Ultra-Long-Term CT Angiography Evaluation of Patients Treated with Covered Stents for Visceral Aneurysms: A Single Center Case Series
Abstract
1. Introduction
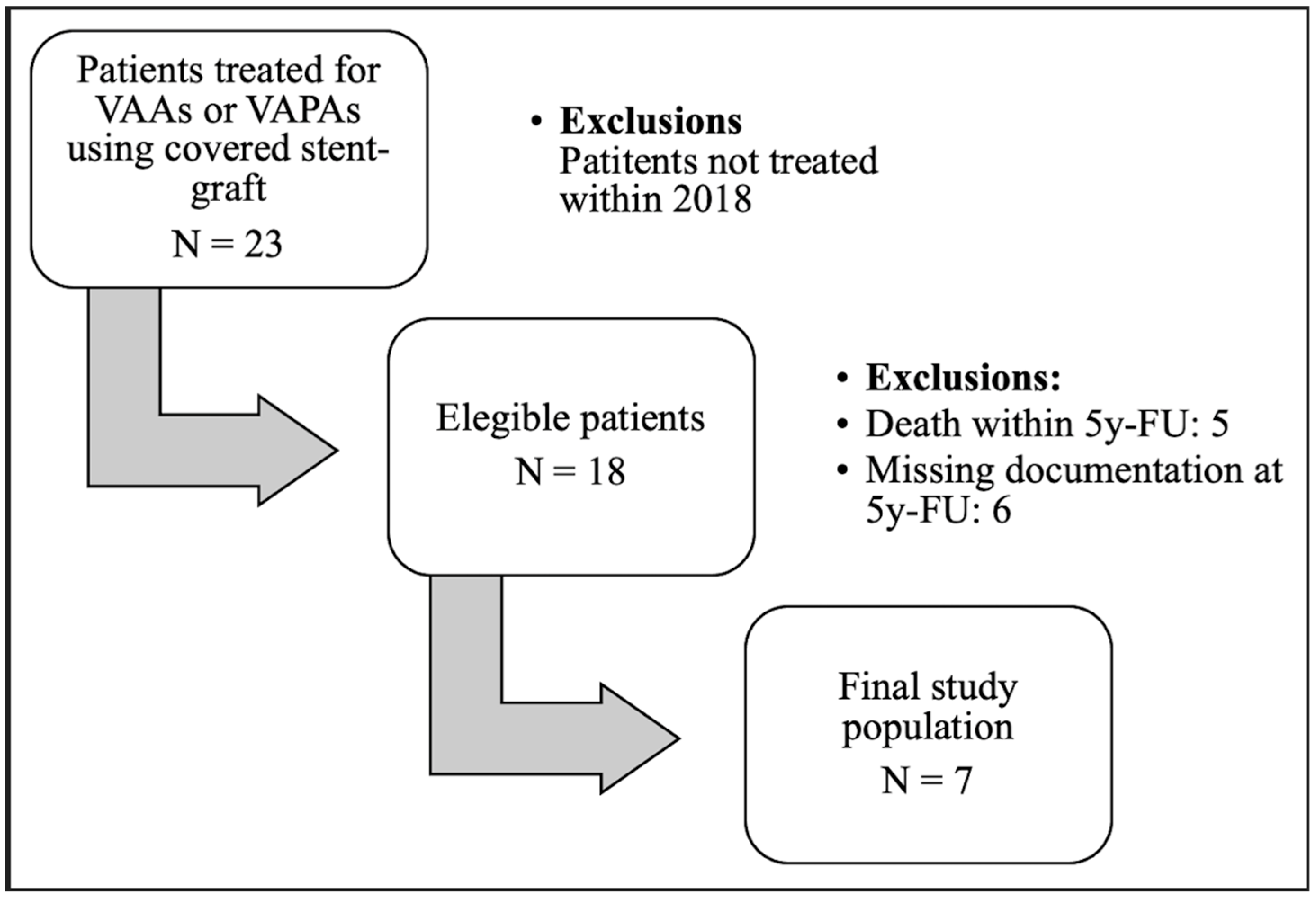
2. Materials and Methods
2.1. Study Design
2.2. Follow-Up and CT Protocol
2.3. Population
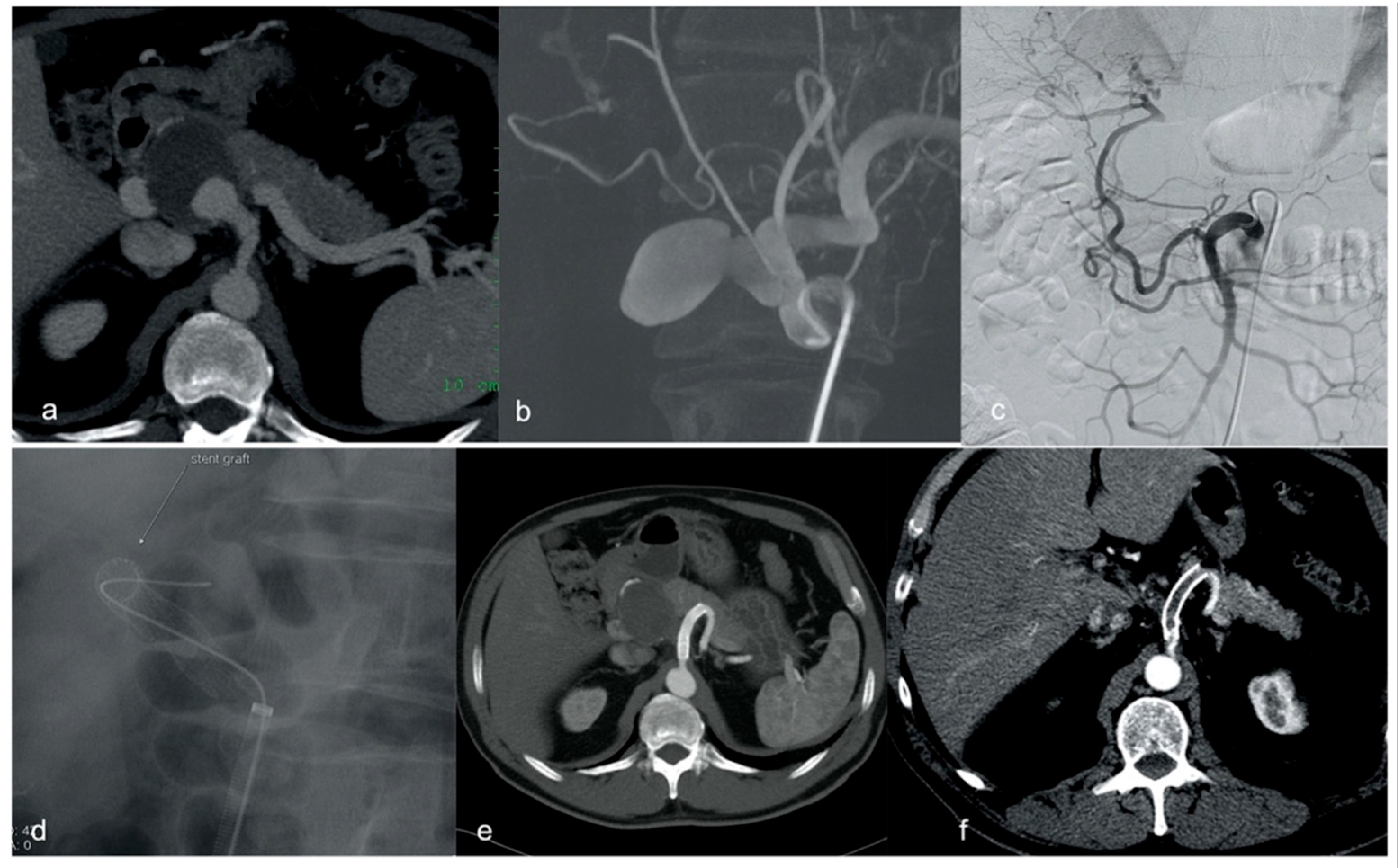
2.4. Procedural Technique
2.5. Outcomes Measures
2.6. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.K.; Hsieh, H.C.; Tsai, F.C.; Chang, S.H.; Lu, M.S.; Ko, P.J. Visceral artery aneurysm: Risk factor analysis and therapeutic opinion. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Johal, M.; Kalaravy, M.; Ali, F.; Barve, R.; Francis, C.T.; Harky, A.; Ahmed, A. Evolving Diagnostic and Therapeutic Options for Visceral Artery Aneurysms. Ann. Vasc. Surg. 2021, 76, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Vittoria De Martini, I.; Pfammatter, T.; Puippe, G.; Clavien, P.A.; Alkadhi, H. Frequency and causes of delayed diagnosis of visceral artery pseudoaneurysms with CT: Lessons learned. Eur. J. Radiol. Open 2020, 7, 100221. [Google Scholar] [CrossRef] [PubMed]
- Juntermanns, B.; Bernheim, J.; Karaindros, K.; Walensi, M.; Hoffmann, J.N. Visceral artery aneurysms. Gefasschirurgie 2018, 23 (Suppl. S1), 19–22. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Colombo, M.; Alparone, M.; Agostini, G.; Bertoglio, L.; Sallemi, C.; Salvioni, M.; Gusmini, S. Endovascular Treatment of Visceral Artery Aneurysms and Pseudoaneurysms in 100 Patients: Covered Stenting vs Transcatheter Embolization. J. Endovasc. Ther. 2017, 24, 709–717. [Google Scholar] [CrossRef]
- Sousa, J.; Costa, D.; Mansilha, A. Visceral artery aneurysms: Review on indications and current treatment strategies. Int. Angiol. 2019, 38, 381–394. [Google Scholar] [CrossRef]
- Venturini, M.; Piacentino, F.; Coppola, A.; Bettoni, V.; Macchi, E.; De Marchi, G.; Curti, M.; Ossola, C.; Marra, P.; Palmisano, A.; et al. Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. J. Clin. Med. 2021, 10, 2520. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Colombo, M.; Panzeri, M.; Gusmini, S.; Sallemi, C.; Salvioni, M.; Lanza, C.; Agostini, G.; Balzano, G.; et al. Endovascular Repair of 40 Visceral Artery Aneurysms and Pseudoaneurysms with the Viabahn Stent-Graft: Technical Aspects, Clinical Outcome and Mid-Term Patency. Cardiovasc. Interv. Radiol. 2018, 41, 385–397. [Google Scholar] [CrossRef]
- Pitton, M.B.; Dappa, E.; Jungmann, F.; Kloeckner, R.; Schotten, S.; Wirth, G.M.; Mittler, J.; Lang, H.; Mildenberger, P.; Kreitner, K.F.; et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur. Radiol. 2015, 25, 2004–2014. [Google Scholar] [CrossRef]
- Rossi, M.; Krokidis, M.; Kashef, E.; Peynircioglu, B.; Tipaldi, M.A. CIRSE Standards of Practice for the Endovascular Treatment of Visceral and Renal Artery Aneurysms and Pseudoaneurysms. Cardiovasc. Interv. Radiol. 2024, 47, 26–35. [Google Scholar] [CrossRef]
- Pratesi, C.; Esposito, D.; Martini, R.; Novali, C.; Zaninelli, A.; Annese, A.L.; Baggi, P.; Bellosta, R.; Bianchini Massoni, C.; Bonardelli, S.; et al. Guidelines on the diagnosis, treatment and management of visceral and renal arteries aneurysms: A joint assessment by the Italian Societies of Vascular and Endovascular Surgery (SICVE) and Medical and Interventional Radiology (SIRM). J. Cardiovasc. Surg 2024, 65, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Belli, A.M.; Markose, G.; Morgan, R. The role of interventional radiology in the management of abdominal visceral artery aneurysms. Cardiovasc Interv. Radiol. 2012, 35, 234–243. [Google Scholar] [CrossRef]
- Batagini, N.C.; El-Arousy, H.; Clair, D.G.; Kirksey, L. Open versus Endovascular Treatment of Visceral Artery Aneurysms and Pseudoaneurysms. Ann. Vasc. Surg. 2016, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rabuffi, P.; Bruni, A.; Antonuccio, E.G.M.; Ambrogi, C.; Vagnarelli, S. Treatment of visceral artery aneurysms and pseudoaneurysms with the use of cerebral flow diverting stents: Initial experience. CVIR Endovasc. 2020, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Tipaldi, M.A.; Krokidis, M.; Orgera, G.; Pignatelli, M.; Ronconi, E.; Laurino, F.; Laughi, A.; Rossi, M. Endovascular management of giant visceral artery aneurysms. Sci. Rep. 2021, 11, 700. [Google Scholar] [CrossRef]
- Cappucci, M.; Zarco, F.; Orgera, G.; López-Rueda, A.; Moreno, J.; Laurino, F.; Barnes, D.; Tipaldi, M.A.; Gomez, F.; Fernandez, M.J.; et al. Endovascular treatment of visceral artery aneurysms and pseudoaneurysms with stent-graft: Analysis of immediate and long-term results. Cir. Esp. 2017, 95, 283–292. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, Z.; Huang, L.; Guo, L.; Lu, W.; Zhang, H.; He, Y.; Tian, L.; Li, D.; Wang, X.; et al. Covered Stents for Treatment of Visceral Artery Aneurysms: A Multicenter Study. J. Vasc. Interv. Radiol. 2022, 33, 640–647. [Google Scholar] [CrossRef]
- Angle, J.F.; Siddiqi, N.H.; Wallace, M.J.; Kundu, S.; Stokes, L.; Wojak, J.C.; Cardella, J.F. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2010, 21, 1479–1486. [Google Scholar] [CrossRef]
- Shukla, A.J.; Eid, R.; Fish, L.; Avgerinos, E.; Marone, L.; Makaroun, M.; Chaer, R.A. Contemporary outcomes of intact and ruptured visceral artery aneurysms. J. Vasc. Surg. 2015, 61, 1442–1447. [Google Scholar] [CrossRef]
- Khairallah, M.K.; Morgan, R.A.; Das, R. Technical considerations of endovascular management of true visceral artery aneurysms. CVIR Endovasc. 2023, 6, 31. [Google Scholar] [CrossRef]
- Loffroy, R.; Favelier, S.; Pottecher, P.; Genson, P.Y.; Estivalet, L.; Gehin, S.; Cercueil, P.J.; Krausé, D. Endovascular management of visceral artery aneurysms: When to watch, when to intervene? World J. Radiol. 2015, 7, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Colombi, D.; Bodini, F.C.; Bossalini, M.; Rossi, B.; Michieletti, E. Extracranial Visceral Artery Aneurysms/Pseudoaneurysms Repaired with Flow Diverter Device Developed for Cerebral Aneurysms: Preliminary Results. Ann. Vasc. Surg. 2018, 53, 272.e1–272.e9. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.É.; Brennan, P.; Maingard, J.T.; Chandra, R.V.; Little, D.M.; Brooks, D.M.; Kok, H.K.; Asadi, H.; Lee, J.M. Treatment of Visceral Artery Aneurysms Using Novel Neurointerventional Devices and Techniques. J. Vasc. Interv. Radiol. 2019, 30, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Tipaldi, M.A.; Pisano, A.; Krokidis, M.; Laurino, F.; Corradini, L.G.; Lucatelli, P.; Venturini, M.; Laghi, A.; Rossi, M. Extravascular Migration of Thrombosed Covered Stents after Endovascular Exclusion of Splenic or Hepatic Artery Aneurysms and Pseudoaneurysms: An Underestimated Phenomenon. J. Vasc. Interv. Radiol. 2021, 32, 317–320. [Google Scholar] [CrossRef]
| Patient | Sex | Age | Type of Lesion | Location | Setting | Size (mm) | Type of Stent |
|---|---|---|---|---|---|---|---|
| 1 | Female | 55 | VAA | Splenic | Elective | 25 | Jostent 5 × 38 mm |
| 2 | Female | 52 | VAA | Splenic | Elective | 20 | Symbiot 5 × 30 mm |
| 3 | Male | 63 | VAPA | Hepatic | Elective | 42 | Viabahn 8 × 50 mm |
| 4 | Male | 27 | VAA | Renal | Elective | 25 | Jostent 5 × 19 mm |
| 5 | Female | 69 | VAA | Splenic | Elective | 25 | Viabahn 6 × 48 mm |
| 6 | Female | 45 | VAPA | Splenic | Emergency | 34 | Viabahn 5 × 50 mm |
| 7 | Male | 65 | VAA | Splenic | Elective | 72 | Viabahn 10 × 100 mm |
| Patient | Vascular Access | Sheath | Procedural Time | Aneurysm Configuration | Aneurysm Location | Anaesthesia | Vascular Access |
|---|---|---|---|---|---|---|---|
| 1 | Femoral | 8F | 90 min | Sacciform large neck | Proximal | Local | Femoral |
| 2 | Brachial | 6F | 120min | Sacciform short neck | Mid-way | Local | Brachial |
| 3 | Femoral | 8F | 100 min | Sacciform large neck | Proximal | Local | Femoral |
| 4 | Femoral | 6F | 80 min | Sacciform large neck | Distal | Local | Femoral |
| 5 | Brachial | 6F | 40 min | Sacciform large neck | Mid-way | Local | Brachial |
| 6 | Femoral | 6F | 80 min | Fusiform | Mid-way | Local | Femoral |
| 7 | Femoral | 8F | 90 min | Sacciform large neck | Proximal | Local | Femoral |
| Patient | Stent | FU-Duration (Months) | Stent-Graft Patency at 5 Years | Stent Occlusion (Months) | Stent Migration (Months) |
|---|---|---|---|---|---|
| 1 | Jostent 5 × 38 mm | 191 | Yes | - | - |
| 2 | Symbiot 5 × 30 mm | 102 | No | 6 | 102 |
| 3 | Viabahn 8 × 50 mm | 139 | Yes | 139 | - |
| 4 | Jostent 5 × 19 mm | 90 | Yes | - | - |
| 5 | Viabahn 6 × 48 mm | 169 | Yes | - | |
| 6 | Viabahn 5 × 50 mm | 79 | No | 6 | 60 |
| 7 | Viabahn 10 × 100 mm | 60 | Yes | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tipaldi, M.A.; Ubaldi, N.; Ronconi, E.; Ortenzi, M.; Arbia, F.; Orgera, G.; Krokidis, M.; Rossi, T.; Sirignano, P.; Rizzo, L.; et al. Ultra-Long-Term CT Angiography Evaluation of Patients Treated with Covered Stents for Visceral Aneurysms: A Single Center Case Series. Diagnostics 2025, 15, 1481. https://doi.org/10.3390/diagnostics15121481
Tipaldi MA, Ubaldi N, Ronconi E, Ortenzi M, Arbia F, Orgera G, Krokidis M, Rossi T, Sirignano P, Rizzo L, et al. Ultra-Long-Term CT Angiography Evaluation of Patients Treated with Covered Stents for Visceral Aneurysms: A Single Center Case Series. Diagnostics. 2025; 15(12):1481. https://doi.org/10.3390/diagnostics15121481
Chicago/Turabian StyleTipaldi, Marcello Andrea, Nicolò Ubaldi, Edoardo Ronconi, Michela Ortenzi, Francesco Arbia, Gianluigi Orgera, Miltiadis Krokidis, Tommaso Rossi, Pasqualino Sirignano, Luigi Rizzo, and et al. 2025. "Ultra-Long-Term CT Angiography Evaluation of Patients Treated with Covered Stents for Visceral Aneurysms: A Single Center Case Series" Diagnostics 15, no. 12: 1481. https://doi.org/10.3390/diagnostics15121481
APA StyleTipaldi, M. A., Ubaldi, N., Ronconi, E., Ortenzi, M., Arbia, F., Orgera, G., Krokidis, M., Rossi, T., Sirignano, P., Rizzo, L., & Rossi, M. (2025). Ultra-Long-Term CT Angiography Evaluation of Patients Treated with Covered Stents for Visceral Aneurysms: A Single Center Case Series. Diagnostics, 15(12), 1481. https://doi.org/10.3390/diagnostics15121481







