Endometriosis and Cardiovascular Disease: Exploring Pathophysiological Interconnections and Risk Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
- -
- Original studies, systematic reviews, or meta-analyses published in peer-reviewed journals within the last 15 years.
- -
- Human studies explicitly investigating the association between endometriosis and cardiovascular outcomes, including atherosclerosis, endothelial dysfunction, oxidative stress, and metabolic alterations.
- -
- Articles available in English and providing sufficient methodological detail.
- -
- Publications limited to in vitro or animal model studies without clinical translation.
- -
- Non-peer-reviewed materials such as editorials, commentaries, conference abstracts, and opinion pieces.
- -
- Studies unrelated to cardiovascular outcomes or lacking in relevance to the pathophysiological mechanisms discussed in this review.
3. Mechanisms Linking Endometriosis and Cardiovascular Disease
3.1. Associations Between Endometriosis and Cardiovascular Risk Factors
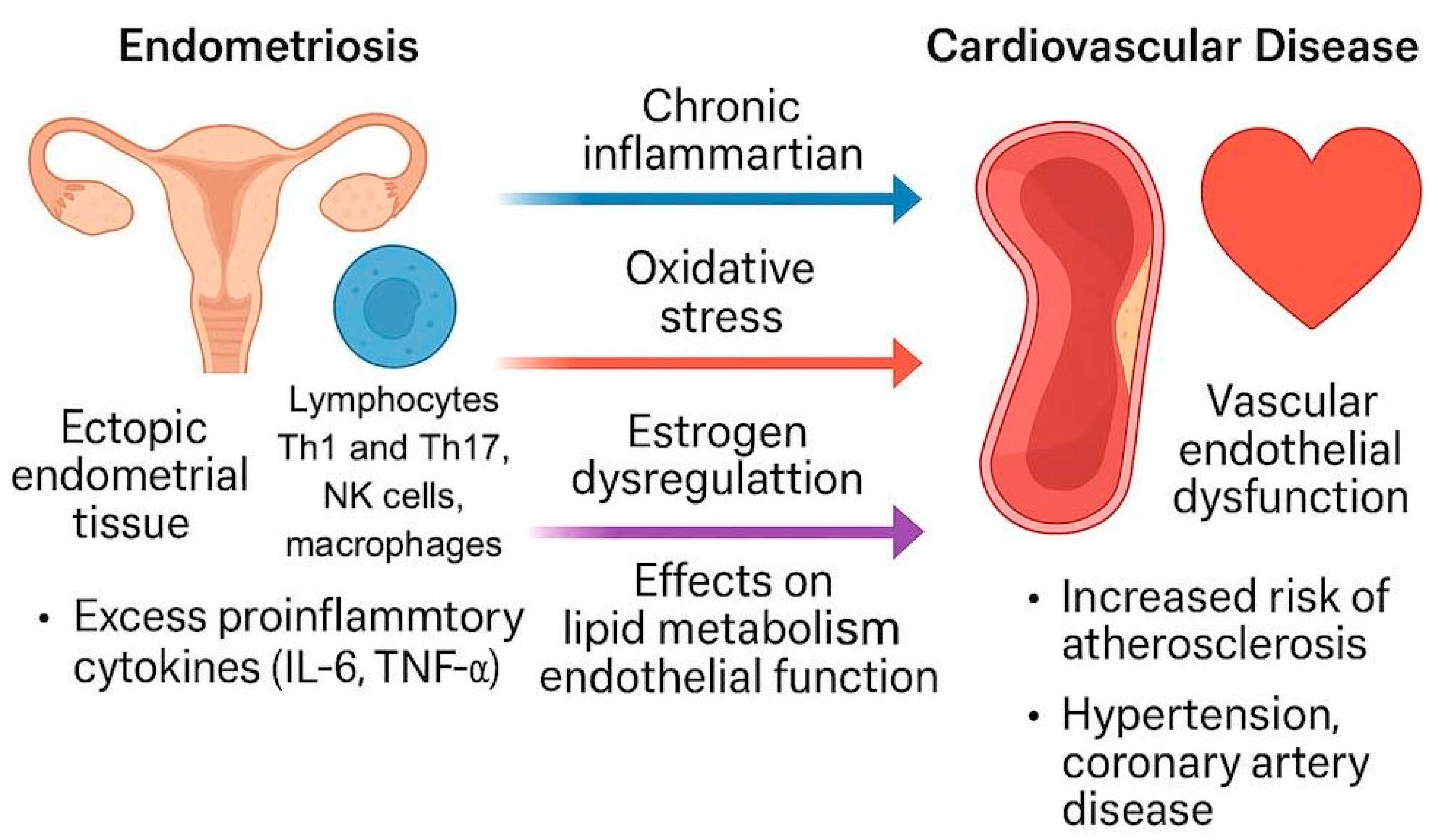
3.2. Endometriosis and Atherosclerosis Shared Pathophysiological Mechanisms (Figure 1)
3.3. Genetic Links Between Cardiovascular Disease and Endometriosis
3.4. Association of Endometriosis with Atherosclerosis and Its Complications
3.5. Supradiaphragmatic Endometriosis: Clinical Cases and Diagnostic Challenges
4. Implications for Women’s Health and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Endometriosis. World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed on 28 March 2025).
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Viganò, D.; Zara, F.; Usai, P. Irritable bowel syndrome and endometriosis: New insights for old diseases. Dig. Liver Dis. 2018, 50, 213–219. [Google Scholar] [CrossRef]
- Endometrioza i Ryzyko Sercowo-Naczyniowe Idą w Parze? Polpharma Dla Ciebie. Available online: https://polpharmadlaciebie.pl/wiedza/biuletyn-stetoskop/wydanie-listopad-2024/endometrioza-i-ryzyko-sercowo-naczyniowe-ida-w-parze (accessed on 28 March 2025).
- Chopyak, V.; Koval, H.D.; Havrylyuk, A.M.; Lishchuk-Yakymovych, K.A.; Potomkina, H.A.; Kurpisz, M. Immunopathogenesis of endometriosis—A novel look at an old problem. Cent. Eur. J. Immunol. 2022, 47, 109–116. [Google Scholar] [CrossRef]
- Cacciottola, L.; Donnez, J.; Dolmans, M.-M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? Int. J. Mol. Sci. 2021, 22, 7138. [Google Scholar] [CrossRef]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef]
- Doroftei, B.; Ilie, O.-D.; Balmus, I.-M.; Ciobica, A.; Maftei, R.; Scripcariu, I.; Simionescu, G.; Grab, D.; Stoian, I.; Ilea, C. Molecular and Clinical Insights on the Complex Interaction between Oxidative Stress, Apoptosis, and Endobiota in the Pathogenesis of Endometriosis. Diagnostics 2021, 11, 1434. [Google Scholar] [CrossRef]
- Gerlach, A. Patients with Endometriosis Have an Increased Risk of Developing Cardiovascular Disease. Pharmacy Times. Available online: https://www.pharmacytimes.com/view/patients-with-endometriosis-have-an-increased-risk-of-developing-cardiovascular-disease (accessed on 28 March 2025).
- Smyk, J.M.; Danielecka, Z.; Kotowska, M.; Zawadka, M.; Andruszkiewicz, P.; Grąt, M.; Główczyńska, R.; Grabowski, M.; Gąsecka, A.; Romejko-Wolniewicz, E. Cardiovascular risks and endothelial dysfunction in reproductive-age women with endometriosis. Sci. Rep. 2024, 14, 24127. [Google Scholar] [CrossRef]
- Ueda, K.; Adachi, Y.; Liu, P.; Fukuma, N.; Takimoto, E. Regulatory Actions of Estrogen Receptor Signaling in the Cardiovascular System. Front. Endocrinol. 2020, 10, 909. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Yamamoto, S.; Maegawa, M.; Kunimi, K.; Matsui, S.; Keyama, K.; Kato, T.; Uemura, H.; Kuwahara, A.; Matsuzaki, T.; et al. Arterial stiffness is increased in young women with endometriosis. J. Obstet. Gynaecol. 2015, 35, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Choi, J.E.; Kwon, Y.J.; Chang, H.J.; Kim, J.O.; Park, D.H.; Park, J.M.; Kim, S.J.; Lee, J.W.; Hong, K.W. Identification of susceptibility loci for cardiovascular disease in adults with hypertension, diabetes, and dyslipidemia. J. Transl. Med. 2021, 19, 85. [Google Scholar] [CrossRef]
- Aherrahrou, R.; Reinberger, T.; Hashmi, S.; Erdmann, J. GWAS breakthroughs: Mapping the journey from one locus to 393 significant coronary artery disease associations. Cardiovasc. Res. 2024, 120, 1508–1530. [Google Scholar] [CrossRef]
- Rafi, U.; Ahmad, S.; Bokhari, S.S.; Iqbal, M.A.; Zia, A.; Khan, M.A.; Roohi, N. Association of Inflammatory Markers/Cytokines with Cardiovascular Risk Manifestation in Patients with Endometriosis. Mediat. Inflamm. 2021, 2021, 3425560. [Google Scholar] [CrossRef]
- Havers-Borgersen, E.; Hartwell, D.; Ekelund, C.; Butt, J.H.; Østergaard, L.; Holgersson, C.; Schou, M.; Køber, L.; Fosbøl, E.L. Endometriosis and Long-Term Cardiovascular Risk: A Nationwide Danish study. Eur. Heart J. 2024, 45, 4734–4743. [Google Scholar] [CrossRef]
- Endometrioza i Mikrobiota: Jakie Są Powiązania? Biocodex Microbiota Institute. Available online: https://www.biocodexmicrobiotainstitute.com/pl/pro/endometrioza-i-mikrobiota-jakie-sa-powiazania (accessed on 28 March 2025).
- Endometrioza. Medycyna Praktyczna. Available online: https://www.mp.pl/pacjent/ginekologia/choroby/88290,endometrioza (accessed on 28 March 2025).
- Terapia Nadciśnienia Tętniczego i Dyslipidemii Zgodnie z Wytycznymi Europejskiego Towarzystwa Kardiologicznego. Choroby Cywilizacyjne w Praktyce Lekarskiej|Czasopismo Dla Kardiologów i Diabetologów. Available online: https://www.kardiologia-i-diabetologia.pl/artykul/terapia-nadcisnienia-tetniczego-i-dyslipidemii-zgodnie-z-wytycznymi-europejskiego-towarzystwa-kardiologicznego (accessed on 28 March 2025).
- Tripathi, A.; Arsha, S.; Thapa, A.; Thapa, S.; Chand, S.; Frishman, W.H.; Aronow, W.S. Cardiovascular Implications of Gynecological Disorders: Bridging the Gap Between Gynecology and Cardiology. Cardiol. Rev. 2024, 10, 1097. [Google Scholar] [CrossRef]
- Okoth, K.; Wang, J.; Zemedikun, D.; Thomas, G.; Nirantharakumar, K.; Adderley, N. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: A retrospective matched cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1598–1609. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circulation 2016, 9, 257–264. [Google Scholar] [CrossRef]
- Farland, L.V.; Degnan, W.J., III; Bell, M.L.; Kasner, S.E.; Liberman, A.L.; Shah, D.K.; Rexrode, K.M.; Missmer, S.A. Laparoscopically Confirmed Endometriosis and Risk of Incident Stroke: A Prospective Cohort Study. Stroke 2022, 53, 3116–3122. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Amirlatifi, N.; Najmi, Z.; Tsuei, A. Thoracic Endometriosis Syndrome: A Comprehensive Review and Multidisciplinary Approach to Management. J. Clin. Med. 2024, 13, 7602. [Google Scholar] [CrossRef] [PubMed]
- McKee, D.C.; Mansour, T.; Wasson, M.N. Thoracic and diaphragmatic endometriosis: An overview of diagnosis and surgical treatment. Curr. Opin. Obstet. Gynecol. 2022, 34, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, H.; Takriti, A.; Sepehrmanesh, Z. Cardiac Involvement Resulting from Thoracic Endometriosis. Cardiovasc. Surg. Interv. 2019, 1, 1002. [Google Scholar]
- Takigawa, Y.; Mizuno, D.; Iga, N.; Fujimoto, N. Catamenial pneumothorax due to heterotopic endometriosis in the pericardium. BMJ Case Rep. 2021, 14, e240335. [Google Scholar] [CrossRef]
- Early Detection Breakthrough in Endometriosis: Oxford University Unveils Promising Imaging Study. Available online: https://www.wrh.ox.ac.uk/news/early-detection-breakthrough-in-endometriosis-oxford-university-unveils-promising-imaging-study (accessed on 28 March 2025).
- Charpentier, E.; Petit, E.; Beranger, S.; Azarine, A. Presumption of pericardial endometriosis using MRI: Case report and review of the literature. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 71–73. [Google Scholar] [CrossRef]
- Nayyar, A.; Saleem, M.I.; Yilmaz, M.; DeFranco, M.; Klein, G.; Elmaliki, K.M.; Kowalsky, E.; Chatterjee, P.K.; Xue, X.; Viswanathan, R.; et al. Menstrual Effluent Provides a Novel Diagnostic Window on the Pathogenesis of Endometriosis. Front. Reprod. Health 2020, 2, 3. [Google Scholar] [CrossRef]
- Sorino, C.; Negri, S.; Spanevello, A.; Feller-Kopman, D. The pleura and the endocrine system. Eur. J. Intern. Med. 2020, 72, 34–37. [Google Scholar] [CrossRef]
- Ceccaroni, M.; Roviglione, G.; Rosenberg, P.; Pesci, A.; Clarizia, R.; Bruni, F.; Zardini, C.; Ruffo, G.; Placci, A.; Crippa, S.; et al. Pericardial, pleural and diaphragmatic endometriosis in association with pelvic peritoneal and bowel endometriosis: A case report and review of the literature. Videosurgery Other Miniinvasive Tech. 2012, 7, 122–131. [Google Scholar] [CrossRef]
- Bachi, A.; Bille, A.; Khazali, S. The Combined Robotic-assisted Laparoscopic and Thoracic Approach in the Management of Diaphragmatic, Pleural, and Pericardial Endometriosis. J. Minim. Invasive Gynecol. 2023, 30, 533–534. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Gilbert, S.; Arendas, K.; Jago, C.A.; Singh, S.S. Laparoscopic excision of pericardial and diaphragmatic endometriosis. Fertil. Steril. 2021, 115, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Endometriosis. Yale Medicine. Available online: https://www.yalemedicine.org/conditions/endometriosis (accessed on 28 March 2025).
- Kido, A.; Himoto, Y.; Moribata, Y.; Kurata, Y.; Nakamoto, Y. MRI in the Diagnosis of Endometriosis and Related Diseases. Korean J. Radiol. 2022, 23, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, K.; Triantafilopoulou, K.; Minadakis, G.; Zatagias, A.; Sapalidis, K.; Kosmidis, C.; Ioannidis, A.; Romanidis, K.; Oikonomou, P.; Sevva, C.; et al. Catamenial pneumothorax recurrence due to endometriosis. Respir. Med. Case Rep. 2020, 30, 101036. [Google Scholar] [CrossRef]
- Ceccaroni, M.; Clarizia, R.; Placci, A. Pericardial, pleural, and diaphragmatic endometriosis. J. Thorac. Cardiovasc. Surg. 2010, 140, 1189–1190. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, H.; Li, Z.; Zhang, J. Endometriosis and aspirin: A systematic review. Front. Endocrinol. 2024, 15, 1409469. [Google Scholar] [CrossRef]
- Uzunlar, O.; Ozyer, S.; Engin-Ustun, Y.; Moraloglu, O.; Gulerman, H.C.; Caydere, M.; Keskin, S.M.; Mollamahmutoglu, L. Effects of repeated propranolol administration in a rat model of surgically induced endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 167–171. [Google Scholar] [CrossRef]
- Long, Q.; Zheng, H.; Liu, X.; Guo, S.W. Perioperative Intervention by β-Blockade and NF-κB Suppression Reduces the Recurrence Risk of Endometriosis in Mice Due to Incomplete Excision. Reprod. Sci. 2019, 26, 697–708. [Google Scholar] [CrossRef]
- Gibran, L.; Maranhão, R.C.; Abrão, M.S.; Baracat, E.C.; Podgaec, S. Could statins constitute a novel treatment for endometriosis? Systematic review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 153–158. [Google Scholar] [CrossRef]
- Sapmaz, T.; Coskun, G.; Saker, D.; Pence, H.H.; Keles, P.; Hayretdag, C.; Kuras, S.; Topkaraoglu, S.; Erdem, E.; Efendic, F.; et al. Effects of metformin, letrozole and atorvastatin on inflammation and apoptosis in experimental peritoneal and ovarian endometriosis in the rat. Pathol.-Res. Pract. 2022, 235, 153951. [Google Scholar] [CrossRef]
| Author (Ref.) | Patient Description | Thoracic Involvement | Symptoms | Diagnosis Method | Management and Outcome |
|---|---|---|---|---|---|
| Takigawa et al. [32] | 46-year-old woman with recurrent catamenial pneumothorax | Pericardium | Cyclical pneumothorax | Surgery + histopathology + IHC (ER/PR positive) | Surgical removal + hormonal therapy → Full symptom resolution, no recurrence |
| Ceccaroni et al. [37] | Extensive endometriosis in multiple sites including thorax | Right-sided diaphragm, pleura, pericardium | Thoracoabdominal discomfort | Surgical exploration | Multidisciplinary surgery tailored to fertility and severity → Successful outcome |
| Bachi et al. [38] | Severe thoracic endometriosis | Diaphragm, pleura, pericardium | Not specified | Robotic-assisted laparoscopy + thoracoscopy | Robotic-assisted laparoscopic and thoracoscopic surgery → Symptomatic relief, no relapse |
| Nguyen et al. [39] | 35-year-old woman with chest and right arm pain | Diaphragm and pericardium | Chest and right upper extremity pain | Intraoperative findings + histological analysis | Robotic-assisted excision → Full symptom resolution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpila, G.; Szczotka, J.; Suchodolski, A.; Szulik, M. Endometriosis and Cardiovascular Disease: Exploring Pathophysiological Interconnections and Risk Mechanisms. Diagnostics 2025, 15, 1458. https://doi.org/10.3390/diagnostics15121458
Szpila G, Szczotka J, Suchodolski A, Szulik M. Endometriosis and Cardiovascular Disease: Exploring Pathophysiological Interconnections and Risk Mechanisms. Diagnostics. 2025; 15(12):1458. https://doi.org/10.3390/diagnostics15121458
Chicago/Turabian StyleSzpila, Gabriela, Julia Szczotka, Alexander Suchodolski, and Mariola Szulik. 2025. "Endometriosis and Cardiovascular Disease: Exploring Pathophysiological Interconnections and Risk Mechanisms" Diagnostics 15, no. 12: 1458. https://doi.org/10.3390/diagnostics15121458
APA StyleSzpila, G., Szczotka, J., Suchodolski, A., & Szulik, M. (2025). Endometriosis and Cardiovascular Disease: Exploring Pathophysiological Interconnections and Risk Mechanisms. Diagnostics, 15(12), 1458. https://doi.org/10.3390/diagnostics15121458






