estimating probability of survival after trauma. UK Trauma and Injury Severity Score. J. Trauma 2001, 51, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zhang, L.; Kong, X.; Herbertl, L. Artificial Intelligence Image-Assisted Knee Ligament Trauma Repair Efficacy Analysis and Postoperative Femoral Nerve Block Analgesia Effect Research. World Neurosurg. 2021, 149, 492–501. [Google Scholar] [CrossRef]
- Pham, T.D.; Holmes, S.B.; Coulthard, P. A review on artificial intelligence for the diagnosis of fractures in facial trauma imaging. Front. Artif. Intell. 2023, 6, 1278529. [Google Scholar] [CrossRef] [PubMed]
- Azad, Z.; Aiman, U.; Shaheen, S. Artificial intelligence in paediatric head trauma: Enhancing diagnostic accuracy for skull fractures and brain haemorrhages. Neurosurg. Rev. 2024, 47, 641. [Google Scholar] [CrossRef]
- Kaike, L.; Castro-Zunti, R.; Ko, S.B.; Jin, G.Y. [Diagnosis of Rib Fracture Using Artificial Intelligence on Chest CT Images of Patients with Chest Trauma]. J. Korean Soc. Radiol. 2024, 85, 769–779. [Google Scholar] [CrossRef]
- Zhao, T.; Meng, X.; Wang, Z.; Hu, Y.; Fan, H.; Han, J.; Zhu, N.; Niu, F. Diagnostic evaluation of blunt chest trauma by imaging-based application of artificial intelligence. Am. J. Emerg. Med. 2024, 85, 35–43. [Google Scholar] [CrossRef]
- Keller, M.; Rohner, M.; Honigmann, P. The potential benefit of artificial intelligence regarding clinical decision-making in the treatment of wrist trauma patients. J. Orthop. Surg. Res. 2024, 19, 579. [Google Scholar] [CrossRef]
- Peng, H.T.; Siddiqui, M.M.; Rhind, S.G.; Zhang, J.; da Luz, L.T.; Beckett, A. Artificial intelligence and machine learning for hemorrhagic trauma care. Mil. Med. Res. 2023, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Q.; Yang, L.; Chen, L. [The application of artificial intelligence in prehospital treatment of spinal cord trauma]. Zhonghua Yi Xue Za Zhi 2024, 104, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Galwankar, S.; Szarpak, L.; Cander, B.; Maj, B.; Pruc, M. The use of artificial intelligence in blunt chest trauma. Am. J. Emerg. Med. 2025, 87, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, W.S.; Kim, D.W.; Seo, S.H.; Kim, J.; Jeong, S.T.; Yon, D.K.; Lee, J. An Artificial Intelligence Model for Predicting Trauma Mortality Among Emergency Department Patients in South Korea: Retrospective Cohort Study. J. Med. Internet Res. 2023, 25, e49283. [Google Scholar] [CrossRef]
- Sun, B.; Lei, M.; Wang, L.; Wang, X.; Li, X.; Mao, Z.; Kang, H.; Liu, H.; Sun, S.; Zhou, F. Prediction of sepsis among patients with major trauma using artificial intelligence: A multicenter validated cohort study. Int. J. Surg. 2025, 111, 467–480. [Google Scholar] [CrossRef]

| Variable | Non-Recovery n = 1562 | Recovery n = 3959 | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 59.11 ± 19.26 | 54.14 ± 20.09 | <0.001 |
| Gender, n (%) | |||
| Female | 498 (31.88%) | 1483 (37.46%) | <0.001 |
| Male | 1064 (68.12%) | 2476 (62.54%) | |
| Mode of Arrival (to the ED), n (%) | |||
| BIBA | 1429 (91.49%) | 3347 (84.54%) | <0.001 |
| PAT | 133 (8.52%) | 612 (15.46%) | |
| GCS, mean ± SD | 10.24 ± 4.78 | 13.70 ± 2.66 | <0.001 |
| ISS, mean ± SD | 26.02 ± 12.15 | 20.83 ± 6.52 | <0.001 |
| Endotracheal Intubation, n (%) | 213 (13.63%) | 139 (3.51%) | <0.001 |
| SBP, mean ± SD | 141.67 ± 47.86 | 146.63 ± 34.80 | <0.001 |
| DBP, mean ± SD | 82.81 ± 26.33 | 86.26 ± 18.51 | <0.001 |
| RR, mean ± SD | 16.85 ± 5.52 | 17.77 ± 2.89 | <0.001 |
| HR, mean ± SD | 87.99 ± 27.01 | 88.80 ± 18.10 | 0.276 |
| Hospital Days, mean ± SD | 22.92 ± 24.76 | 14.35 ± 14.92 | <0.001 |
| Algorithm | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| XGBoost | 0.716 | 0.751 | 0.627 | 0.748 |
| Random Forest | 0.709 | 0.751 | 0.603 | 0.743 |
| LightGBM | 0.705 | 0.750 | 0.591 | 0.738 |
| Logistic Regression | 0.708 | 0.750 | 0.601 | 0.732 |
| MLP | 0.709 | 0.750 | 0.603 | 0.731 |
| Outcomes and Predictive Models | Accuracy | Sensitivity | Specificity | AUC | p-Value |
|---|---|---|---|---|---|
| AI prediction (XGBoost) | 0.716 | 0.751 | 0.627 | 0.748 | |
| Injury Severity Score | 0.713 | 0.771 | 0.567 | 0.689 | <0.001 |
| Glasgow Coma Scale | 0.663 | 0.657 | 0.678 | 0.719 | <0.001 |
| Study | This Study | [35] | [36] |
|---|---|---|---|
| Patient number | 5521 | 921 | 961 |
| Types of Patient Origin | Adult inpatient with major trauma (database built by our trauma center) | Korean National Emergency Department Information System (NEDIS) data set | Major trauma at their hospital + a database in the United States |
| Outcome | Recovery (whether a major trauma patient achieves recovery within 2 months after the injury) | In-hospital mortality | Sepsis |
| Study method | Five machine leaning methods | Various machine learning models with an ensemble approach via 5-fold cross-validation | Six machine leaning methods |
| Real world implementation | Yes A predictive application with AI models was implemented and integrated into the existing HIS | Yes Deployed their final AI model on a public website | Yes Medical providers can access the application by clicking on the URL provided in the study |
| Input data | 10 patient demographic features: including ISS, GCS, vital signs, BIBA or PAT, endotracheal intubation | 10 patient demographic features: including Korean Triage and Acuity Scale, GCS, vital signs | 12 patient demographic features: including ISS, GCS, vital signs, smoking, trauma part, lab. data |
| Testing results (AUC) | Recovery (0.731–0.748) | In-hospital mortality (0.7675) | Sepsis (0.787–0.932) |
| Year | 2025 | 2023 | 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.-C.; Liu, C.-F.; Yeh, C.-C. “Could She/He Walk Out of the Hospital?”: Implementing AI Models for Recovery Prediction and Doctor-Patient Communication in Major Trauma. Diagnostics 2025, 15, 1582. https://doi.org/10.3390/diagnostics15131582
Cheng L-C, Liu C-F, Yeh C-C. “Could She/He Walk Out of the Hospital?”: Implementing AI Models for Recovery Prediction and Doctor-Patient Communication in Major Trauma. Diagnostics. 2025; 15(13):1582. https://doi.org/10.3390/diagnostics15131582
Chicago/Turabian StyleCheng, Li-Chin, Chung-Feng Liu, and Chin-Choon Yeh. 2025. "“Could She/He Walk Out of the Hospital?”: Implementing AI Models for Recovery Prediction and Doctor-Patient Communication in Major Trauma" Diagnostics 15, no. 13: 1582. https://doi.org/10.3390/diagnostics15131582
APA StyleCheng, L.-C., Liu, C.-F., & Yeh, C.-C. (2025). “Could She/He Walk Out of the Hospital?”: Implementing AI Models for Recovery Prediction and Doctor-Patient Communication in Major Trauma. Diagnostics, 15(13), 1582. https://doi.org/10.3390/diagnostics15131582





