Causal Relationship Between Cerebrospinal Fluid Metabolites and Intervertebral Disc Disease: A Bidirectional Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
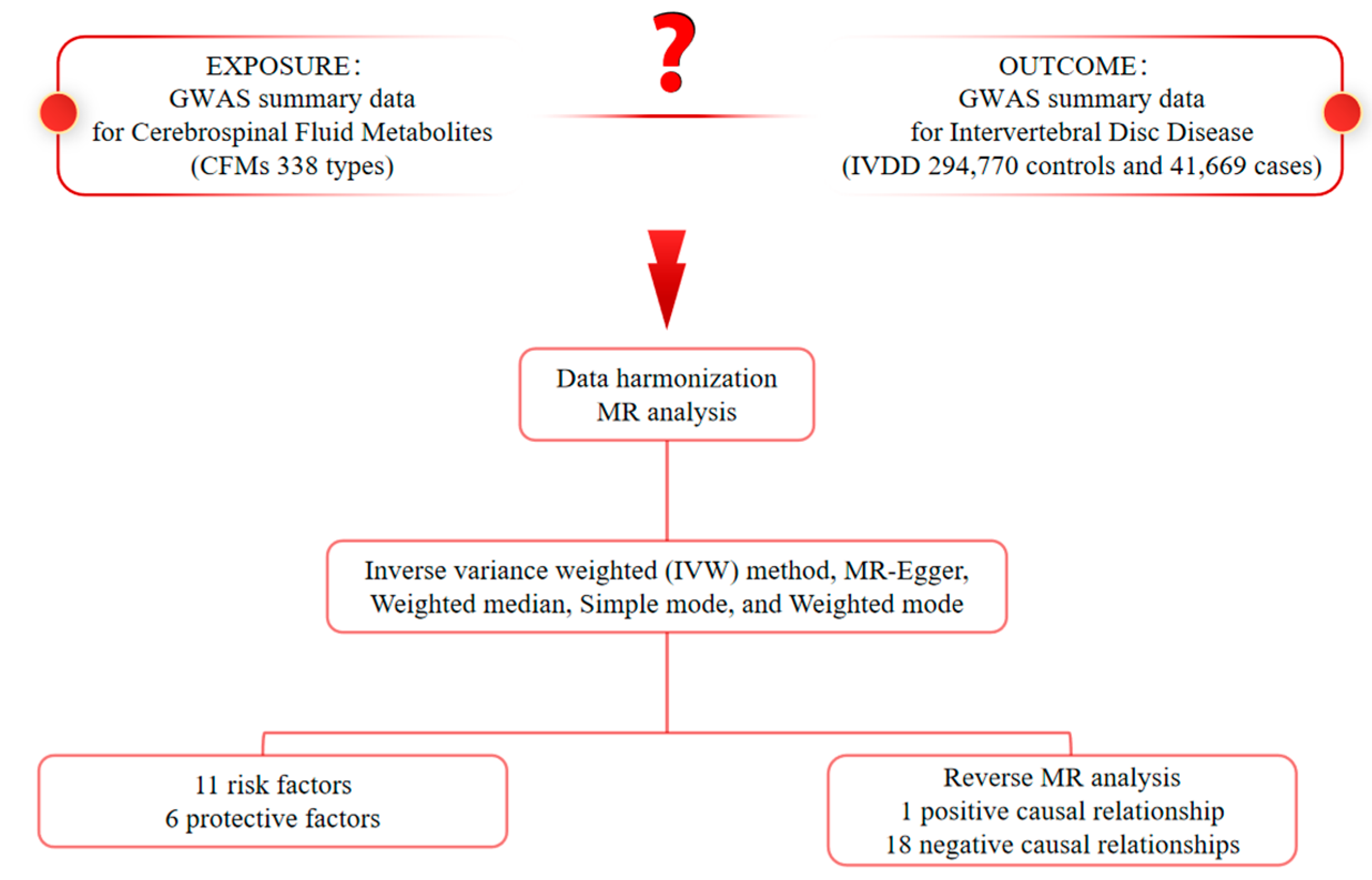
2.1. Study Design
2.2. Genome-Wide Association Study (GWAS) Data Sources for IVDD
2.3. CFMs GWAS Data Sources
2.4. Selection of Instrumental Variables (IVs)
2.5. Statistical Analysis
3. Results
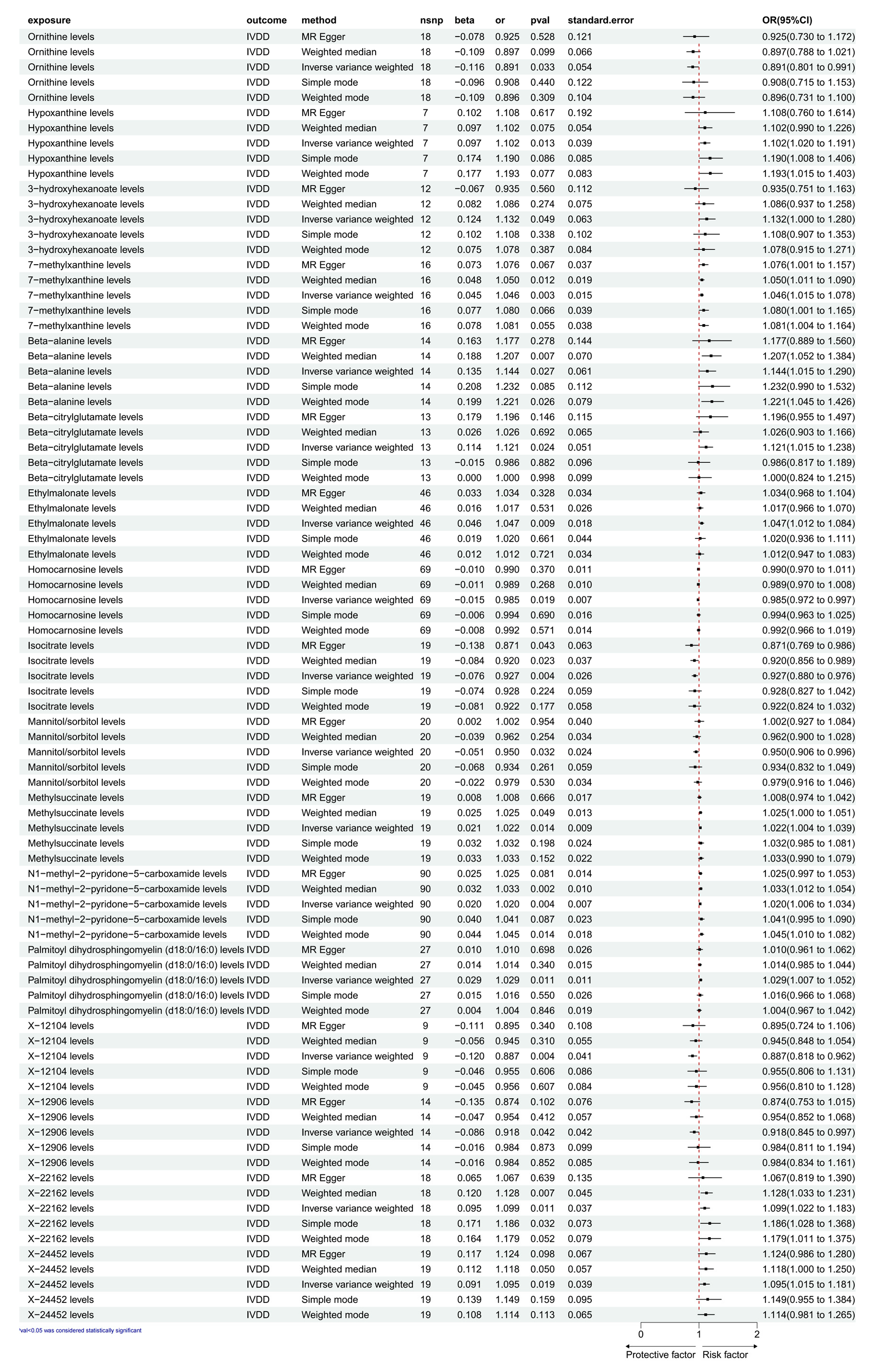
3.1. Exploration of the Causal Effect of CFM on IVDD
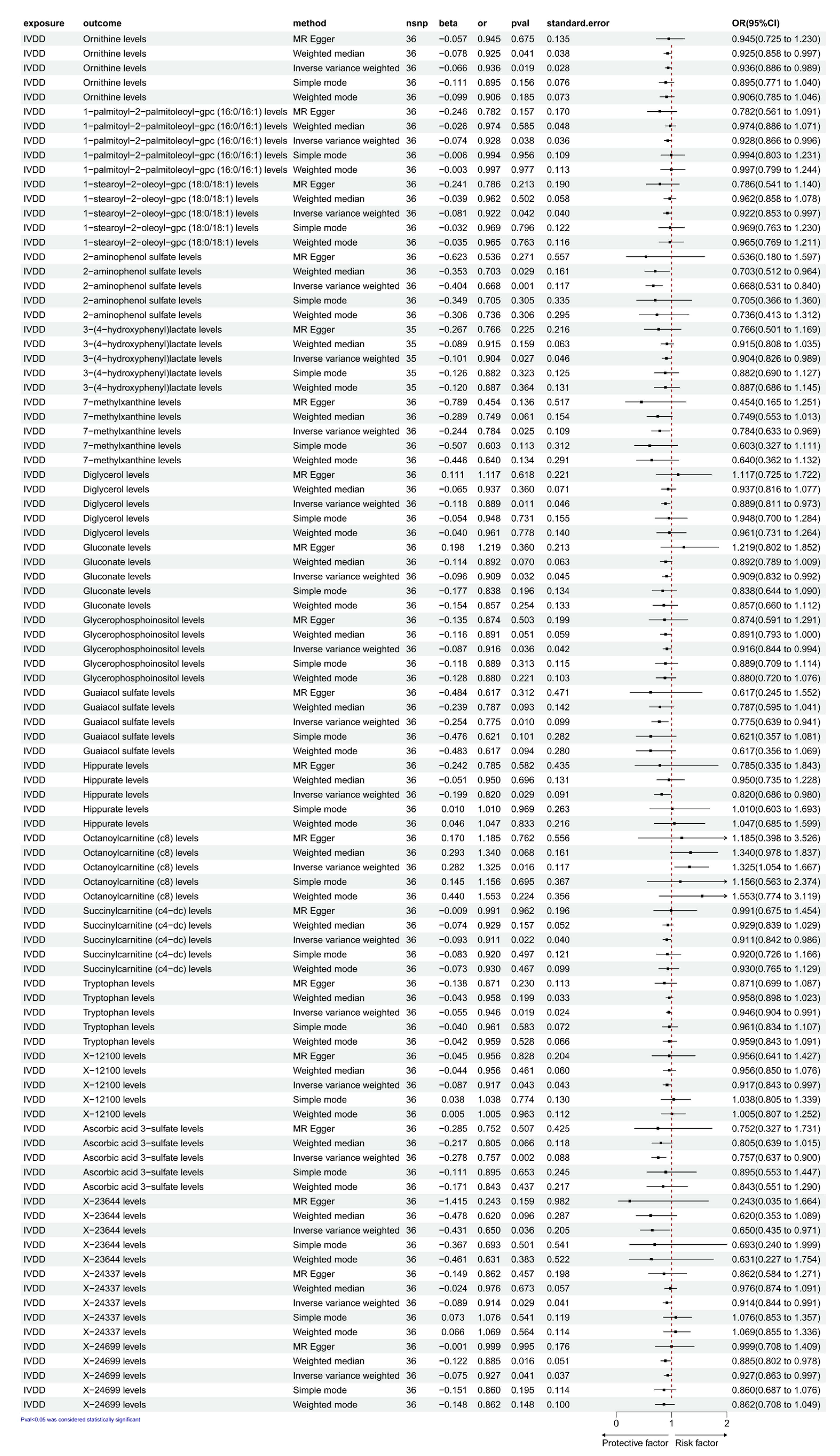
3.2. Exploration of the Causal Effect of IVDD on CFM
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P.; Lancet Low Back Pain Series Working Group. Low back pain: A call for action. Lancet 2018, 391, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Zafonte, R.D. Sciatica. N. Engl. J. Med. 2015, 372, 1240–1248. [Google Scholar] [CrossRef]
- Kague, E.; Turci, F.; Newman, E.; Yang, Y.; Brown, K.R.; Aglan, M.S.; Otaify, G.A.; Temtamy, S.A.; Ruiz-Perez, V.L.; Cross, S.; et al. 3D assessment of intervertebral disc degeneration in zebrafish identifies changes in bone density that prime disc disease. Bone Res. 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Y.; Liao, Z.; Liu, H.; Zhang, S.; Zhong, D.; Qiu, X.; Chen, T.; Su, D.; Ke, X.; et al. Self-amplifying loop of NF-κB and periostin initiated by PIEZO1 accelerates mechano-induced senescence of nucleus pulposus cells and intervertebral disc degeneration. Mol. Ther. 2022, 30, 3241–3256. [Google Scholar] [CrossRef]
- Ma, K.; Chen, S.; Li, Z.; Deng, X.; Huang, D.; Xiong, L.; Shao, Z. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthr. Cartil. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Ji, L.; Wang, Y.; Lu, T.; Yang, J.; Luo, C.; Qiu, B. Identification of blood metabolites linked to the risk of intervertebral disc diseases: A comprehensive Mendelian randomization study. Postgrad. Med. J. 2023, 99, 1148–1153. [Google Scholar] [CrossRef]
- Luo, Z.; Wei, Z.; Zhang, G.; Chen, H.; Li, L.; Kang, X. Achilles’ Heel-The Significance of Maintaining Microenvironmental Homeostasis in the Nucleus Pulposus for Intervertebral Discs. Int. J. Mol. Sci. 2023, 24, 16592. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiao, X.; Wang, Z.G.; Yuan, K.; Yang, Y.Q.; Wang, Y.; Li, Y.T.; Wang, T.C.; Kan, T.Y.; Wang, J.; et al. D-mannose alleviates intervertebral disc degeneration through glutamine metabolism. Mil. Med. Res. 2024, 11, 28. [Google Scholar] [CrossRef]
- Simrén, J.; Ashton, N.J.; Blennow, K.; Zetterberg, H. An update on fluid biomarkers for neurodegenerative diseases: Recent success and challenges ahead. Curr. Opin. Neurobiol. 2020, 61, 29–39. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Panyard, D.J.; Kim, K.M.; Darst, B.F.; Deming, Y.K.; Zhong, X.; Wu, Y.; Kang, H.; Carlsson, C.M.; Johnson, S.C.; Asthana, S.; et al. Cerebrospinal fluid metabolomics identifies 19 brain-related phenotype associations. Commun. Biol. 2021, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Holmes, M.V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods 2019, 10, 486–496. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.; Chai, K. Systemic immune-inflammation index is associated with disease activity in patients with ankylosing spondylitis. J. Clin. Lab. Anal. 2021, 35, e23964. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, D.; Zhang, D.; Zuo, X.; Yao, L.; Liu, T.; Ge, X.; He, C.; Zhou, Y.; Shen, Z. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry 2023, 23, 590. [Google Scholar] [CrossRef]
- Jin, P.; Xing, Y.; Xiao, B.; Wei, Y.; Yan, K.; Zhao, J.; Tian, W. Diabetes and intervertebral disc degeneration: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1100874. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S. Disc morphology in health and disease. Biochem. Soc. Trans. 2002, 30 Pt 6, 864–869. [Google Scholar] [CrossRef]
- Elmasry, S.; Asfour, S.; de Rivero Vaccari, J.P.; Travascio, F. Effects of Tobacco Smoking on the Degeneration of the Intervertebral Disc: A Finite Element Study. PLoS ONE 2015, 10, e0136137. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Jt. Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef]
- Feng, C.; Liu, H.; Yang, M.; Zhang, Y.; Huang, B.; Zhou, Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle 2016, 15, 1674–1684. [Google Scholar] [CrossRef]
- Sakai, D.; Grad, S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv. Drug Deliv. Rev. 2015, 84, 159–171. [Google Scholar] [CrossRef]
- Patil, P.; Niedernhofer, L.J.; Robbins, P.D.; Lee, J.; Sowa, G.; Vo, N. Cellular senescence in intervertebral disc aging and degeneration. Curr. Mol. Biol. Rep. 2018, 4, 180–190. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cell Mater. 2015, 30, 104–116; discussion 116–117. [Google Scholar] [CrossRef]
- Frapin, L.; Clouet, J.; Delplace, V.; Fusellier, M.; Guicheux, J.; Le Visage, C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv. Drug Deliv. Rev. 2019, 149, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef]
- Patil, P.; Dong, Q.; Wang, D.; Chang, J.; Wiley, C.; Demaria, M.; Lee, J.; Kang, J.; Niedernhofer, L.J.; Robbins, P.D.; et al. Systemic clearance of p16INK4a-positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell 2019, 18, e12927. [Google Scholar] [CrossRef]
- Horvatić, A.; Gelemanović, A.; Pirkić, B.; Smolec, O.; Beer Ljubić, B.; Rubić, I.; Eckersall, P.D.; Mrljak, V.; McLaughlin, M.; Samardžija, M.; et al. Multi-Omics Approach to Elucidate Cerebrospinal Fluid Changes in Dogs with Intervertebral Disc Herniation. Int. J. Mol. Sci. 2021, 22, 11678. [Google Scholar] [CrossRef]
- Kosek, E.; Finn, A.; Ultenius, C.; Hugo, A.; Svensson, C.; Ahmed, A.S. Differences in neuroimmune signalling between male and female patients suffering from knee osteoarthritis. J. Neuroimmunol. 2018, 321, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Gasche, Y.; Fujimura, M.; Morita-Fujimura, Y.; Copin, J.C.; Kawase, M.; Massengale, J.; Chan, P.H. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: A possible role in blood-brain barrier dysfunction. J. Cereb. Blood Flow Metab. 1999, 19, 1020–1028. [Google Scholar] [CrossRef]
- Romanic, A.M.; White, R.F.; Arleth, A.J.; Ohlstein, E.H.; Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998, 29, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Gårseth, M.; Sonnewald, U.; White, L.R.; Rød, M.; Nygaard, Ø.; Zwart, J.A. Metabolic changes in the cerebrospinal fluid of patients with lumbar disc herniation or spinal stenosis. J. Neurosci. Res. 2002, 69, 692–695. [Google Scholar] [CrossRef]
- Wada, K.; Tanaka, T.; Kumagai, G.; Kudo, H.; Asari, T.; Chiba, D.; Ota, S.; Kamei, K.; Takeda, O.; Nakaji, S.; et al. A study of the factors associated with cervical spinal disc degeneration, with a focus on bone metabolism and amino acids, in the Japanese population: A cross sectional study. BMC Musculoskelet. Disord. 2018, 19, 153. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, X.; Lv, J.; Mei, Y.; Luo, Y.; Li, F.; Liu, Z. Analysis of Key Differential Metabolites in Intervertebral Disc Degeneration Based on Untargeted Metabolomics. JOR Spine 2025, 8, e70032. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Winlove, C.P. Pathophysiology of the intervertebral disc and the challenges for MRI. J. Magn. Reason. Imaging 2007, 25, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Toczylowska, B.; Woznica, M.; Zieminska, E.; Krolicki, L. Metabolic Biomarkers Differentiate a Surgical Intervertebral Disc from a Nonsurgical Intervertebral Disc. Int. J. Mol. Sci. 2023, 24, 10572. [Google Scholar] [CrossRef] [PubMed]
| Exposure | Outcome | Egger Intercept | SE | p Val |
|---|---|---|---|---|
| Ornithine levels | IVDD | −0.003 | 0.007 | 0.731 |
| Hypoxanthine levels | 0.000 | 0.017 | 0.979 | |
| 3-hydroxyhexanoate levels | 0.013 | 0.007 | 0.076 | |
| 7-methylxanthine levels | −0.007 | 0.009 | 0.414 | |
| Beta-alanine levels | −0.002 | 0.009 | 0.830 | |
| Beta-citrylglutamate levels | −0.006 | 0.009 | 0.539 | |
| Ethylmalonate levels | 0.002 | 0.005 | 0.657 | |
| Homocarnosine levels | −0.002 | 0.003 | 0.495 | |
| Isocitrate levels | 0.007 | 0.007 | 0.296 | |
| Mannitol/sorbitol levels | −0.010 | 0.006 | 0.109 | |
| Methylsuccinate levels | 0.006 | 0.006 | 0.363 | |
| N1-methyl-2-pyridone-5-carboxamide levels | −0.002 | 0.005 | 0.683 | |
| Palmitoyl dihydrosphingomyelin (d18:0/16:0) levels | 0.005 | 0.006 | 0.425 | |
| X-12104 levels | −0.001 | 0.008 | 0.928 | |
| X-12906 levels | 0.006 | 0.008 | 0.454 | |
| X-22162 levels | 0.003 | 0.011 | 0.819 | |
| X-24452 levels | −0.002 | 0.005 | 0.639 |
| Exposure | Outcome | Method | Q | Q_df | Q_p Val |
|---|---|---|---|---|---|
| Ornithine levels | IVDD | MR-Egger | 24.505 | 16 | 0.079 |
| Ornithine levels | Inverse variance weighted | 24.692 | 17 | 0.102 | |
| Hypoxanthine levels | MR-Egger | 6.170 | 5 | 0.290 | |
| Hypoxanthine levels | Inverse variance weighted | 6.171 | 6 | 0.404 | |
| 3-hydroxyhexanoate levels | MR-Egger | 11.638 | 10 | 0.310 | |
| 3-hydroxyhexanoate levels | Inverse variance weighted | 16.204 | 11 | 0.134 | |
| 7-methylxanthine levels | MR-Egger | 19.801 | 14 | 0.137 | |
| 7-methylxanthine levels | Inverse variance weighted | 20.803 | 15 | 0.143 | |
| Beta-alanine levels | MR-Egger | 21.812 | 12 | 0.040 | |
| Beta-alanine levels | Inverse variance weighted | 21.900 | 13 | 0.057 | |
| Beta-citrylglutamate levels | MR-Egger | 15.722 | 11 | 0.152 | |
| Beta-citrylglutamate levels | Inverse variance weighted | 16.297 | 12 | 0.178 | |
| Ethylmalonate levels | MR-Egger | 46.878 | 44 | 0.355 | |
| Ethylmalonate levels | Inverse variance weighted | 47.091 | 45 | 0.387 | |
| Homocarnosine levels | MR-Egger | 59.626 | 67 | 0.727 | |
| Homocarnosine levels | Inverse variance weighted | 60.097 | 68 | 0.742 | |
| Isocitrate levels | MR-Egger | 8.416 | 17 | 0.957 | |
| Isocitrate levels | Inverse variance weighted | 9.579 | 18 | 0.945 | |
| Mannitol/sorbitol levels | MR-Egger | 15.336 | 18 | 0.639 | |
| Mannitol/sorbitol levels | Inverse variance weighted | 18.183 | 19 | 0.510 | |
| Methylsuccinate levels | MR-Egger | 12.798 | 17 | 0.750 | |
| Methylsuccinate levels | Inverse variance weighted | 13.672 | 18 | 0.750 | |
| N1-methyl-2-pyridone-5-carboxamide levels | MR-Egger | 95.730 | 88 | 0.269 | |
| N1-methyl-2-pyridone-5-carboxamide levels | Inverse variance weighted | 95.913 | 89 | 0.289 | |
| Palmitoyl dihydrosphingomyelin (d18:0/16:0) levels | MR-Egger | 33.292 | 25 | 0.124 | |
| Palmitoyl dihydrosphingomyelin (d18:0/16:0) levels | Inverse variance weighted | 34.168 | 26 | 0.131 | |
| X-12104 levels | MR-Egger | 6.234 | 7 | 0.513 | |
| X-12104 levels | Inverse variance weighted | 6.243 | 8 | 0.620 | |
| X-12906 levels | MR-Egger | 6.728 | 12 | 0.875 | |
| X-12906 levels | Inverse variance weighted | 7.327 | 13 | 0.885 | |
| X-22162 levels | MR-Egger | 23.990 | 16 | 0.090 | |
| X-22162 levels | Inverse variance weighted | 24.071 | 17 | 0.118 | |
| X-24452 levels | MR-Egger | 11.820 | 17 | 0.811 | |
| X-24452 levels | Inverse variance weighted | 12.048 | 18 | 0.845 |
| Exposure | Outcome | Egger Intercept | SE | p Val |
|---|---|---|---|---|
| IVDD | Ornithine levels | −0.001 | 0.007 | 0.946 |
| 1-palmitoyl-2-palmitoleoyl-gpc (16:0/16:1) levels | 0.010 | 0.009 | 0.309 | |
| 1-stearoyl-2-oleoyl-gpc (18:0/18:1) levels | 0.009 | 0.010 | 0.394 | |
| 2-aminophenol sulfate levels | 0.012 | 0.031 | 0.690 | |
| 3-(4-hydroxyphenyl)lactate levels | 0.009 | 0.012 | 0.438 | |
| 7-methylxanthine levels | 0.031 | 0.028 | 0.288 | |
| Diglycerol levels | −0.013 | 0.012 | 0.296 | |
| Gluconate levels | −0.016 | 0.012 | 0.168 | |
| Glycerophosphoinositol levels | 0.003 | 0.011 | 0.808 | |
| Guaiacol sulfate levels | 0.013 | 0.026 | 0.622 | |
| Hippurate levels | 0.002 | 0.024 | 0.920 | |
| Octanoylcarnitine (c8) levels | 0.006 | 0.031 | 0.838 | |
| Succinylcarnitine (c4-dc) levels | −0.005 | 0.011 | 0.665 | |
| Tryptophan levels | 0.005 | 0.006 | 0.459 | |
| X-12100 levels | −0.002 | 0.011 | 0.835 | |
| Ascorbic acid 3-sulfate levels | 0.000 | 0.023 | 0.986 | |
| X-23644 levels | 0.055 | 0.054 | 0.313 | |
| X-24337 levels | 0.003 | 0.011 | 0.760 | |
| X-24699 levels | −0.004 | 0.010 | 0.668 |
| Exposure | Outcome | Method | Q | Q_df | Q_p Val |
|---|---|---|---|---|---|
| IVDD | Ornithine levels | MR-Egger | 27.226 | 34 | 0.788 |
| Ornithine levels | Inverse variance weighted | 27.231 | 35 | 0.823 | |
| 1-palmitoyl-2-palmitoleoyl-gpc (16:0/16:1) levels | MR-Egger | 38.023 | 34 | 0.291 | |
| 1-palmitoyl-2-palmitoleoyl-gpc (16:0/16:1) levels | Inverse variance weighted | 39.216 | 35 | 0.286 | |
| 1-stearoyl-2-oleoyl-gpc (18:0/18:1) levels | MR-Egger | 34.950 | 34 | 0.423 | |
| 1-stearoyl-2-oleoyl-gpc (18:0/18:1) levels | Inverse variance weighted | 35.716 | 35 | 0.435 | |
| 2-aminophenol sulfate levels | MR-Egger | 27.470 | 34 | 0.778 | |
| 2-aminophenol sulfate levels | Inverse variance weighted | 27.632 | 35 | 0.808 | |
| 3-(4-hydroxyphenyl)lactate levels | MR-Egger | 21.051 | 33 | 0.947 | |
| 3-(4-hydroxyphenyl)lactate levels | Inverse variance weighted | 21.667 | 34 | 0.950 | |
| 7-methylxanthine levels | MR-Egger | 28.475 | 34 | 0.735 | |
| 7-methylxanthine levels | Inverse variance weighted | 29.638 | 35 | 0.724 | |
| Diglycerol levels | MR-Egger | 34.053 | 34 | 0.465 | |
| Diglycerol levels | Inverse variance weighted | 35.182 | 35 | 0.460 | |
| Gluconate levels | MR-Egger | 20.209 | 34 | 0.971 | |
| Gluconate levels | Inverse variance weighted | 22.190 | 35 | 0.954 | |
| Glycerophosphoinositol levels | MR-Egger | 31.422 | 34 | 0.595 | |
| Glycerophosphoinositol levels | Inverse variance weighted | 31.481 | 35 | 0.639 | |
| Guaiacol sulfate levels | MR-Egger | 24.569 | 34 | 0.883 | |
| Guaiacol sulfate levels | Inverse variance weighted | 24.816 | 35 | 0.899 | |
| Hippurate levels | MR-Egger | 27.661 | 34 | 0.770 | |
| Hippurate levels | Inverse variance weighted | 27.671 | 35 | 0.806 | |
| Octanoylcarnitine (c8) levels | MR-Egger | 29.801 | 34 | 0.674 | |
| Octanoylcarnitine (c8) levels | Inverse variance weighted | 29.843 | 35 | 0.715 | |
| Succinylcarnitine (c4-dc) levels | MR-Egger | 43.452 | 34 | 0.128 | |
| Succinylcarnitine (c4-dc) levels | Inverse variance weighted | 43.696 | 35 | 0.149 | |
| Tryptophan levels | MR-Egger | 35.026 | 34 | 0.419 | |
| Tryptophan levels | Inverse variance weighted | 35.603 | 35 | 0.440 | |
| X-12100 levels | MR-Egger | 24.151 | 34 | 0.895 | |
| X-12100 levels | Inverse variance weighted | 24.196 | 35 | 0.915 | |
| Ascorbic acid 3-sulfate levels | MR-Egger | 36.414 | 34 | 0.357 | |
| Ascorbic acid 3-sulfate levels | Inverse variance weighted | 36.414 | 35 | 0.403 | |
| X-23644 levels | MR-Egger | 37.286 | 34 | 0.320 | |
| X-23644 levels | Inverse variance weighted | 38.437 | 35 | 0.317 | |
| X-24337 levels | MR-Egger | 40.106 | 34 | 0.218 | |
| X-24337 levels | Inverse variance weighted | 40.218 | 35 | 0.250 | |
| X-24699 levels | MR-Egger | 33.458 | 34 | 0.494 | |
| X-24699 levels | Inverse variance weighted | 33.645 | 35 | 0.533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Xia, T.; Zhou, X.; Xing, X.; Zhu, Y.; Zhang, Y.; Xiong, L. Causal Relationship Between Cerebrospinal Fluid Metabolites and Intervertebral Disc Disease: A Bidirectional Mendelian Randomization Study. Diagnostics 2025, 15, 1526. https://doi.org/10.3390/diagnostics15121526
Xiao J, Xia T, Zhou X, Xing X, Zhu Y, Zhang Y, Xiong L. Causal Relationship Between Cerebrospinal Fluid Metabolites and Intervertebral Disc Disease: A Bidirectional Mendelian Randomization Study. Diagnostics. 2025; 15(12):1526. https://doi.org/10.3390/diagnostics15121526
Chicago/Turabian StyleXiao, Jiheng, Tianyi Xia, Xianglong Zhou, Xin Xing, Yanbin Zhu, Yingze Zhang, and Liming Xiong. 2025. "Causal Relationship Between Cerebrospinal Fluid Metabolites and Intervertebral Disc Disease: A Bidirectional Mendelian Randomization Study" Diagnostics 15, no. 12: 1526. https://doi.org/10.3390/diagnostics15121526
APA StyleXiao, J., Xia, T., Zhou, X., Xing, X., Zhu, Y., Zhang, Y., & Xiong, L. (2025). Causal Relationship Between Cerebrospinal Fluid Metabolites and Intervertebral Disc Disease: A Bidirectional Mendelian Randomization Study. Diagnostics, 15(12), 1526. https://doi.org/10.3390/diagnostics15121526






