18F-FDG PET/CT Impact on Malignant Melanoma Patients Undergoing Staging and Restaging: A Single-University-Center Experience in a Real-World Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging Technique
2.3. Therapy Options
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Fang, Y.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; Yang, X.; Lu, M. Spatiotemporal Trends of the Global Burden of Melanoma in 204 Countries and Territories from 1990 to 2019: Results from the 2019 Global Burden of Disease Study. Neoplasia 2022, 24, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Forsea, A.M.; Del Marmol, V.; De Vries, E.; Bailey, E.E.; Geller, A.C. Melanoma Incidence and Mortality in Europe: New Estimates, Persistent Disparities: Melanoma Incidence and Mortality Disparities in Europe. Br. J. Dermatol. 2012, 167, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Pandeya, N.; Ragaini, B.E.; Whiteman, D.C. International patterns and trends in the incidence of melanoma and cutaneous squamous cell carcinoma, 1989–2020. Br. J. Dermatol. 2024, 190, 492–500. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Kristiansen, I.S.; Thapa, S. Increasing Melanoma Incidence with Unchanged Mortality: More Sunshine, Better Treatment, Increased Diagnostic Activity, Overdiagnosis or Lowered Diagnostic Threshold? Br. J. Dermatol. 2024, 191, 365–374. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/toda (accessed on 7 February 2025).
- Institute of Public Health of Serbia “Dr Milan Jovanović Batut” (2024. Belgrade) “Malignant Tumours in Republic of Serbia 2022”. Serbian Cancer Registry. Available online: https://www.batut.org.rs/download/publikacije/MaligniTumoriURepubliciSrbiji2022.pdf (accessed on 9 March 2025).
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus Ipilimumab or Nivolumab Alone versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Mesnard, C.; Bodet-Milin, C.; Eugène, T.; Nguyen, J.; Khammari, A.; Dréno, B. Predictive Value of FDG-PET Imaging for Relapse in Metastatic Melanoma Patients Treated with Immunotherapy. Acad. Dermatol. Venereol. 2020, 34, 2261–2267. [Google Scholar] [CrossRef]
- Swetter, S.M.; Thompson, J.A.; Albertini, M.R.; Barker, C.A.; Baumgartner, J.; Boland, G.; Chmielowski, B.; DiMaio, D.; Durham, A.; Fields, R.C.; et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 364–376. [Google Scholar] [CrossRef]
- Perng, P.; Marcus, C.; Subramaniam, R.M. 18 F-FDG PET/CT and Melanoma: Staging, Immune Modulation and Mutation-Targeted Therapy Assessment, and Prognosis. Am. J. Roentgenol. 2015, 205, 259–270. [Google Scholar] [CrossRef]
- Petersen, H.; Holdgaard, P.C.; Madsen, P.H.; Knudsen, L.M.; Gad, D.; Gravergaard, A.E.; Rohde, M.; Godballe, C.; Engelmann, B.E.; Bech, K.; et al. FDG PET/CT in Cancer: Comparison of Actual Use with Literature-Based Recommendations. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Krug, B.; Crott, R.; Roch, I.; Lonneux, M.; Beguin, C.; Baurain, J.-F.; Pirson, A.-S.; Borght, T.V. Cost-Effectiveness Analysis of FDG PET-CT in the Management of Pulmonary Metastases from Malignant Melanoma. Acta Oncol. 2010, 49, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Laks, S. Surveillance Imaging for Metastasis in High-Risk Melanoma: Importance in Individualized Patient Care and Survivorship. Melanoma Manag. 2019, 6, MMT12. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, M.J.; Joe, A.Y.; Jaeger, U.; Huber, A.; Matthies, A.; Bucerius, J.; Roedel, R.; Strunk, H.; Bieber, T.; Biersack, H.-J.; et al. Diagnostic Performance of Whole Body Dual Modality18 F-FDG PET/CT Imaging for N- and M-Staging of Malignant Melanoma: Experience With 250 Consecutive Patients. JCO 2006, 24, 1178–1187. [Google Scholar] [CrossRef]
- Tomasi, G.; Rosso, L. PET Imaging: Implications for the Future of Therapy Monitoring with PET/CT in Oncology. Curr. Opin. Pharmacol. 2012, 12, 569–575. [Google Scholar] [CrossRef]
- Balch, C.M.; Soong, S.-J.; Gershenwald, J.E.; Thompson, J.F.; Reintgen, D.S.; Cascinelli, N.; Urist, M.; McMasters, K.M.; Ross, M.I.; Kirkwood, J.M.; et al. Prognostic Factors Analysis of 17,600 Melanoma Patients: Validation of the American Joint Committee on Cancer Melanoma Staging System. JCO 2001, 19, 3622–3634. [Google Scholar] [CrossRef]
- Krug, B.; Crott, R.; Lonneux, M.; Baurain, J.-F.; Pirson, A.-S.; Vander Borght, T. Role of PET in the Initial Staging of Cutaneous Malignant Melanoma: Systematic Review. Radiology 2008, 249, 836–844. [Google Scholar] [CrossRef]
- Jiménez-Requena, F.; Delgado-Bolton, R.C.; Fernández-Pérez, C.; Gambhir, S.S.; Schwimmer, J.; Pérez-Vázquez, J.M.; Carreras-Delgado, J.L. Meta-Analysis of the Performance of 18F-FDG PET in Cutaneous Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 284–300. [Google Scholar] [CrossRef]
- El-Shourbagy, K.H.; Mashaly, E.M.; Khodair, S.A.; Houseni, M.M.; Abou Khadrah, R.S. PET/CT in Restaging, Prognosis, and Recurrence in Patients with Malignant Melanoma. Egypt. J. Radiol. Nucl. Med. 2020, 51, 167. [Google Scholar] [CrossRef]
- Zamani-Siahkali, N.; Mirshahvalad, S.A.; Pirich, C.; Beheshti, M. Diagnostic Performance of [18F]F-FDG Positron Emission Tomography (PET) in Non-Ophthalmic Malignant Melanoma: A Systematic Review and Meta-Analysis of More Than 10,000 Melanoma Patients. Cancers 2024, 16, 215. [Google Scholar] [CrossRef]
- Eldon, M.; Kjerkegaard, U.K.; Ørndrup, M.H.; Sjøgren, P.; Stolle, L.B. Role of FDG-PET/CT in Stage 1–4 Malignant Melanoma Patients. Eur. J. Plast. Surg. 2017, 40, 47–52. [Google Scholar] [CrossRef]
- Xing, Y.; Bronstein, Y.; Ross, M.I.; Askew, R.L.; Lee, J.E.; Gershenwald, J.E.; Royal, R.; Cormier, J.N. Contemporary Diagnostic Imaging Modalities for the Staging and Surveillance of Melanoma Patients: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2011, 103, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Wieder, H.A.; Tekin, G.; Rosenbaum-Krumme, S.; Klode, J.; Altenbernd, J.; Bockisch, A.; Nagarajah, J. 18FDG-PET to assess recurrence and long term survival in patients with malignant melanoma. Nuklearmedizin 2013, 52, 198–203. [Google Scholar] [CrossRef]
- Cachin, F.; Miot-Noirault, E.; Gillet, B.; Isnardi, V.; Labeille, B.; Payoux, P.; Meyer, N.; Cammilleri, S.; Gaudy, C.; Razzouk-Cadet, M.; et al. 123 I-BZA2 as a Melanin-Targeted Radiotracer for the Identification of Melanoma Metastases: Results and Perspectives of a Multicenter Phase III Clinical Trial. J. Nucl. Med. 2014, 55, 15–22. [Google Scholar] [CrossRef]
- Smith, B.; Church-Martin, J.; Abed, H.; Lloyd, E.; Hardwicke, J.T. False Positive Rate from Prospective Studies of PET-CT in Cutaneous Malignant Melanoma: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2024, 131, 102849. [Google Scholar] [CrossRef]
- Pfannenberg, C.; Aschoff, P.; Schanz, S.; Eschmann, S.M.; Plathow, C.; Eigentler, T.K.; Garbe, C.; Brechtel, K.; Vonthein, R.; Bares, R.; et al. Prospective Comparison of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Whole-Body Magnetic Resonance Imaging in Staging of Advanced Malignant Melanoma. Eur. J. Cancer 2007, 43, 557–564. [Google Scholar] [CrossRef]
- Tos, T.; Klyver, H.; Drzewiecki, K.T. Extensive Screening for Primary Tumor Is Redundant in Melanoma of Unknown Primary. J. Surg. Oncol. 2011, 104, 724–727. [Google Scholar] [CrossRef]
- Danielsen, M.; Højgaard, L.; Kjær, A.; Fischer, B.M. Positron Emission Tomography in the Follow-up of Cutaneous Malignant Melanoma Patients: A Systematic Review. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 17–28. [Google Scholar]
- Avilés Izquierdo, J.A.; Molina López, I.; Sobrini Morillo, P.; Márquez Rodas, I.; Mercader Cidoncha, E. Utility of PET/CT in Patients with Stage I–III Melanoma. Clin. Transl. Oncol. 2020, 22, 1414–1417. [Google Scholar] [CrossRef]
- Martin, J.; Smith, B.; Abed, H.; Hardwicke, J. 530 False Positive Rate from Pet-Ct in Cutaneous Malignant Melanoma—A Systematic Review and Meta-Analysis. Br. J. Surg. 2023, 110 (Suppl. S7), znad258.474. [Google Scholar] [CrossRef]
- Frary, E.C.; Gad, D.; Bastholt, L.; Hess, S. The Role of FDG-PET/CT in Preoperative Staging of Sentinel Lymph Node Biopsy-Positive Melanoma Patients. EJNMMI Res. 2016, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Meyer, N.; Zerdoud, S.; Julian, A.; Chevreau, C.; Payoux, P.; Courbon, F. Fluorodeoxyglucose Positron Emission Tomography Fails to Detect Distant Metastases at Initial Staging of Melanoma Patients with Metastatic Involvement of Sentinel Lymph Node: FDG PET in Melanoma Patients with Positive SLNB. Br. J. Dermatol. 2011, 164, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Dieng, M.; Khanna, N.; Nguyen, M.; Zeng, J.; Nijhuis, A.A.G.; Nieweg, O.E.; Einstein, A.J.; Emmett, L.; Lord, S.J.; et al. Performance of Long-Term CT and PET/CT Surveillance for Detection of Distant Recurrence in Patients with Resected Stage IIIA–D Melanoma. Ann. Surg. Oncol. 2021, 28, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Yao, X.; Abadir, W.; Baetz, T.D.; Easson, A.; Knight, G.; McWhirter, E.; Nessim, C.; Rosen, C.F.; Sun, A.; et al. Surveillance Evaluations in Patients with Stage I, II, III, or Resectable IV Melanoma Who Were Treated with Curative Intent: A Systematic Review. Surg. Oncol. 2024, 54, 102077. [Google Scholar] [CrossRef]
- Ravichandran, S.; Nath, N.; Jones, D.C.; Li, G.; Suresh, V.; Brys, A.K.; Hanks, B.A.; Beasley, G.M.; Salama, A.K.S.; Howard, B.A.; et al. The Utility of Initial Staging PET-CT as a Baseline Scan for Surveillance Imaging in Stage II and III Melanoma. Surg. Oncol. 2020, 35, 533–539. [Google Scholar] [CrossRef]
- Mena, E.; Taghipour, M.; Sheikhbahaei, S.; Mirpour, S.; Xiao, J.; Subramaniam, R.M. 18F-FDG PET/CT and Melanoma: Value of Fourth and Subsequent Posttherapy Follow-up Scans for Patient Management. Clin. Nucl. Med. 2016, 41, e403–e409. [Google Scholar] [CrossRef]
- Nijhuis, A.A.G.; Dieng, M.; Khanna, N.; Lord, S.J.; Dalton, J.; Menzies, A.M.; Turner, R.M.; Allen, J.; Saw, R.P.M.; Nieweg, O.E.; et al. False-Positive Results and Incidental Findings with Annual CT or PET/CT Surveillance in Asymptomatic Patients with Resected Stage III Melanoma. Ann. Surg. Oncol. 2019, 26, 1860–1868. [Google Scholar] [CrossRef]
- Koskivuo, I.; Kemppainen, J.; Giordano, S.; Seppänen, M.; Veräjänkorva, E.; Vihinen, P.; Minn, H. Whole Body PET/CT in the Follow-up of Asymptomatic Patients with Stage IIB-IIIB Cutaneous Melanoma. Acta Oncol. 2016, 55, 1355–1359. [Google Scholar] [CrossRef]
- Baker, J.J.; Meyers, M.O.; Frank, J.; Amos, K.D.; Stitzenberg, K.B.; Ollila, D.W. Routine Restaging PET/CT and Detection of Initial Recurrence in Sentinel Lymph Node Positive Stage III Melanoma. Am. J. Surg. 2014, 207, 549–554. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA A Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Plym, A.; Ullenhag, G.J.; Breivald, M.; Lambe, M.; Berglund, A. Clinical Characteristics, Management and Survival in Young Adults Diagnosed with Malignant Melanoma: A Population-Based Cohort Study. Acta Oncol. 2014, 53, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, N.; Jansen, L.; Holleczek, B.; Waldmann, A.; Luttmann, S.; Emrich, K.; Hauschild, A.; Brenner, H.; Katalinic, A.; the GEKID Survival Working Group. Up-to-Date Results on Survival of Patients with Melanoma in Germany: Survival of Patients with Melanoma in Germany. Br. J. Dermatol. 2012, 167, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Bay, C.; Kejs, A.M.T.; Storm, H.H.; Engholm, G. Incidence and Survival in Patients with Cutaneous Melanoma by Morphology, Anatomical Site and TNM Stage: A Danish Population-Based Register Study 1989–2011. Cancer Epidemiol. 2015, 39, 1–7. [Google Scholar] [CrossRef]
- Costa Svedman, F.; Pillas, D.; Kaur, M.; Linder, R.; Hansson, J.; Aliki, T. Stage-Specific Survival and Recurrence in Patients with Cutaneous Malignant Melanoma in Europe – a Systematic Review of the Literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef]
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]. Surveillance Research Program, National Cancer Institute; Data Source(s): SEER Incidence Data, November 2023 Submission (1975–2021), SEER 22 Registries (Excluding Illinois and Massachusetts). Expected Survival Life Tables by Socio-Economic Standards. [Updated: 2024 November 5]. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html (accessed on 23 February 2025).
- Malik, D.; Sood, A.; Mittal, B.R.; Basher, R.K.; Bhattacharya, A.; Singh, G. Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Restaging and Prognosis of Recurrent Melanoma after Curative Surgery. World J. Nucl. Med. 2019, 18, 176–182. [Google Scholar] [CrossRef]
- Crispo, A.; Corradin, M.T.; Giulioni, E.; Vecchiato, A.; Del Fiore, P.; Queirolo, P.; Spagnolo, F.; Vanella, V.; Caracò, C.; Tosti, G.; et al. Real Life Clinical Management and Survival in Advanced Cutaneous Melanoma: The Italian Clinical National Melanoma Registry Experience. Front. Oncol. 2021, 11, 672797. [Google Scholar] [CrossRef]
- Pavlick, A.C.; Fecher, L.; Ascierto, P.A.; Sullivan, R.J. Frontline Therapy for BRAF -Mutated Metastatic Melanoma: How Do You Choose, and Is There One Correct Answer? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 564–571. [Google Scholar] [CrossRef]
- Rigo, R.; Doherty, J.; Koczka, K.; Kong, S.; Ding, P.Q.; Cheng, T.; Cheung, W.Y.; Monzon, J.G. Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis. Curr. Oncol. 2021, 28, 3978–3986. [Google Scholar] [CrossRef]
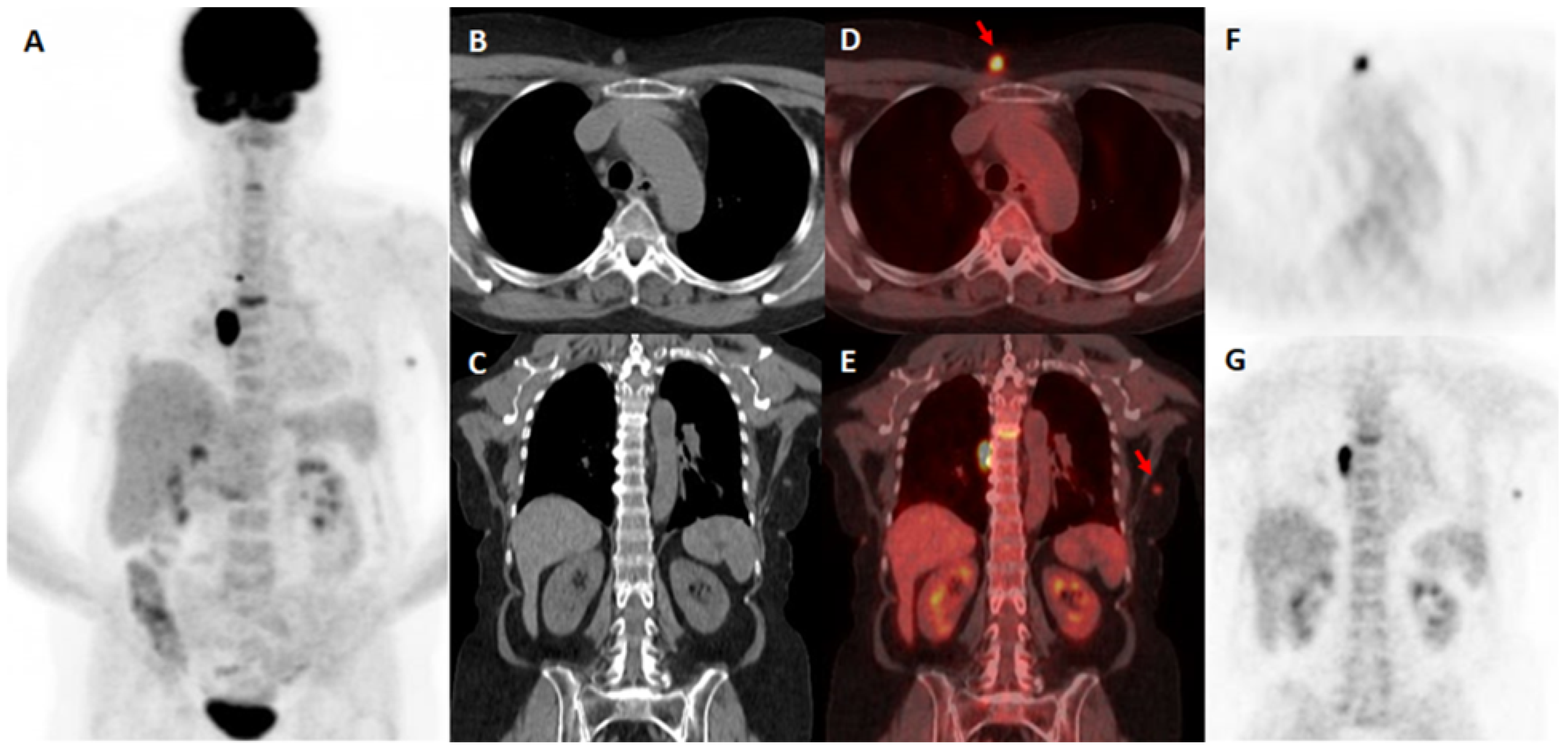
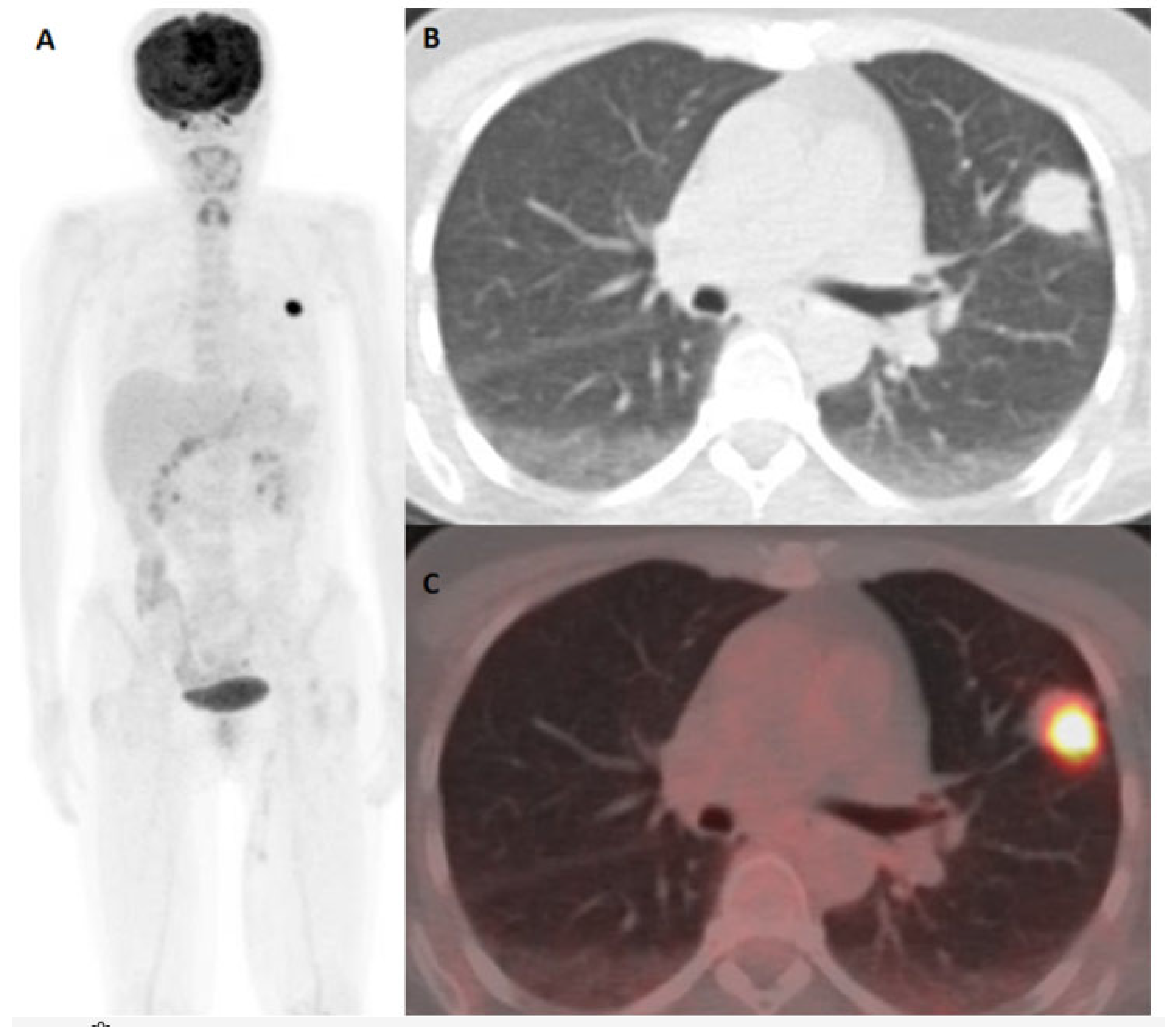

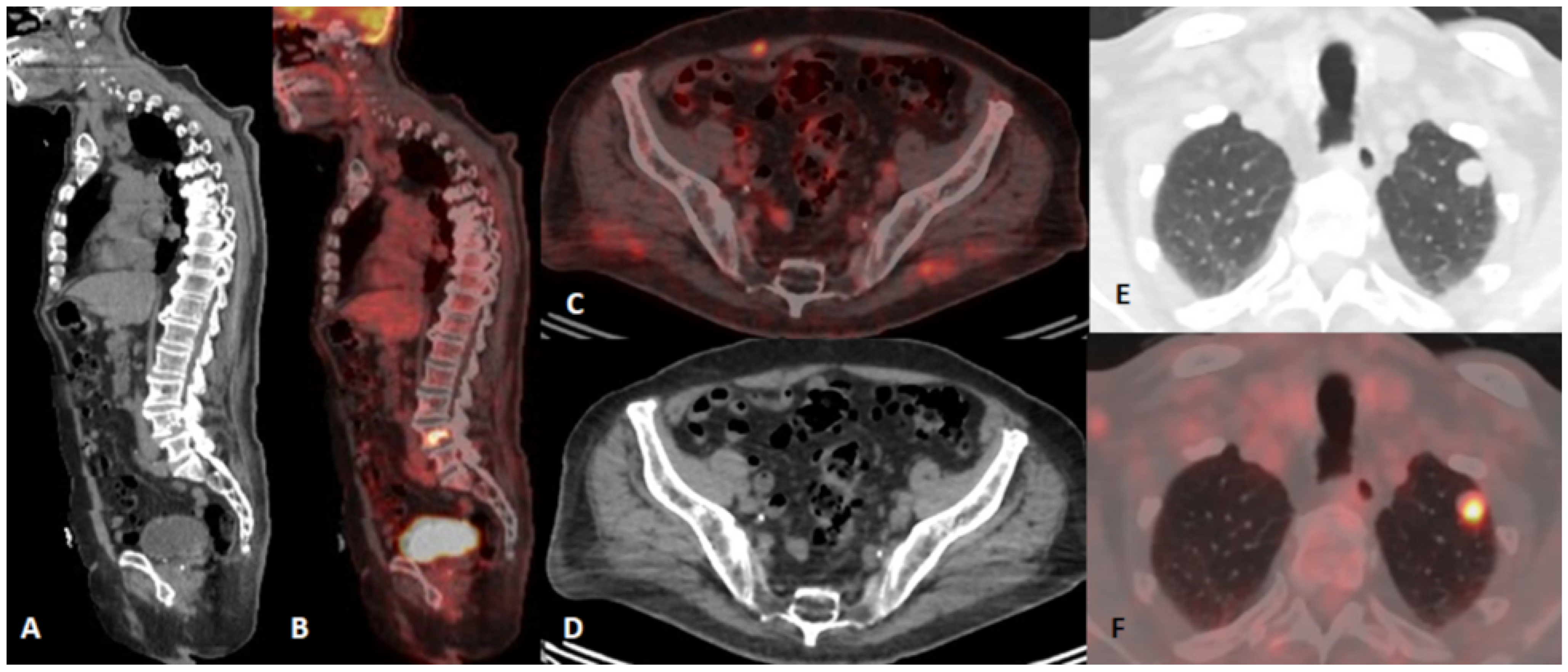
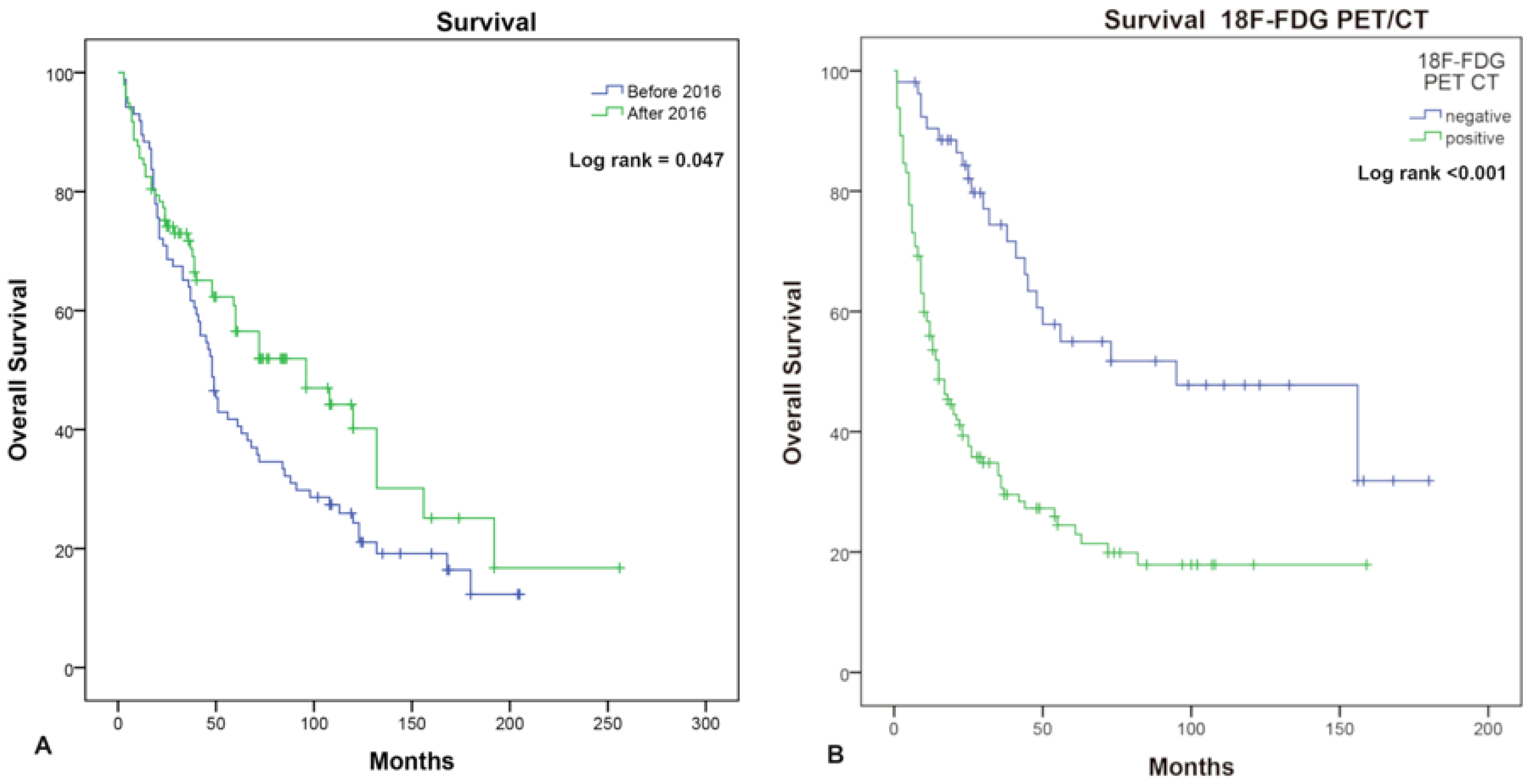
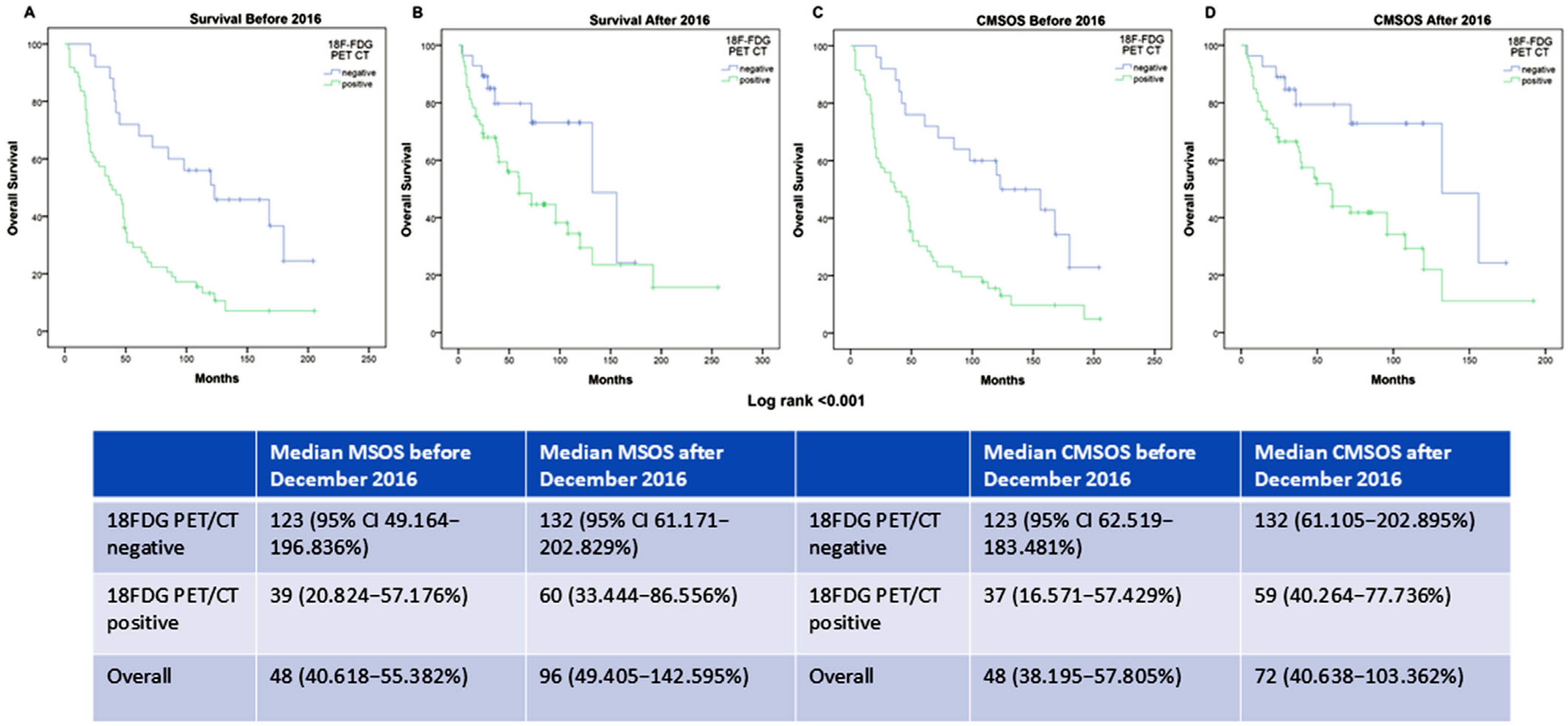
| Characteristics | Number |
|---|---|
| Gender (No) | 189 |
| Men | 103 (54.5%) |
| Women | 86 (45.5%) |
| Tumoral thickness, intervals in mm (T) (No) | 185 |
| T1 (a + b) | 1 + 6 (0.5 + 3.2%) |
| T2 (a + b) | 6 + 21 (3.2 + 11.1%) |
| T3 (a + b) | 17 + 38 (9 + 20.1%) |
| T4 (a + b) | 6 + 37 (3.2 + 19.6%) |
| Tx (unknown primary) | 53 (28%) |
| AJCC stage (No) | 185 |
| I | 5 (2.6%) |
| II | 59 (31.2%) |
| III | 15 (7.9%) |
| IV | 106 (56.1%) |
| Treated before December 2016 | 90 (47.6%) |
| Treated after December 2016 | 99 (52.4%) |
| Age at diagnosis (mean, median, range) | 53.22 ± 14.62; 54; 19–88 |
| Age at 18F-FDG PET/CT (mean, median, range) | 55.67 ± 14.28; 57; 21–88 |
| Melanoma type: | |
| Cutaneous | 183 (96.82%) |
| Uveal | 4 (2.12%) |
| Mucosal | 2 (1.06%) |
| Folow-up (mean, median, range) | 65.5 ± 56.41; 48; 3–312 months |
| Follow-up time from 18F-FDG PET/CT exam (mean, median, range) | 35.14 ± 39.16 21; 1–180 months |
| Time frame from initial diagnosis until 18F-FDG PET/CT (mean, median, range) | 29.34 ± 37.07; 12; 1–228 months |
| Outcome | |
| Dead | 124 (65.6%) |
| Alive | 65 (34.4%) |
| 18F-FGD PET/CT per patient/per scan | |
| Negative | 56 (29.6%)/63 (29.7%) |
| Positive | 133 (70.4%)/149 (70.4%) |
| True positive | 96/109 |
| False positive | 32/35 |
| True negative | 53/60 |
| False negative | 8/8 |
| Per Patient Number | Per Scan Number | Stage I and II | Stage III and IV | Staging Indication Group | Restaging Indication Group | Therapy Evaluation Indication Group | |
|---|---|---|---|---|---|---|---|
| Sensitivity (95%CI) | 92.38% (85.54–96.65%) | 93.22% (87.08–97.03%) | 90.48% (69.62–98.83%) | 92.59% (84.57–97.23%) | 90.24% (76.87–97.28%) | 93.65% (84.53–98.24%) | 92.31% (63.97–99.81%) |
| Specificity (95%CI) | 61.9% (50.66–72.29%) | 62.77% (52.18–72.52%) | 69.77% (53.87–82.82%) | 50% (33.38–66.62%) | 68.18% (52.42–81.39%) | 56.1% (39.75–71.53%) | 83.33% (35.88–99.58%) |
| AUC * (95% CI) | 0.86 (0.714–0.942) | 0.865 (0.779–0.949) | 0.872 (0.801–0.942) | 0.819 (0.713–0.926) | 0.865 (0.792–0.938) | 0.846 (0.748–0.944) | 0.9261 (0.878–0.974) |
| Whole Group (n = 189) | MSM * (n = 183) | CMSM ** (n = 177) | Stage I and II (n = 64) | Stage III and IV (n = 121) | 18F-FDG PET/CT Staging (n = 84) | 18F-FDG PET/CT Restaging (n = 94) | |
|---|---|---|---|---|---|---|---|
| OS from initial diagnosis (months) (95% CI) | 61 (45.447–76.553) | 60 (45.547–74.453) | 60 (42.681–77.319) | 132 (73.024–190.976) | 42 (32.794–51.206) | 28 (14.340–41.660) | 94 (59.942–129.058) |
| Therapy | Time Point (Before/After December 2016) | 18FDG PET/CT | Stage | Age | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | 0.098 | 0.068 | 0.0001 | 0.002 | 0.82 | 0.914 | ||||||
| HR (95% CI for Exp(B)) | 0.905 (0.805–1.018) | 0.699 (0.475–1.027) | 2.659 (1.627–4.346) | 1.331 (1.107–1.6) | 1.002 (0.988–1.016) | 1.021 (0.702–1.484) | ||||||
| No Th | Radio Th | Chemo Th | Immuno Th | Targeted Th | Combined Th | Surgery | Time point | FDG | Stage | Age | Gender | |
| p value | 0.00001 | 0.00001 | 0.081 | 0.001 | 0.013 | 0.05 | 0.01 | 0.243 | 0.081 | 0.017 | 0.206 | 0.604 |
| Adjusted HR 95% CI for Exp(B) | 0.206 (0.093–0.457) | / | 0.521 (0.25–1.084) | 0.218 (0.09–0.527) | 0.37 (0.168–0.814) | 0.23 (0.083–0.639) | 0.241 (0.101–0.574) | 0.757 (0.475–1.208) | 1.633 (0.941–2.834) | 1.251 (1.041–1.504) | 1.01 (0.995–1.025) | 1.107 (0.754–1.624) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucic, S.; Spirovski, M.; Nikolin, B.; Stojanovic, D.; Peter, A.; Gajic, B.; Cimbaljevic, V.; Lucic, M.A. 18F-FDG PET/CT Impact on Malignant Melanoma Patients Undergoing Staging and Restaging: A Single-University-Center Experience in a Real-World Setting. Diagnostics 2025, 15, 1560. https://doi.org/10.3390/diagnostics15121560
Lucic S, Spirovski M, Nikolin B, Stojanovic D, Peter A, Gajic B, Cimbaljevic V, Lucic MA. 18F-FDG PET/CT Impact on Malignant Melanoma Patients Undergoing Staging and Restaging: A Single-University-Center Experience in a Real-World Setting. Diagnostics. 2025; 15(12):1560. https://doi.org/10.3390/diagnostics15121560
Chicago/Turabian StyleLucic, Silvija, Milena Spirovski, Borislava Nikolin, Dragana Stojanovic, Andrea Peter, Branislava Gajic, Vanja Cimbaljevic, and Milos A. Lucic. 2025. "18F-FDG PET/CT Impact on Malignant Melanoma Patients Undergoing Staging and Restaging: A Single-University-Center Experience in a Real-World Setting" Diagnostics 15, no. 12: 1560. https://doi.org/10.3390/diagnostics15121560
APA StyleLucic, S., Spirovski, M., Nikolin, B., Stojanovic, D., Peter, A., Gajic, B., Cimbaljevic, V., & Lucic, M. A. (2025). 18F-FDG PET/CT Impact on Malignant Melanoma Patients Undergoing Staging and Restaging: A Single-University-Center Experience in a Real-World Setting. Diagnostics, 15(12), 1560. https://doi.org/10.3390/diagnostics15121560






