Growth Assessment and Nutritional Status in Children with Congenital Adrenal Hyperplasia—A Cross-Sectional Study from a Vietnamese Tertiary Pediatric Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical and Biochemical Assessments
- 25-hydroxyvitamin D [25(OH)D]: deficiency: <50 nmol/L; insufficiency: 50–72.5 nmol/L [45].
- Total serum calcium, 2.2–2.7 mmol/L; ionized calcium, 1.12–1.23 mmol/L. Serum phosphate levels were interpreted using age-specific reference ranges as follows: 1.25–2.10 mmol/L for children aged 1–3 years, 1.20–1.80 mmol/L for ages 4–11 years, 0.95–1.75 mmol/L for ages 12–15 years, and 0.90–1.50 mmol/L for ages 16–19 years [46].
- 17-hydroxyprogesterone (17-OHP) and testosterone levels were interpreted according to age- and sex-specific reference standards.
2.3. Data Analysis
3. Results
3.1. Description of the Study Sample
3.2. Growth Assessment and Nutritional Status
3.3. Sociodemographic Factors
3.3.1. Age
3.3.2. Gender Differences
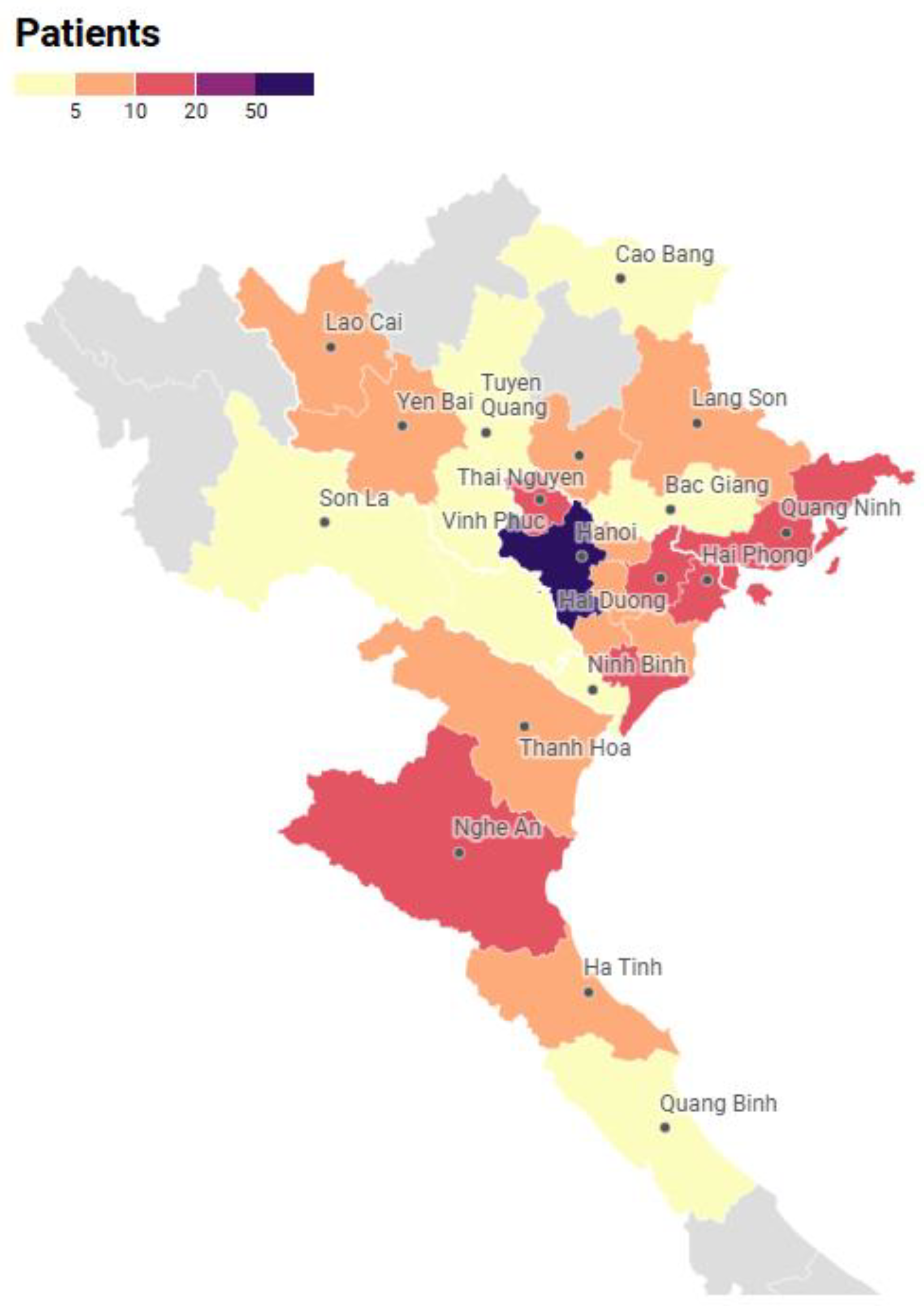
3.3.3. Residential Area
3.4. Disease-Related Factors
3.4.1. Clinical Phenotype
3.4.2. Clinical Disease Control Indicators
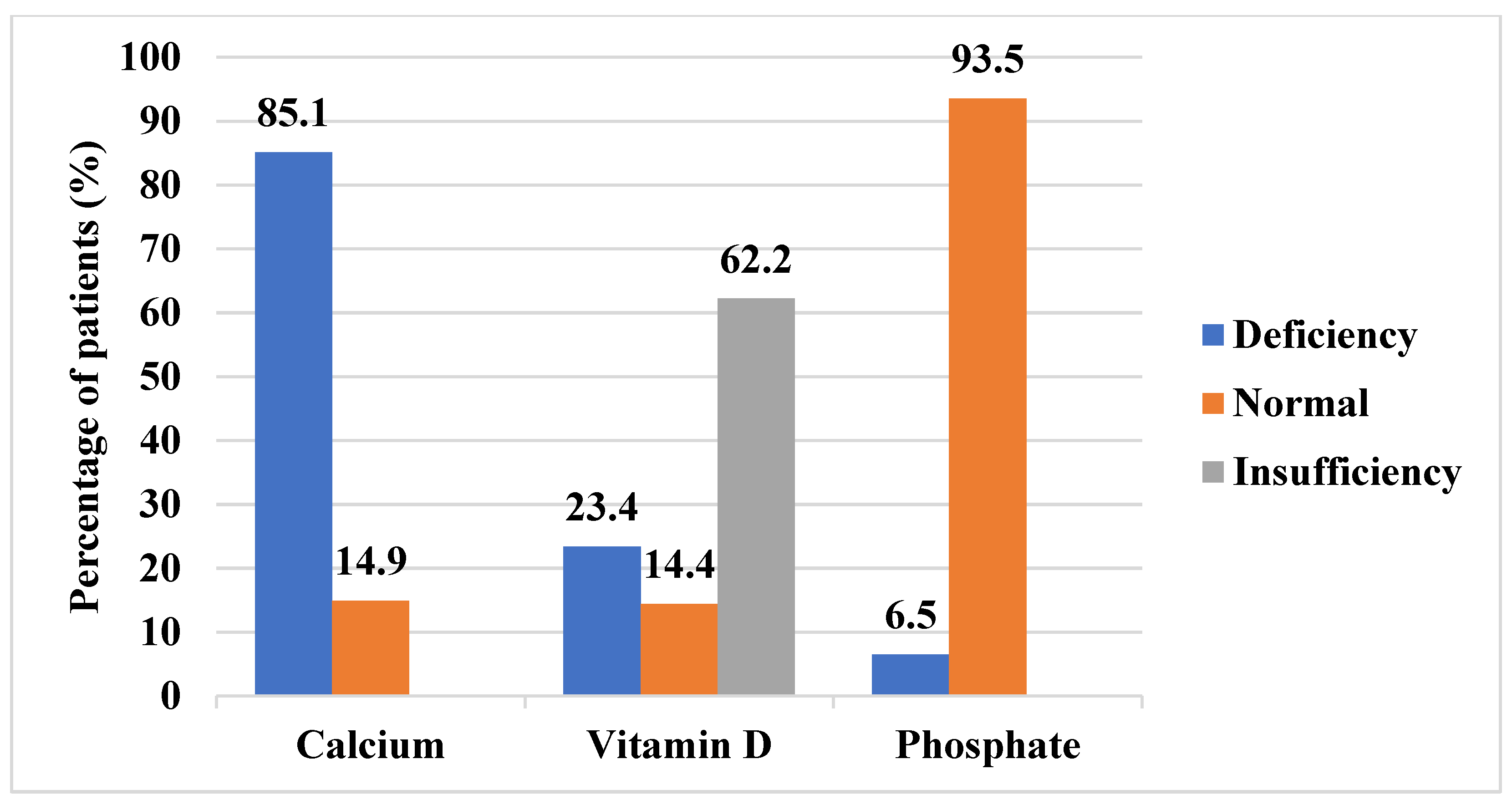
3.4.3. Biochemical Markers
3.5. Treatment-Related Factors
3.6. Associations Between Subclinical Nutritional and Growth Outcomes
4. Discussion
4.1. Growth and Nutritional Outcome
4.2. Related Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| BA | Bone age |
| BMI | Body mass index |
| CA | Chronological age |
| CAH | Congenital adrenal hyperplasia |
| CPP | Central precocious puberty |
| ELISA | Enzyme-linked immunosorbent assay |
| HAZ | Height-for-age |
| SD | Standard deviation |
| SDS | Standard deviation score |
| SV | Simple virilizing |
| SW | Salt-wasting |
| WHO | World Health Organization |
| 17OHP | 17-hydroxyprogesterone |
| 25(OH)D | 25-hydroxyvitamin D |
References
- Gruñeiro-Papendieek, L.; Chiesa, A.; Mendez, V.; Prieto, L. Neonatal Screening for Congenital Adrenal Hyperplasia: Experience and Results in Argentina. J. Pediatr. Endocrinol. Metab. 2008, 21, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Zambrana, A.N.; Sheets, L.R. Ethnic and National Differences in Congenital Adrenal Hyperplasia Incidence: A Systematic Review and Meta-Analysis. Horm. Res. Paediatr. 2023, 96, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Momodu, I.I.; Singh, G. Congenital Adrenal Hyperplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Speiser, P.W.; White, P.C. Congenital Adrenal Hyperplasia. N. Engl. J. Med. 2003, 349, 776–788. [Google Scholar] [CrossRef]
- Muthusamy, K.; Elamin, M.B.; Smushkin, G.; Murad, M.H.; Lampropulos, J.F.; Elamin, K.B.; Abu Elnour, N.O.; Gallegos-Orozco, J.F.; Fatourechi, M.M.; Agrwal, N.; et al. Adult Height in Patients with Congenital Adrenal Hyperplasia: A Systematic Review and Metaanalysis. J. Clin. Endocrinol. Metab. 2010, 95, 4161–4172. [Google Scholar] [CrossRef]
- Alzanbagi, M.A.; Milyani, A.A.; Al-Agha, A.E. Growth Characteristics in Children with Congenital Adrenal Hyperplasia. Saudi Med. J. 2018, 39, 674–678. [Google Scholar] [CrossRef]
- Finkielstain, G.P.; Kim, M.S.; Sinaii, N.; Nishitani, M.; Van Ryzin, C.; Hill, S.C.; Reynolds, J.C.; Hanna, R.M.; Merke, D.P. Clinical Characteristics of a Cohort of 244 Patients with Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2012, 97, 4429–4438. [Google Scholar] [CrossRef]
- Huang, M.; Ma, H.; Du, M.; Chen, H.; Li, Y.; Chen, Q.; Zhang, J.; Guo, S. Metabolic Status in Children with Classic Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. In Proceedings of the ESPE Abstracts; Bioscientifica: Bristol, UK, 2023; Volume 97. [Google Scholar]
- Bonfig, W.; Dalla Pozza, S.B.; Schmidt, H.; Pagel, P.; Knorr, D.; Schwarz, H.P. Hydrocortisone Dosing during Puberty in Patients with Classical Congenital Adrenal Hyperplasia: An Evidence-Based Recommendation. J. Clin. Endocrinol. Metab. 2009, 94, 3882–3888. [Google Scholar] [CrossRef]
- Merke, D.P.; Auchus, R.J. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N. Engl. J. Med. 2020, 383, 1248–1261. [Google Scholar] [CrossRef]
- Halper, A.; Sanchez, B.; Hodges, J.S.; Kelly, A.S.; Dengel, D.; Nathan, B.M.; Petryk, A.; Sarafoglou, K. Bone Mineral Density and Body Composition in Children with Congenital Adrenal Hyperplasia. Clin. Endocrinol. 2018, 88, 813–819. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E. Chrousos Syndrome: From Molecular Pathogenesis to Therapeutic Management. Eur. J. Clin. Investig. 2015, 45, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Anisowicz, S.K.; Vogt, K.S. Congenital Adrenal Hyperplasia. Pediatr. Ann. 2025, 54, e74–e77. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, A.; Cicognani, A.; Baldazzi, L.; Barbaro, M.; Baronio, F.; Gennari, M.; Bal, M.; Cassio, A.; Kontaxaki, K.; Cacciari, E. CYP21 Genotype, Adult Height, and Pubertal Development in 55 Patients Treated for 21-Hydroxylase Deficiency. J. Clin. Endocrinol. Metab. 2003, 88, 5680–5688. [Google Scholar] [CrossRef] [PubMed]
- Stikkelbroeck, N.M.M.L.; Van’T Hof-Grootenboer, B.A.E.; Hermus, A.R.M.M.; Otten, B.J.; Van’T Hof, M.A. Growth Inhibition by Glucocorticoid Treatment in Salt Wasting 21-Hydroxylase Deficiency: In Early Infancy and (Pre)Puberty. J. Clin. Endocrinol. Metab. 2003, 88, 3525–3530. [Google Scholar] [CrossRef]
- Abdel Meguid, S.E.; Soliman, A.T.; De Sanctis, V.; Abougabal, A.M.S.; Ramadan, M.A.E.F.; Hassan, M.; Hamed, N.; Ahmed, S. Growth and Metabolic Syndrome (MetS) Criteria in Young Children with Classic Congenital Adrenal Hyperplasia (CAH) Treated with Corticosteroids (CS). Acta Bio-Medica Atenei Parm. 2022, 93, e2022304. [Google Scholar] [CrossRef]
- Nimkarn, S.; Lin-Su, K.; New, M.I. Steroid 21 Hydroxylase Deficiency Congenital Adrenal Hyperplasia. Pediatr. Clin. N. Am. 2011, 58, 1281–1300. [Google Scholar] [CrossRef]
- Kurahashi, I.; Matsunuma, A.; Kawane, T.; Abe, M.; Horiuchi, N. Dexamethasone Enhances Vitamin D-24-Hydroxylase Expression in Osteoblastic (UMR-106) and Renal (LLC-PK1) Cells Treated with 1α,25-Dihydroxyvitamin D3. Endocrine 2002, 17, 109–118. [Google Scholar] [CrossRef]
- Nguyen, T.G.; Nguyen, P.D. Treatment Outcome and Some Affecting Factors of Congenital Adrenal Hyperplasia. J. Med. Res. 2011, 74, 102–106. [Google Scholar]
- Eitel, K.B.; Fechner, P.Y. Barriers to the Management of Classic Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. J. Clin. Endocrinol. Metab. 2025, 110, S67–S73. [Google Scholar] [CrossRef]
- Therrell, B.L.; Padilla, C.D.; Borrajo, G.J.C.; Khneisser, I.; Schielen, P.C.J.I.; Knight-Madden, J.; Malherbe, H.L.; Kase, M. Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020–2023). Int. J. Neonatal Screen. 2024, 10, 38. [Google Scholar] [CrossRef]
- Vietnam+ (VietnamPlus) Prenatal, Newborn Screening Programme Helps Improve Population Quality. Available online: https://en.vietnamplus.vn/prenatal-newborn-screening-programme-helps-improve-population-quality-post250241.vnp (accessed on 12 June 2025).
- WHO. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; de Onis, M., Ed.; WHO child growth standards; WHO Press: Geneva, Switzerland, 2006; ISBN 978-92-4-154693-5. [Google Scholar]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.N.; Spinola-Castro, A.M.; Kochi, C.; Kopacek, C.; Silva, P.C.A.d.; Guerra-Júnior, G. Central Precocious Puberty: Revisiting the Diagnosis and Therapeutic Management. Arch. Endocrinol. Metab. 2016, 60, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.; Rajpert-De Meyts, E. Regulation of Meiotic Entry and Gonadal Sex Differentiation in the Human: Normal and Disrupted Signaling. Biomol. Concepts 2014, 5, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Buttterfield, D.A. Unraveling the Complexity of Neurodegeneration in Brains of Subjects with Down Syndrome: Insights from Proteomics. Proteom. Clin. Appl. 2014, 8, 73–85. [Google Scholar] [CrossRef]
- Avramis, V.I.; Sencer, S.; Periclou, A.P.; Sather, H.; Bostrom, B.C.; Cohen, L.J.; Ettinger, A.G.; Ettinger, L.J.; Franklin, J.; Gaynon, P.S.; et al. A Randomized Comparison of nativeEscherichia Coli Asparaginase and Polyethylene Glycol Conjugated Asparaginase for Treatment of Children with Newly Diagnosed Standard-Risk Acute Lymphoblastic Leukemia: A Children’s Cancer Group Study. Blood 2002, 99, 1986–1994. [Google Scholar] [CrossRef]
- Korkmaz, O.; Sari, G.; Mecidov, I.; Ozen, S.; Goksen, D.; Darcan, S. The Gonadotropin-Releasing Hormone Analogue Therapy May Not Impact Final Height in Precocious Puberty of Girls with Onset of Puberty Aged 6–8 Years. J. Clin. Med. Res. 2019, 11, 133–136. [Google Scholar] [CrossRef]
- Macedo, D.B.; Cukier, P.; Mendonca, B.B.; Latronico, A.C.; Brito, V.N. Advances in the etiology, diagnosis and treatment of central precocious puberty. Arq. Bras. Endocrinol. Metabol. 2014, 58, 108–117. [Google Scholar] [CrossRef]
- Chaudhary, S.; Walia, R.; Bhansali, A.; Dayal, D.; Sachdeva, N.; Singh, T.; Bhadada, S.K. FSH-Stimulated Inhibin B (FSH-iB): A Novel Marker for the Accurate Prediction of Pubertal Outcome in Delayed Puberty. J. Clin. Endocrinol. Metab. 2021, 106, e3495–e3505. [Google Scholar] [CrossRef]
- Soriano-Guillén, L.; Argente, J. Central precocious puberty: Epidemiology, etiology, diagnosis and treatment. An. Pediatr. Barc. Spain 2003 2011, 74, e1–e336. [Google Scholar] [CrossRef]
- Tomlinson, C.; Macintyre, H.; Dorrian, C.; Ahmed, S.; Wallace, A. Testosterone Measurements in Early Infancy. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F558–F559. [Google Scholar] [CrossRef]
- Johannsen, T.H.; Main, K.M.; Ljubicic, M.L.; Jensen, T.K.; Andersen, H.R.; Andersen, M.S.; Petersen, J.H.; Andersson, A.-M.; Juul, A. Sex Differences in Reproductive Hormones During Mini-Puberty in Infants with Normal and Disordered Sex Development. J. Clin. Endocrinol. Metab. 2018, 103, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.F. Physiology of Puberty in Boys and Girls and Pathological Disorders Affecting Its Onset. J. Adolesc. 2019, 71, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testicular Growth and Development in Puberty. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Sultan, C.; Gaspari, L.; Maimoun, L.; Kalfa, N.; Paris, F. Disorders of Puberty. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 62–89. [Google Scholar] [CrossRef]
- Muerköster, A.-P.; Frederiksen, H.; Juul, A.; Andersson, A.-M.; Jensen, R.C.; Glintborg, D.; Kyhl, H.B.; Andersen, M.S.; Timmermann, C.A.G.; Jensen, T.K. Maternal Phthalate Exposure Associated with Decreased Testosterone/LH Ratio in Male Offspring during Mini-Puberty. Odense Child Cohort. Environ. Int. 2020, 144, 106025. [Google Scholar] [CrossRef]
- Latronico, A.C.; Brito, V.N.; Carel, J.-C. Causes, Diagnosis, and Treatment of Central Precocious Puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Willemsen, R.H.; Elleri, D.; Williams, R.M.; Ong, K.K.; Dunger, D.B. Pros and Cons of GnRHa Treatment for Early Puberty in Girls. Nat. Rev. Endocrinol. 2014, 10, 352–363. [Google Scholar] [CrossRef]
- Spaziani, M.; Lecis, C.; Tarantino, C.; Sbardella, E.; Pozza, C.; Gianfrilli, D. The Role of Scrotal Ultrasonography from Infancy to Puberty. Andrology 2021, 9, 1306–1321. [Google Scholar] [CrossRef]
- Lloyd, C.; McHugh, K. The Role of Radiology in Head and Neck Tumours in Children. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2010, 10, 49–61. [Google Scholar] [CrossRef]
- World Health Organization. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumfer-ence-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age: Methods and Development; World Health Organization Child Growth Standard: Geneva, Switzerland, 2007. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Kliegman, R.M.; Blum, N.J.; Tasker, R.C.; Wilson, K.M.; St. Geme, J.W.; Schuh, A.M.; Mack, C.L.; Deardorff, M.A.; Nelson, W.E. (Eds.) Nelson Textbook of Pediatrics, 22nd ed.; Elsevier: Philadelphia, PA, USA, 2025; ISBN 978-0-323-88305-4. [Google Scholar]
- Hindmarsh, P.C. Management of the Child with Congenital Adrenal Hyperplasia. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Gidlöf, S.; Hogling, D.E.; Lönnberg, H.; Ritzén, M.; Lajic, S.; Nordenström, A. Growth and Treatment in Congenital Adrenal Hyperplasia: An Observational Study from Diagnosis to Final Height. Horm. Res. Paediatr. 2024, 97, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Troger, T.; Sommer, G.; Lang-Muritano, M.; Konrad, D.; Kuhlmann, B.; Zumsteg, U.; Flück, C.E. Characteristics of Growth in Children with Classic Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency During Adrenarche and Beyond. J. Clin. Endocrinol. Metab. 2022, 107, e487–e499. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Chandrashekhar, S.R.; Gemmell, I.; O’Shea, E.; Jones, J.; Banerjee, I.; Amin, R.; Clayton, P. Gender Dichotomy in Long Term Growth Trajectories of Children with 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia. Horm. Res. Paediatr. 2011, 75, 206–212. [Google Scholar] [CrossRef]
- Völkl, T.M.K.; Simm, D.; Beier, C.; Dörr, H.G. Obesity Among Children and Adolescents with Classic Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. Pediatrics 2006, 117, e98–e105. [Google Scholar] [CrossRef]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of Glucocorticoid-Induced Insulin Resistance. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef]
- Kim, M.S.; Fraga, N.R.; Minaeian, N.; Geffner, M.E. Components of Metabolic Syndrome in Youth with Classical Congenital Adrenal Hyperplasia. Front. Endocrinol. 2022, 13, 848274. [Google Scholar] [CrossRef]
- Ben Simon, A.; Brener, A.; Segev-Becker, A.; Yackobovitch-Gavan, M.; Uretzky, A.; Schachter Davidov, A.; Alaev, A.; Oren, A.; Eyal, O.; Weintrob, N.; et al. Body Composition in Children and Adolescents with Non-Classic Congenital Adrenal Hyperplasia and the Risk for Components of Metabolic Syndrome: An Observational Study. Front. Endocrinol. 2022, 13, 1022752. [Google Scholar] [CrossRef]
- Ministry of Health Vietnam. General Nutrition Survey 2019–2020; Medical Publishing House: Hanoi, Vietnam, 2021. [Google Scholar]
- Bomberg, E.M.; Addo, O.Y.; Kyllo, J.; Gonzalez-Bolanos, M.T.; Ltief, A.M.; Pittock, S.; Himes, J.H.; Miller, B.S.; Sarafoglou, K. The Relation of Peripubertal and Pubertal Growth to Final Adult Height in Children with Classic Congenital Adrenal Hyperplasia. J. Pediatr. 2015, 166, 743–750. [Google Scholar] [CrossRef]
- Demirel, F.; Kara, O.; Tepe, D.; Esen, I. Bone Mineral Density and Vitamin D Status in Children and Adolescents with Congenital Adrenal Hyperplasia. Turk. J. Med. Sci. 2014, 44, 109–114. [Google Scholar] [CrossRef]
- Laillou, A.; Wieringa, F.; Tran, T.N.; Van, P.T.; Le, B.M.; Fortin, S.; Le, T.H.; Pfanner, R.M.; Berger, J. Hypovitaminosis D and Mild Hypocalcaemia Are Highly Prevalent among Young Vietnamese Children and Women and Related to Low Dietary Intake. PLoS ONE 2013, 8, e63979. [Google Scholar] [CrossRef] [PubMed]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [PubMed]
| Salt-Wasting | Simple Virilizing | Total | ||
|---|---|---|---|---|
| Sociodemographic | n | 160 | 41 | 201 |
| Female, n (%) | 50.6 (81) | 56.1 (23) | 104 (51.7) | |
| Age (year) | 8.7 ± 4.3 | 9.8 ± 3.6 | 9.8 (1.1–16.5) | |
| Urban area, n (%) | 92 (57.5) | 20 (48.8) | 112 (55.7) | |
| Clinical characteristics | Height SDS | −0.9 (−4.0–2.9) | 0.4 (−2.6–4.3) | −0.7 (−4.0–4.3) |
| Normal, n (%) | 130 (81.2) | 38 (92.7) | 168 (83.6) | |
| Moderate stunting, n (%) | 21 (13.1) | 3 (7.3) | 24 (11.9) | |
| Severe stunting, n (%) | 9 (5.6) | 0 (0) | 9 (4.5) | |
| BMI SDS | 1.4 (−2.7–5.2) | 1.2 (−1.0–3.4) | 1.4 (−2.7–5.2) | |
| Thinness, n (%) | 3 (1.9) | 0 (0) | 3 (1.5) | |
| Normal, n (%) | 71 (44.4) | 20 (48.8) | 91 (45.3) | |
| Overweight, n (%) | 41 (25.6) | 8 (19.5) | 49 (24.4) | |
| Obesity, n (%) | 45 (28.1) | 13 (31.7) | 58 (28.9) | |
| Cushingoid appearance, n (%) | 13 (8.1) | 4 (9.8) | 17 (8.5) | |
| Hyperpigmentation, n (%) | 44 (27.5) | 6 (14.6) | 50 (24.9) | |
| Virilization, n (%) | 23 (14.4) | 8 (19.5) | 31 (15.4) | |
| Central precocious puberty, n (%) | 27 (16.9) | 20 (48.8) | 47 (23.4) | |
| Biochemical and radiological markers | 17OHP (nmol/L) | 52.4 (0–1182) | 57.1 (2.0–925) | 52.4 (0–1182) |
| Testosterone (nmol/L) | 0.24 (0.1–26.3) | 0.78 (0.1–29.8) | 0.24 (0.1–29.8) | |
| Bone age (BA) − Chronological age (CA) (year) 1 | 0.9 (−8.3–6.5) | 2.5 (−1.4–8.6) | 1.4 (−8.3–8.6) | |
| Advanced bone age (BA > CA), n (%) | 70 (43.8) | 34 (82.9) | 104 (51.7) | |
| Age-appropriate bone age (BA = CA), n (%) | 71 (44.4) | 6 (14.6) | 77 (38.3) | |
| Delayed bone age (BA < CA), n (%) | 19 (11.9) | 1 (2.4) | 20 (10) | |
| Treatment-related factors | Age at diagnosis and treatment (year) 2 | 0 (0–3) | 3 (0–9) | 0 (0–9) |
| Hydrocortisone (mg/m2/day) | 16.3 ± 4.4 | 18.7 ± 3.7 | 16.8 ± 4.4 | |
| Duration of glucocorticoid therapy (year) | 8.6 ± 4.3 | 6.7 ± 3.6 | 8.2 ± 4.2 |
| Independent Factor | Variable Group | Subgroup | n | Median Age (Years) | p-Value |
|---|---|---|---|---|---|
| Age at evaluation | Hypocalcemia | Yes | 171 | 0 | 0.24 |
| No | 30 | 0 | |||
| Hypophosphatemia | Yes | 9 | 1.5 | 0.039 * | |
| No | 129 | 0 | |||
| Vitamin D Status | Deficiency | 47 | 10.3 | 0.001 * | |
| Insufficiency | 125 | 10.1 | |||
| Sufficiency | 29 | 5.6 | |||
| BMI Classification | Thinness | 3 | 2.5 | <0.001 * | |
| Normal | 91 | 7.5 | |||
| Overweight | 49 | 10.8 | |||
| Obesity | 58 | 10.5 | |||
| Height-for-Age | Severe stunting | 9 | 4.8 | 0.183 | |
| Moderate stunting | 24 | 12.7 | |||
| Normal | 168 | 9.7 | |||
| BA-CA Classification | BA > CA | 104 | 10.9 | <0.001 * | |
| BA = CA | 77 | 5.9 | |||
| BA < CA | 20 | 4.6 |
| Cushingoid Appearance | Acanthosis Nigricans | Hyperpigmentation | Virilization | Central Precocious Puberty | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| Hypocalcemia | 1 (FET) | 0.803 (0.22–2.98) | 0.704 (FET) | 2.59 (0.33–20.43) | 0.012 (Χ2) * | 5.46 (1.25–23.83) | 0.054 (FET) | 6.17 (0.81–47.07) | 0.019 (Χ2) * | 5 (1.145–21.84) |
| Hypophosphatemia | 1 (FET) | NA | 1 (FET) | NA | 0.121 (FET) | 3.357 (0.85–13.22) | 0.626 (FET) | 1.65 (0.319–8.572) | 0.041 (FET) * | 4.31 (1.086–17.103) |
| p-value | Cramer’s V | p-value | Cramer’s V | p-value | Cramer’s V | p-value | Cramer’s V | p-value | Cramer’s V | |
| Vitamin D Status | 0.933 (FET) | 0.053 | 0.741 (FET) | 0.073 | 0.131 (Χ2) | 0.142 | 0.128 (FET) | 0.139 | 0.142 (Χ2) | 0.139 |
| BMI Classification | 0.001 (FET) * | 0.262 | < 0.001 (FET) * | 0.334 | 0.045 (FET) * | 0.201 | 0.779 (FET) | 0.083 | 0.392 (FET) | 0.132 |
| Height-for-age | 0.64 (FET) | 0.059 | 0.852 (FET) | 0.08 | 0.74 (FET) | 0.069 | 0.206 (FET) | 0.129 | 0.168 (FET) | 0.138 |
| BA-CA Classification | 0.43 (FET) | 0.088 | 0.205 (FET) | 0.135 | 0.005 (FET) * | 0.227 | 0.004 (FET) * | 0.231 | <0.001 (FET) * | 0.324 |
| Outcome Variable | p-Value | Effect Size | |
|---|---|---|---|
| Glucocorticoid treatment duration | Hypocalcemia | <0.001 * | r = 0.447 |
| Hypophosphatemia | 0.942 | r = 0.006 | |
| Vitamin D status | 0.007 * | η2 = 0.04 | |
| BMI classification | <0.001 * | η2 = 0.08 | |
| Height-for-age classification | 0.108 | η2 = 0.01 | |
| BA-CA classification | <0.001 * | η2 = 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.H.; Le, K.M.; Tran, T.A.T.; Nguyen, K.N.; Can, T.B.N.; Bui, P.T.; Tran, D.T.; Vu, C.D. Growth Assessment and Nutritional Status in Children with Congenital Adrenal Hyperplasia—A Cross-Sectional Study from a Vietnamese Tertiary Pediatric Center. Diagnostics 2025, 15, 1534. https://doi.org/10.3390/diagnostics15121534
Nguyen TTH, Le KM, Tran TAT, Nguyen KN, Can TBN, Bui PT, Tran DT, Vu CD. Growth Assessment and Nutritional Status in Children with Congenital Adrenal Hyperplasia—A Cross-Sectional Study from a Vietnamese Tertiary Pediatric Center. Diagnostics. 2025; 15(12):1534. https://doi.org/10.3390/diagnostics15121534
Chicago/Turabian StyleNguyen, Thi Thuy Hong, Khanh Minh Le, Thi Anh Thuong Tran, Khanh Ngoc Nguyen, Thi Bich Ngoc Can, Phuong Thao Bui, Dat Tien Tran, and Chi Dung Vu. 2025. "Growth Assessment and Nutritional Status in Children with Congenital Adrenal Hyperplasia—A Cross-Sectional Study from a Vietnamese Tertiary Pediatric Center" Diagnostics 15, no. 12: 1534. https://doi.org/10.3390/diagnostics15121534
APA StyleNguyen, T. T. H., Le, K. M., Tran, T. A. T., Nguyen, K. N., Can, T. B. N., Bui, P. T., Tran, D. T., & Vu, C. D. (2025). Growth Assessment and Nutritional Status in Children with Congenital Adrenal Hyperplasia—A Cross-Sectional Study from a Vietnamese Tertiary Pediatric Center. Diagnostics, 15(12), 1534. https://doi.org/10.3390/diagnostics15121534






