19-Gauge Versus 22-Gauge Franseen Needles, Comparison of the Histological Diagnostic Capability of Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Autoimmune Pancreatitis: A Multicenter Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. EUS-FNB
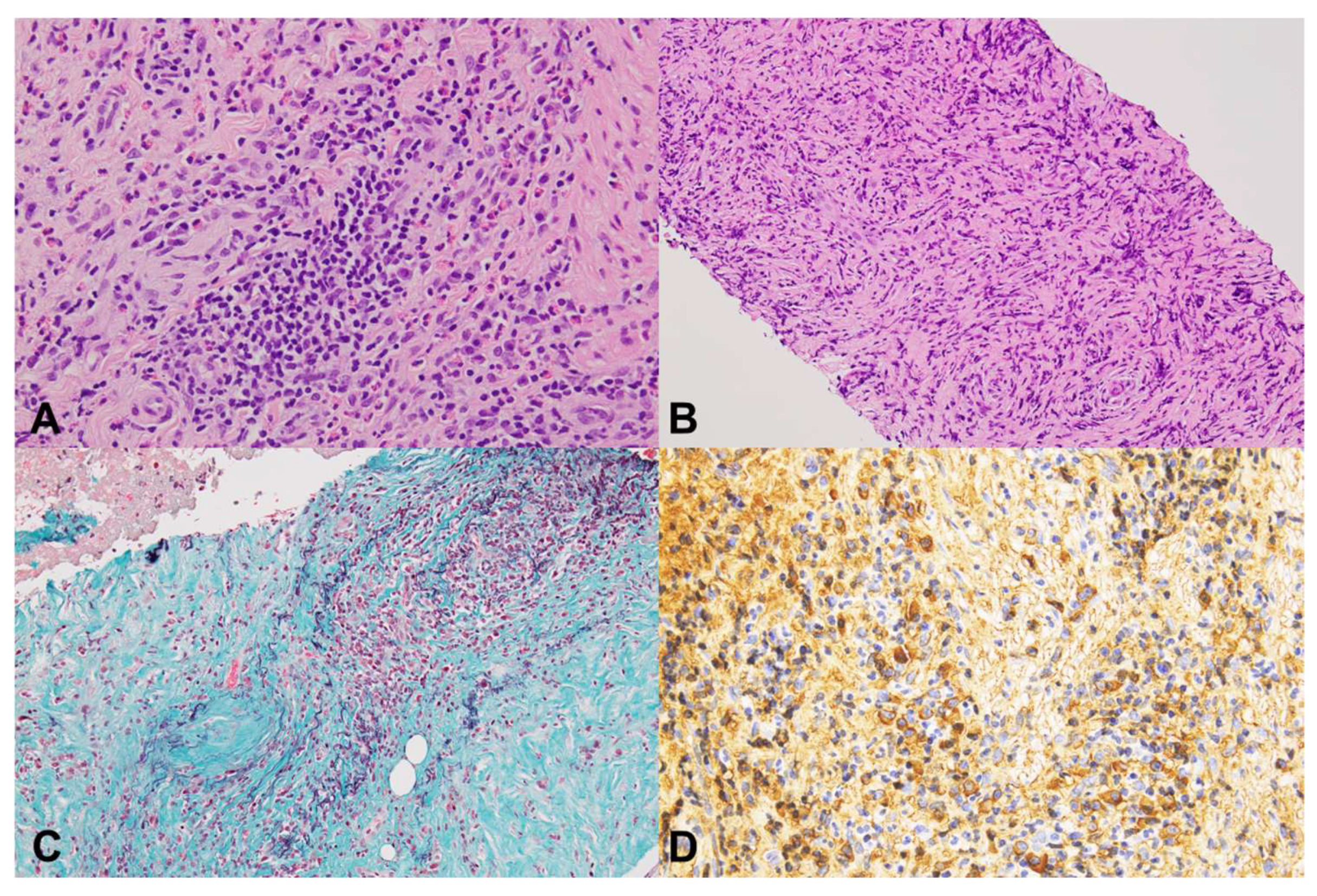
2.3. Pathological Evaluations
2.4. Study Definitions
2.5. Study Outcomes and Statistical Analysis
3. Results
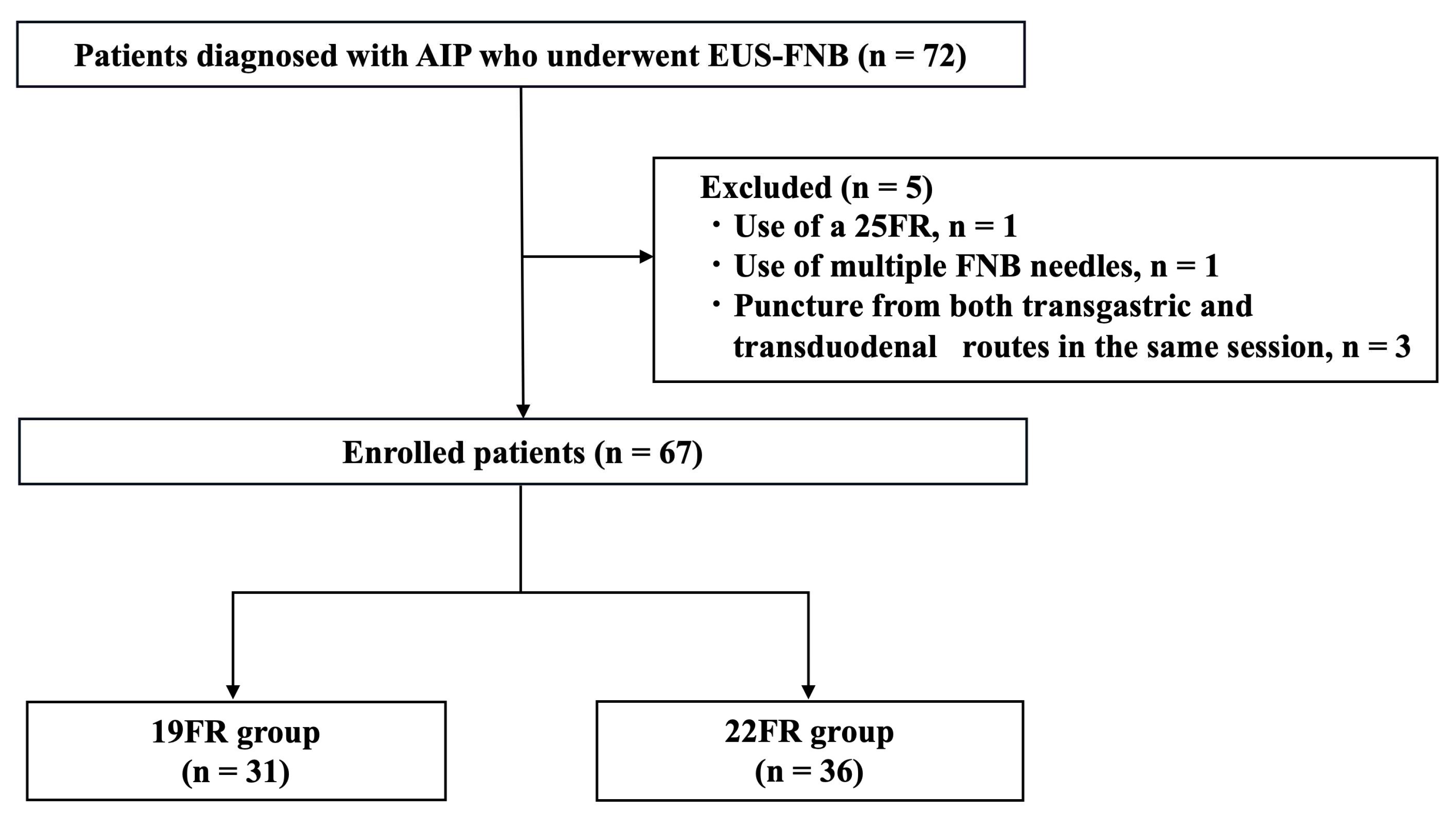
3.1. Patient’s Identification and Characteristics
3.2. EUS-FNB Procedures
3.3. Comparison of Histological Outcomes Between 19FR and 22FR
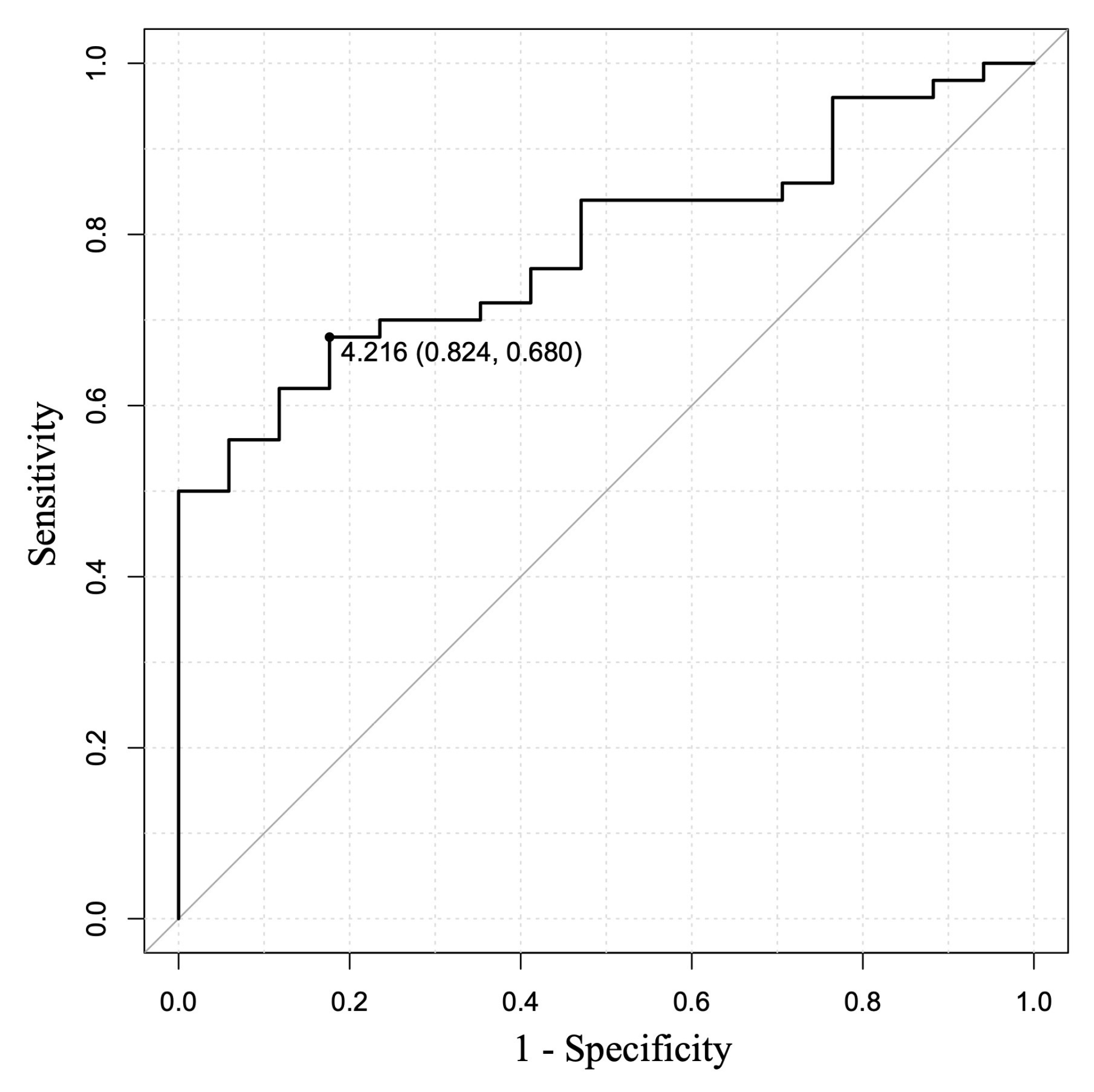
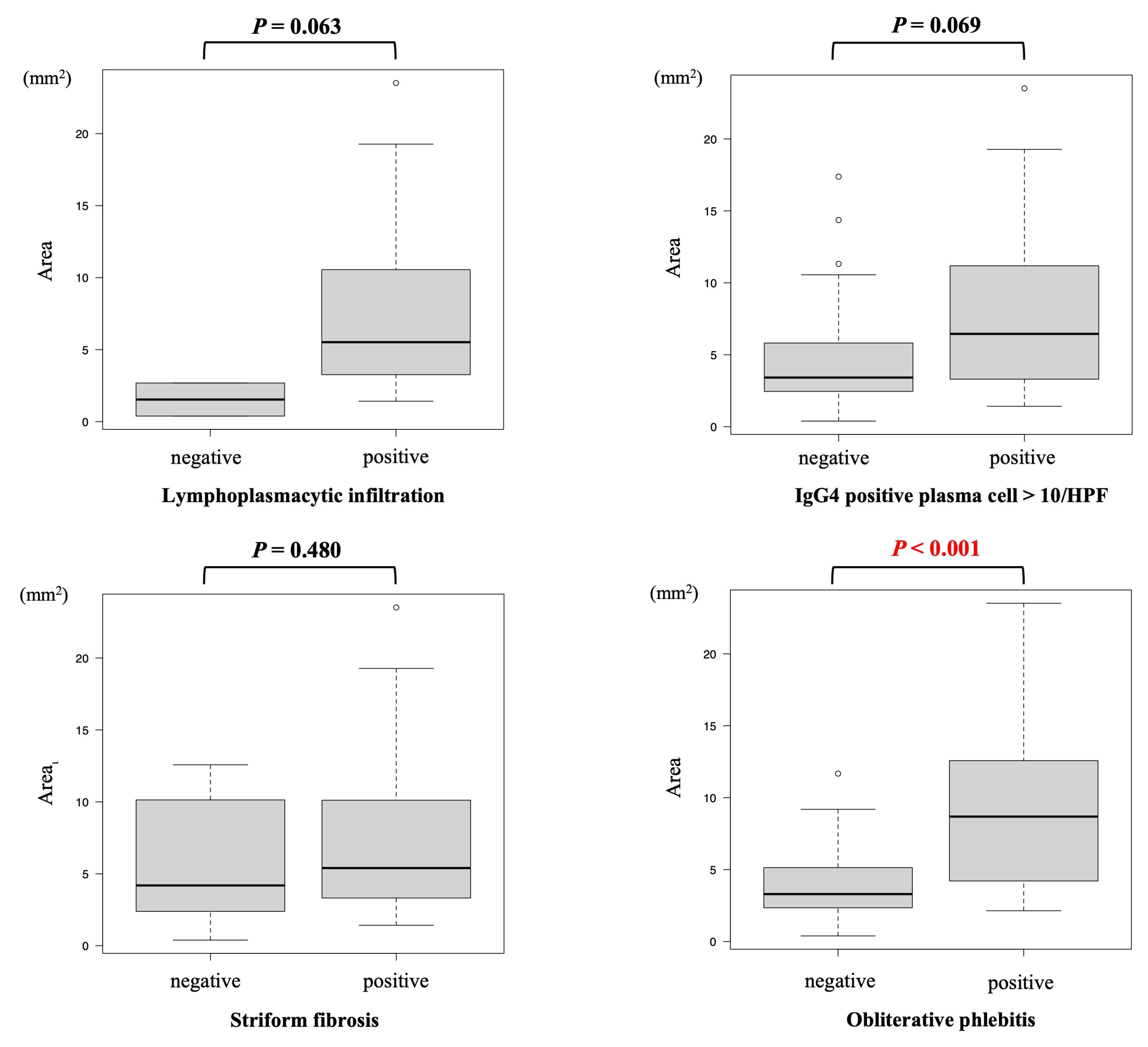
3.4. Relationship of Histological Tissue Area and Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIP | Autoimmune pancreatitis |
| EUS-FNA | Endoscopic ultrasound–guided fine needle aspiration |
| EUS-FNB | Endoscopic ultrasound–guided fine needle biopsy |
| EVG | Elastica van Gieson |
| FR | Franseen needle |
| HE | Hematoxylin and eosin |
| HPF | High-power field |
| ICDC | International Consensus Diagnostic Criteria |
| IDCP | Idiopathic duct-centric pancreatitis |
| LPSP | Lymphoplasmacytic sclerosing pancreatitis |
| OOI | Other organ involvement |
| ROC | Receiver operating characteristic |
References
- Yoshida, K.; Toki, F.; Takeuchi, T.; Watanabe, S.; Shiratori, K.; Hayashi, N. Chronic Pancreatitis Caused by an Autoimmune Abnormality. Proposal of the Concept of Autoimmune Pancreatitis. Dig. Dis. Sci. 1995, 40, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Rivera, M.; Ting, D.T.; Deshpande, V. The histological diagnosis of IgG4-related disease on small biopsies: Challenges and pitfalls. Histopathology 2019, 74, 688–698. [Google Scholar] [CrossRef]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.H.; Klöppel, G.; Lerch, M.M.; Löhr, M.; et al. International Consensus Diagnostic Criteria for Autoimmune Pancreatitis Guidelines of the International Association of Pancreatology. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef]
- Hart, P.A.; Zen, Y.; Chari, S.T. Recent Advances in Autoimmune Pancreatitis. Gastroenterology 2015, 149, 39–51. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Pancreas Society; the Research Committee of Intractable Disease for IgG4-Related Disease Supported by the Japanese Ministry of Health, Labor and Welfare. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2020. Suizo 2020, 35, 465–550. (In Japanese) [Google Scholar] [CrossRef]
- Notohara, K.; Nishimori, I.; Mizuno, N.; Okazaki, K.; Ito, T.; Kawa, S.; Egawa, S.; Kihara, Y.; Kanno, A.; Masamune, A.; et al. Clinicopathological Features of Type 2 Autoimmune Pancreatitis in Japan. Pancreas 2015, 44, 1072–1077. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Meng, X.; Li, R.; Leung, P.S.C.; Gershwin, M.E.; Zhang, S.; Sun, S.; Song, J. Autoimmune pancreatitis type 2 (idiopathic duct-centric pancreatitis): A comprehensive review. J. Autoimmun. 2023, 140, 103121. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Doi, S.; Ando, N.; Nakashima, M.; Adachi, S.; Hirose, Y.; Mukai, T.; Iwata, K.; Tomita, E.; et al. Use of Samples From Endoscopic Ultrasound-Guided 19-Gauge Fine-Needle Aspiration in Diagnosis of Autoimmune Pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 316–322. [Google Scholar] [CrossRef]
- Kurita, A.; Yasukawa, S.; Zen, Y.; Yoshimura, K.; Ogura, T.; Ozawa, E.; Okabe, Y.; Asada, M.; Nebiki, H.; Shigekawa, M.; et al. Comparison of a 22-gauge Franseen-tip needle with a 20-gauge forward-bevel needle for the diagnosis of type 1 autoimmune pancreatitis: A prospective, randomized, controlled, multicenter study (COMPAS study). Gastrointest. Endosc. 2020, 91, 373–381.e2. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Suhara, H.; Hayashi, D.; Hiramatsu, T.; Matsubara, H.; Suzuki, T.; Kuwahara, T.; Ishikawa, E.; et al. Usefulness of endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of autoimmune pancreatitis using a 22-gauge Franseen needle: A prospective multicenter study. Endoscopy 2020, 52, 978–985. [Google Scholar] [CrossRef]
- Ko, S.W.; Song, T.J.; Oh, D.; Yoon, S.B.; Oh, C.H.; Park, J.S.; Chang, J.H.; Yoon, J.H. Comparison of clinical/histological outcomes according to puncture sites in endoscopic ultrasound-guided fine needle biopsy for large pancreatic masses: Multicenter randomized prospective pilot study. Dig. Endosc. 2024, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Miwata, T.; Nagai, H.; Ikeda, E.; Ando, K.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Tamada, K.; Fukushima, N.; et al. Endoscopic ultrasound-guided pancreatic sampling for the histopathological diagnosis of autoimmune pancreatitis. Dig. Endosc. 2022, 34, 420–427. [Google Scholar] [CrossRef] [PubMed]
- de Pretis, N.; Crinò, S.F.; Frulloni, L. The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis. Diagnostics 2021, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Notohara, K.; Kamisawa, T.; Kanno, A.; Naitoh, I.; Iwasaki, E.; Shimizu, K.; Kuraishi, Y.; Motoya, M.; Kodama, Y.; Kasashima, S.; et al. Efficacy and limitations of the histological diagnosis of type 1 autoimmune pancreatitis with endoscopic ultrasound-guided fine needle biopsy with large tissue amounts. Pancreatology 2020, 20, 834–843. [Google Scholar] [CrossRef]
- Huh, G.; Song, T.J. A new era for autoimmune pancreatitis diagnosis: Fine-needle biopsy outperforms fine-needle aspiration in endoscopic ultrasound-guided tissue acquisition. Clin. Endosc. 2025, 58, 406–408. [Google Scholar] [CrossRef]
- Kurita, Y.; Kubota, K.; Harada, J.; Honda, Y.; Yamazaki, Y.; Iizuka, T.; Nihei, S.; Hasegawa, S.; Hosono, K.; Kobayashi, N.; et al. Endoscopic ultrasound-guided fine-needle biopsy needle can facilitate histological diagnosis of type 1 autoimmune pancreatitis. J. Hepatobiliary Pancreat. Sci. 2025, 32, 238–245. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Ueki, T.; Noma, Y.; Omonishi, K.; Ohno, K.; Kawahara, S.; Oda, T.; Kato, H.; Okada, H. Utility of a 21-gauge Menghini-type biopsy needle with the rolling method for an endoscopic ultrasound-guided histological diagnosis of autoimmune pancreatitis: A retrospective study. BMC Gastroenterol. 2021, 21, 21. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Wang, X.; Wan, D.; Xu, J.; Jiang, M.; Liu, Y.; Liu, C.; Tu, Y.; Huang, H.; et al. EUS-guided fine-needle biopsy versus fine-needle aspiration for histopathological evidence for type 1 autoimmune pancreatitis: A single-center retrospective study in China. Endosc. Ultrasound 2024, 13, 351–360. [Google Scholar] [CrossRef]
- Chhoda, A.; Rustagi, T. EUS-guided needle biopsy for autoimmune pancreatitis. Clin. J. Gastroenterol. 2020, 13, 669–677. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Mukai, T.; Doi, S.; Nakashima, M.; Uemura, S.; Mabuchi, M.; Shimizu, M.; Hatano, Y.; Hara, A.; et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study). Gastrointest. Endosc. 2015, 81, 177–185. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Moon, S.H.; Song, T.J.; Kim, J.H.; Kim, M.H. Endoscopic ultrasound-guided fine needle aspiration versus biopsy for diagnosis of autoimmune pancreatitis: Systematic review and comparative meta-analysis. Dig. Endosc. 2021, 33, 1024–1033. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamao, K.; Mizutani, Y.; Iida, T.; Uetsuki, K.; Shimoyama, Y.; Nakamura, M.; Furukawa, K.; Yamamura, T.; Kawashima, H. A prospective study on the histological evaluation of type 1 autoimmune pancreatitis using endoscopic ultrasound-guided fine needle biopsy with a 19-gauge Franseen needle. J. Hepatobiliary Pancreat. Sci. 2024, 31, 581–590. [Google Scholar] [CrossRef]
- Crinò, S.F.; Conti Bellocchi, M.C.; Di Mitri, R.; Inzani, F.; Rimbaș, M.; Lisotti, A.; Manfredi, G.; Teoh, A.Y.B.; Mangiavillano, B.; Sendino, O.; et al. Wet-suction versus slow-pull technique for endoscopic ultrasound-guided fine-needle biopsy: A multicenter, randomized, crossover trial. Endoscopy 2023, 55, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, J.O.; Durko, Ł.; Malecka-Wojciesko, E. The Latest Advancements in Diagnostic Role of Endosonography of Pancreatic Lesions. J. Clin. Med. 2023, 12, 4630. [Google Scholar] [CrossRef]
- Takahashi, K.; Yasuda, I.; Hanaoka, T.; Hayashi, Y.; Motoo, I.; Kajiura, S.; Ando, T.; Fujinami, H.; Tajiri, K.; Imura, J.; et al. Comparison of Histological Sample Volumes among Various Endoscopic Ultrasound-Guided Biopsy Needles. J. Clin. Med. 2021, 10, 3560. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ohno, E.; Mizutani, Y.; Iida, T.; Uetsuki, K.; Yashika, J.; Yamada, K.; Gibo, N.; Aoki, T.; Kataoka, K.; et al. Usefulness of Macroscopic On-Site Evaluation Using a Stereomicroscope during EUS-FNB for Diagnosing Solid Pancreatic Lesions. Can. J. Gastroenterol. Hepatol. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Barresi, L.; Cannizzaro, R.; Antonini, F.; Triantafyllou, K.; Tziatzios, G.; Muscatiello, N.; Hart, P.A.; Wani, S. Diagnostic yield of endoscopic ultrasound-guided tissue acquisition in autoimmune pancreatitis: A systematic review and meta-analysis. Endosc. Int. Open 2021, 09, E66–E75. [Google Scholar] [CrossRef]
- Oppong, K.W.; Maheshwari, P.; Nayar, M.K.; Darne, A.; Parkinson, D.; Leeds, J.S.; Haugk, B. Utility of endoscopic ultrasound-guided fine-needle biopsy in the diagnosis of type 1 autoimmune pancreatitis. Endosc. Int. Open 2020, 08, E1855–E1861. [Google Scholar] [CrossRef]
- Miyabe, K.; Notohara, K.; Nakazawa, T.; Hayashi, K.; Naitoh, I.; Okumura, F.; Shimizu, S.; Yoshida, M.; Yamashita, H.; Takahashi, S.; et al. Histological evaluation of obliterative phlebitis for the diagnosis of autoimmune pancreatitis. J. Gastroenterol. 2014, 49, 715–726. [Google Scholar] [CrossRef]
- Kanno, A.; Yasuda, I.; Irisawa, A.; Hara, K.; Ashida, R.; Iwashita, T.; Takenaka, M.; Katanuma, A.; Takikawa, T.; Kubota, K.; et al. Adverse events of endoscopic ultrasound-guided fine-needle aspiration for histologic diagnosis in Japanese tertiary centers: Multicenter retrospective study. Dig. Endosc. 2021, 33, 1146–1157. [Google Scholar] [CrossRef] [PubMed]


| 19 FR | 22 FR | p-Value | |
|---|---|---|---|
| (n = 31) | (n = 36) | ||
| Sex, male, n (%) | 29 (93.5) | 26 (72.2) | 0.028 |
| Age, yo, median (range) | 74 (21–87) | 69 (45–80) | 0.365 |
| Chief complaint, n (%) | 0.139 | ||
| Pancreatic enlargement | 23 (74.2) | 16 (44.4) | |
| Jaundice or liver dysfunction | 6 (19.4) | 13 (36.1) | |
| Abdominal pain | 2 (6.5) | 4 (11.1) | |
| Others | 0 | 3 (8.3) | |
| Pancreatic enlargement, n (%) | 0.243 | ||
| Diffuse (≥2/3) | 11 (35.5) | 16 (44.4) | |
| Segmental (1/3–2/3) | 10 (32.3) | 5 (13.9) | |
| Focal (<1/3) | 10 (32.3) | 15 (41.7) | |
| Capsule-like rim, n (%) | 20 (64.5) | 14 (38.9) | 0.051 |
| Serum IgG4, mg/dL, median (range) | 359 (8–2710) | 406 (75.1–1690) | 0.930 |
| IgG4 ≥ 135 mg/dL, n (%) | 25 (80.6) | 31 (86.1) | 0.742 |
| IgG4 ≥ 270 mg/dL, n (%) | 20 (64.5) | 23 (63.9) | 1.000 |
| Other organ involvement, n (%) | |||
| Sclerosing cholangitis | 6 (19.4) | 10 (27.8) | 0.567 |
| Retroperitoneal fibrosis | 9 (29) | 7 (19.4) | 0.401 |
| IgG4-related respiratory disease | 0 | 2 (5.6) | 0.495 |
| Sialadenitis | 0 | 2 (5.6) | 0.495 |
| Dacryoadenitis | 0 | 2 (5.6) | 0.495 |
| Hypophysitis | 1 (3.2) | 1 (2.8) | 1.000 |
| Ulcerative colitis | 1 (3.2) | 0 | 1.000 |
| IgG4-related kidney disease | 0 | 1 (2.8) | 1.000 |
| Diabetes Mellitus, n (%) | 12 (38.7) | 17 (47.2) | 0.622 |
| Treatment, n (%) | |||
| Steroid administration | 17 (54.8) | 26 (72.2) | 0.202 |
| 19 FR | 22 FR | p-Value | |
|---|---|---|---|
| (n = 31) | (n = 36) | ||
| Route for puncture, n (%) | <0.001 | ||
| Transgastric | 30 (96.8) | 21 (58.3) | |
| Transduodenal | 1 (3.2) | 15 (41.7) | |
| Number of passes, median (range) | 2 (1–5) | 2 (1–4) | 0.543 |
| Adverse events, n (%) | |||
| Bleeding | 1 (3.2) | 0 | 0.460 |
| 19 FR | 22 FR | p-Value | |
|---|---|---|---|
| (n = 31) | (n = 36) | ||
| Specimen adequacy, n (%) | 31 (100) | 35 (97.2) | 1.000 |
| Pathological findings, n (%) | |||
| Lymphoplasmacytic infiltration | 31 (100) | 34 (94.4) | 0.495 |
| Storiform fibrosis | 26 (83.9) | 26 (72.2) | 0.379 |
| Obliterative phlebitis | 24 (77.4) | 14 (38.9) | 0.003 |
| IgG4 positive plasma cells > 10/HPF | 22 (71.0) | 24 (66.7) | 0.795 |
| ICDC diagnostic level, n (%) | |||
| Level 0 (items ≤ 1) | 0 | 3 (8.3) | 0.243 |
| Level 1 (items ≥ 3) | 28 (90.3) | 22 (61.1) | 0.010 |
| Level 2 (2 items) | 3 (9.7) | 11 (30.6) | 0.068 |
| Level 1 or 2 (items ≥ 2) | 31 (100) | 33 (91.7) | 0.243 |
| Tissue area, mm2/session, median (range) | 9.19 (2.14–23.51) | 3.36 (0.39–12.58) | <0.001 |
| Tissue area, mm2/pass, median (range) | 4.82 (0.72–12.67) | 1.83 (0.20–12.56) | <0.001 |
| Items of Histological Findings, n (%) | Tissue Area, mm2, Median (Range) | |
|---|---|---|
| 0 | 2 (3.0) | 1.54 (0.39–2.68) |
| 1 | 1 (1.5) | 2.99 |
| 2 | 14 (20.9) | 3.38 (1.58–7.25) |
| 3 | 28 (41.8) | 5.88 (1.42–17.28) |
| 4 | 22 (32.8) | 8.41 (2.16–23.51) |
| Histological Finding | Tissue Area, mm2, Median, (Range) | p-Value | ||
|---|---|---|---|---|
| Lymphoplasmacytic infiltration | positive | 5.52 (1.42–23.51) |  | 0.063 |
| negative | 1.54 (0.39–2.68) | |||
| Storiform fibrosis | positive | 5.40 (1.42–23.51) |  | 0.480 |
| negative | 4.19 (0.39–12.58) | |||
| Obliterative phlebitis | positive | 8.69 (2.14–23.51) |  | <0.001 |
| negative | 3.30 (0.39–11.68) | |||
| IgG4 positive plasma cell > 10/HPF | positive | 6.45 (1.42–23.51) |  | 0.069 |
| negative | 3.42 (0.39–17.38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwata, S.; Iwashita, T.; Ohashi, Y.; Senju, A.; Tezuka, R.; Uemura, S.; Yoshida, K.; Maruta, A.; Iwasa, Y.; Okuno, M.; et al. 19-Gauge Versus 22-Gauge Franseen Needles, Comparison of the Histological Diagnostic Capability of Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Autoimmune Pancreatitis: A Multicenter Retrospective Cohort Study. Diagnostics 2025, 15, 1496. https://doi.org/10.3390/diagnostics15121496
Iwata S, Iwashita T, Ohashi Y, Senju A, Tezuka R, Uemura S, Yoshida K, Maruta A, Iwasa Y, Okuno M, et al. 19-Gauge Versus 22-Gauge Franseen Needles, Comparison of the Histological Diagnostic Capability of Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Autoimmune Pancreatitis: A Multicenter Retrospective Cohort Study. Diagnostics. 2025; 15(12):1496. https://doi.org/10.3390/diagnostics15121496
Chicago/Turabian StyleIwata, Shota, Takuji Iwashita, Yosuke Ohashi, Akihiko Senju, Ryuichi Tezuka, Shinya Uemura, Kensaku Yoshida, Akinori Maruta, Yuhei Iwasa, Mitsuru Okuno, and et al. 2025. "19-Gauge Versus 22-Gauge Franseen Needles, Comparison of the Histological Diagnostic Capability of Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Autoimmune Pancreatitis: A Multicenter Retrospective Cohort Study" Diagnostics 15, no. 12: 1496. https://doi.org/10.3390/diagnostics15121496
APA StyleIwata, S., Iwashita, T., Ohashi, Y., Senju, A., Tezuka, R., Uemura, S., Yoshida, K., Maruta, A., Iwasa, Y., Okuno, M., Iwata, K., Miyazaki, T., & Shimizu, M. (2025). 19-Gauge Versus 22-Gauge Franseen Needles, Comparison of the Histological Diagnostic Capability of Endoscopic Ultrasound-Guided Fine-Needle Biopsy for Autoimmune Pancreatitis: A Multicenter Retrospective Cohort Study. Diagnostics, 15(12), 1496. https://doi.org/10.3390/diagnostics15121496







