Determination of the Stage of Periodontitis with 20 ng/mL Cut-Off aMMP-8 Mouth Rinse Test and Polynomial Functions in a Mobile Application
Abstract
1. Introduction
2. Methods and Materials
2.1. Framework and Hypothesis
2.2. Periodontitis Classification
2.3. Staging of Periodontitis
2.4. aMMP-8 Point-of-Care Testing (POCT)
2.5. Dental Plaque
2.6. Missing Teeth
2.7. Sample Data and Parameters
2.8. Logistic Regression and Assumptions
2.9. Sample Size
2.10. Statistical Analysis
3. Results
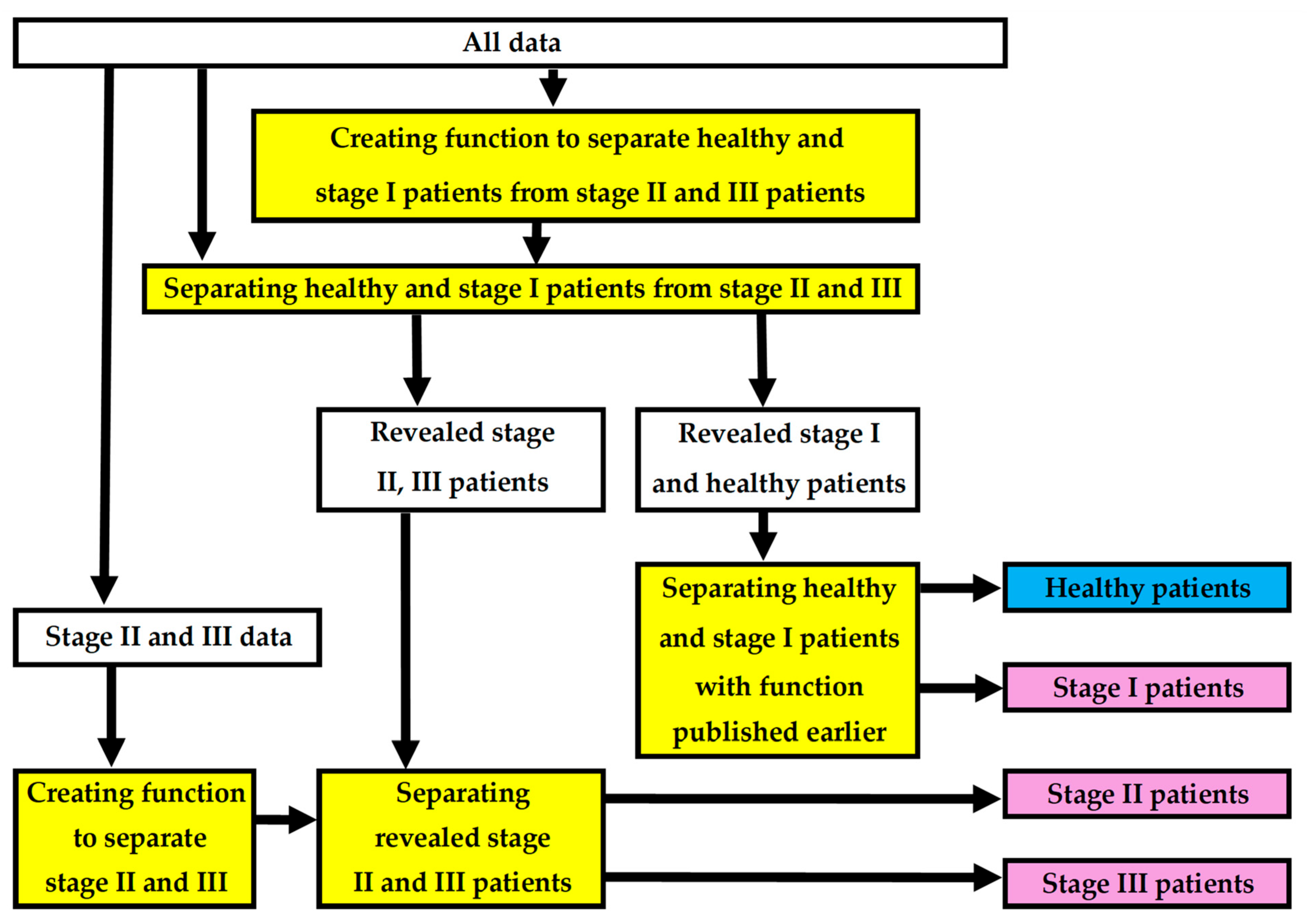
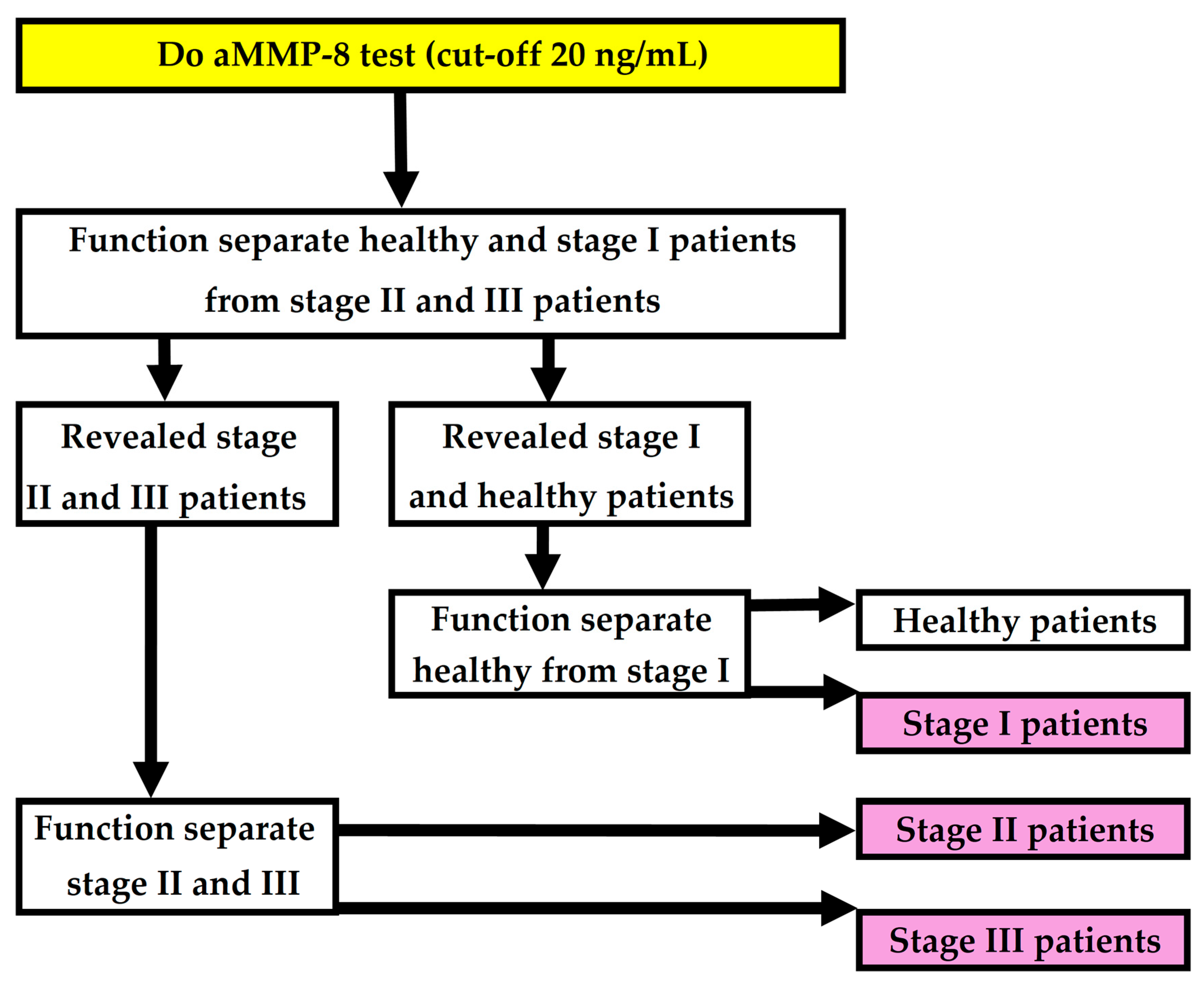
3.1. Created New Two Functions
- PERIOSTAGE I: The phi coefficient φ = 0.52; F1 score 0.83; chi-square test χ2 (1, N = 149) = 3.46, p = 0.06; AUC = 0.842 (95% CI = 0.774–0.910), sensitivity 82/104 = 0.79; specificity 34/45 = 0.76; accuracy 116/149 = 0.78; Youden’s index = 0.5441, cut-off 0.598.
- PERIOSTAGE II/III: The phi coefficient φ = 0.41; F1 score 0.56; chi-square test χ2 (1, N = 104) = 2.94, p = 0.09, AUC = 0.779 (95% CI = 0.680–0.878), sensitivity 15/23 = 0.65; specificity 65/81 = 0.80; accuracy 80/104 = 0.77; Youden’s index = 0.4545, cut-off 0.326).
3.2. Forming of the Complete Model
3.3. Performance of the Complete Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob. Heart 2020, 15, 1. [Google Scholar] [CrossRef]
- Stødle, I.H.; Sen, A.; Høvik, H.; Verket, A.; Koldsland, O.C. Association between periodontitis stages and self-reported diseases in a Norwegian population: The HUNT study. BMC Oral Health 2023, 23, 999. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants; Merete Aass, A.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontol. 2000 2020, 82, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 1475. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Kinane, D.F.; Darby, I.B.; Said, S.; Luoto, H.; Sorsa, T.; Tikanoja, S.; Mäntylä, P. Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J. Periodontal Res. 2003, 38, 400–404. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Aji, N.R.A.S.; Yucel-Lindberg, T.; Räisänen, I.T.; Kuula, H.; Nieminen, M.T.; Mc Crudden, M.T.C.; Listyarifah, D.; Lundmark, A.; Lundy, F.T.; Gupta, S.; et al. In Vivo Regulation of Active Matrix Metalloproteinase-8 (aMMP-8) in Periodontitis: From Transcriptomics to Real-Time Online Diagnostics and Treatment Monitoring. Diagnostics 2024, 14, 1011. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M.; Ryan, M.E.; Giannobile, W.V.; Payne, J.; Sorsa, T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 1998, 12, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.K.; Hill, M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef]
- Kopp, S.L.; Ramseier, C.A.; Ratka-Krüger, P.; Woelber, J.P. Motivational interviewing as an adjunct to periodontal therapy—A systematic review. Front. Psychol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Penttala, M.; Sorsa, T.; Thomas, J.T.; Grigoriadis, A.; Sakellari, D.; Gupta, S.; Pärnänen, P.; Pätilä, T.; Räisänen, I.T. Periodontitis Home Screening with Mouth Rinse Cut-Off 20 Ng/mL aMMP-8 Test and Mobile Application. Diagnostics 2025, 15, 296. [Google Scholar] [CrossRef]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.-R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Aji, N.R.A.S.; Sahni, V.; Penttala, M.T.; Sakellari, D.; Grigoriadis, A.; Pätilä, T.; Pärnänen, P.; Neefs, D.; Pfützner, A.; Gupta, S.; et al. Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments. Dent. J. 2025, 13, 127. [Google Scholar] [CrossRef]
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838. [Google Scholar] [CrossRef]
- Buduneli, N.; Bıyıkoğlu, B.; Kinane, D.F. Utility of gingival crevicular fluid components for periodontal diagnosis. Periodontol. 2000 2024, 95, 156–175. [Google Scholar] [CrossRef]
- Gupta, S.; Sahni, V.; Räisänen, I.T.; Grigoriadis, A.; Sakellari, D.; Gieselmann, D.-R.; Sorsa, T. Linking oral microbial proteolysis to aMMP-8 PoC diagnostics along with the stage and grade of periodontitis: A cross-sectional study. Oral. Dis. 2023, 29, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontol. 2000 2011, 55, 36. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 WorldWorkshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Li, Y.; Kung, J.C.K.; Shi, J.; Wu, X.; Lam, S.L.T.; Deng, K.; Zhang, X.; Lai, H.; Pelekos, G.; Jin, L.; et al. Diagnostic Accuracy of a Point-Of-Care aMMP-8 Test for Discriminating Periodontal Health Status in Adults: Validation Trials and Updated Meta-Analysis. J. Clin. Periodontol. 2025, 52, 510–529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, W.; Aitken, S.; Sodek, J.; McCulloch, C.A. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: Role of active enzyme in human periodontitis. J. Periodontal Res. 1995, 30, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.; Mancini, S.; Laschinger, C.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Activation of neutrophil collagenase in perio- dontitis. Infect. Immun. 1999, 67, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Romanelli, R.; Laschinger, C.A.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J. Periodontol. 1999, 70, 1292–1302. [Google Scholar] [CrossRef]
- Aji, N.R.A.S.; Räisänen, I.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden, M.T.C.; Goyal, L.; Sorsa, T.; Gupta, S. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease. J. Clin. Periodontol. 2021, 48, 1051–1065. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Authors’ Response: “Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease”. J. Clin. Periodontol. 2021, 48, 1499–1500. [Google Scholar] [CrossRef]
- Deng, K.; Wei, S.; Xu, M.; Shi, J.; Lai, H.; Tonetti, M.S. Diagnostic accuracy of active matrix metalloproteinase-8 point-of-care test for the discrimination of periodontal health status: Comparison of saliva and oral rinse samples. J. Periodontal Res. 2022, 57, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lin, T.; Sáenz-Ravello, G.; Gao, H.; Zhang, Y.; Tonetti, M.S.; Deng, K. Diagnostic accuracy of salivary active matrix metalloproteinase (aMMP)-8 point-of-care test for detecting periodontitis in adults: A systematic review and meta-analysis. J. Clin. Periodontol. 2024, 51, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Reda, R.; Zanza, A.; Xhajanka, E.; Patil, S.; Di Nardo, D.; Testarelli, L. Relationship between gingival and peri-implant sulcular fluid active matrix metalloproteinase-8 concentration and clinical indices in healthy and diseased conditions. Explor. Med. 2024, 5, 243–256. [Google Scholar] [CrossRef]
- Tschesche, H.; Wenzel, H. Neutrophil Collagenase. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 725–734. [Google Scholar]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar]
- Bornes, R.; Montero, J.; Correia, A.; Marques, T.; Rosa, N. Peri-implant diseases diagnosis, prognosis and dental implant monitoring: A narrative review of novel strategies and clinical impact. BMC Oral Health 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Reda, R.; Zanza, A.; Miccoli, G.; Nardo, D.D.; Testarelli, L. Can Peri-Implant Marginal Bone Loss Progression and a-MMP-8 Be Considered Indicators of the Subsequent Onset of Peri-Implantitis? A 5-Year Study. Diagnostics 2022, 12, 2599. [Google Scholar] [CrossRef]
- Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Tervahartiala, T.; Sorsa, T.; Sakellari, D. Prediabetes/diabetes screening strategy at the periodontal clinic. Clin. Exp. Dent. Res. 2021, 7, 85–92. [Google Scholar] [CrossRef]
- Räisänen, I.T.; Lähteenmäki, H.; Gupta, S.; Grigoriadis, A.; Sahni, V.; Suojanen, J.; Seppänen, H.; Tervahartiala, T.; Sakellari, D.; Sorsa, T. An aMMP-8 point-of-care and questionnaire based real-time diagnostic toolkit for medical practitioners. Diagnostics 2021, 11, 711. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.; Gul, S.S.; Sha, A.; Chapple, I.L. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Allison, P. Logistic Regression Using SAS: Theory and Application; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- O’brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Long, S.J. Regression models for categorical and limited dependent variables. In Advanced Quantitative Techniques in the Social Sciences; SAGE Publications: Thousand Oaks, CA, USA, 1997. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community–implications for health and disease. BMC Oral Health 2006, 1, S14. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Armfield, J.M.; Stewart, J.F.; Spencer, A.J. The vicious cycle of dental fear: Exploring the interplay between oral health, service utilization and dental fear. BMC Oral Health 2007, 7, 1–15. [Google Scholar] [CrossRef]
- Haukka, A.; Heikkinen, A.M.; Haukka, J.; Kaila, M. Oral health indices predict individualised recall interval. Clin. Exp. Dent. Res. 2020, 6, 585–595. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Beklen, A.; Sali, N.; Yavuz, M.B. The impact of smoking on periodontal status and dental caries. Tob. Induc. Dis. 2022, 20, 72. [Google Scholar] [CrossRef]
- Nociti Jr, F.H.; Casati, M.Z.; Duarte, P.M. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontol. 2000 2015, 67, 187–210. [Google Scholar] [CrossRef]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Makrilakis, K.; Kalpourtzi, N.; Ioannidis, I.; Iraklianou, S.; Raptis, A.; Sotiropoulos, A.; Gavana, M.; Vantarakis, A.; Kantzanou, M.; Hadjichristodoulou, C.; et al. Prevalence of diabetes and pre-diabetes in Greece. Results of the First National Survey of Morbidity and Risk Factors (EMENO) study. Diabetes Res. Clin. Pract. 2021, 172, 108646. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, D.; Coresh, J.; Selvin, E. Undiagnosed Diabetes in U.S. Adults: Prevalence and Trends. Diabetes Care 2022, 45, 1994–2002. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Dawson, D.A., 3rd; Emecen Huja, P.; Pandruvada, S.; Basu, A.; Nguyen, L.; Zhang, Y.; Gonzalez, O.A. Age and Periodontal Health—Immunological View. Curr. Oral Health Rep. 2018, 5, 229–241. [Google Scholar] [CrossRef]
- Holde, G.E.; Oscarson, N.; Trovik, T.A.; Tillberg, A.; Jönsson, B. Periodontitis prevalence and severity in adults: A cross-sectional study in Norwegian circumpolar communities. J. Periodontol. 2017, 88, 1012–1022. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhao, C.; Wang, J.; Zhou, H. Association between weight-adjusted waist index and periodontitis: A population-based study. PLoS ONE 2024, 19, e0305010. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Xu, M.; Li, R.; Zhang, J.; Zhu, S.; He, Z.; Chen, M.; Wang, G. Association of weight-adjusted-waist index with asthma prevalence and the age of first asthma onset in United States adults. Front. Endocrinol. 2023, 14, 1116621. [Google Scholar] [CrossRef]
- Munoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef]
- Caton, J.; Ryan, M.E. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol. Res. 2011, 63, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontol. 2000 2013, 62, 95–162. [Google Scholar] [CrossRef] [PubMed]
- Jundaeng, J.; Chamchong, R.; Nithikathkul, C. Periodontitis diagnosis: A review of current and future trends in artificial intelligence. Technol. Health Care 2025, 33, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Shafi, I.; Fatima, A.; Afzal, H.; Díez, I.d.l.T.; Lipari, V.; Breñosa, J.; Ashraf, I. A Comprehensive Review of Recent Advances in Artificial Intelligence for Dentistry E-Health. Diagnostics 2023, 13, 2196. [Google Scholar] [CrossRef]
- Bashir, N.Z.; Rahman, Z.; Chen, S.L. Systematic comparison of machine learning algorithms to develop and validate predictive models for periodontitis. J. Clin. Periodontol. 2022, 49, 958–969. [Google Scholar] [CrossRef]
| Patient’s Data | Periodontal Status | |||
|---|---|---|---|---|
| No Evidence of Periodontitis (n = 31) | Stage I (n = 14) | Stage II (n = 81) | Stage III (n = 23) | |
| Gender | ||||
| Female | 11 | 13 | 39 | 12 |
| Male | 20 | 1 | 42 | 11 |
| Smoking status | ||||
| Tobacco smoker | 3 | 7 | 24 | 10 |
| Non-smoker | 28 | 7 | 57 | 13 |
| Diabetic status | ||||
| Diabetic | 0 | 0 | 3 | 4 |
| Non-diabetic | 31 | 14 | 78 | 19 |
| Annual dental visit | ||||
| Yes | 19 | 13 | 45 | 13 |
| No | 12 | 1 | 36 | 10 |
| Age (yrs.) | 43 ± 11 | 62 ±8 | 55 ±10 | 56 ±10 |
| Body mass index (kg/m2) | 30.6 ± 4.5 | 28.6 ± 4.4 | 30.5 ± 4.7 | 29.3 ± 5.9 |
| Weight (kg) | 93 ± 17 | 78 ± 11 | 89 ± 17 | 85 ± 23 |
| Height (cm) | 174 ± 10 | 165 ± 6 | 171 ± 9 | 169 ± 10 |
| Waist circumference (cm) | 100 ± 17 | 98 ± 12 | 103 ± 14 | 105 ± 21 |
| Waist-to-height ratio (cm/cm) | 0.57 ± 0.08 | 0.60 ± 0.09 | 0.60 ± 0.08 | 0.62 ± 0.11 |
| aMMP-8 levels | ||||
| aMMP-8 ≥ 20 ng/mL | 2 | 2 | 31 | 17 |
| aMMP-8 < 20 ng/mL | 29 | 12 | 50 | 6 |
| Stage of periodontitis status | ||||
| No evidence of periodontitis | 31 | 0 | 0 | 0 |
| Stage I | 0 | 14 | 0 | 0 |
| Stage Il | 0 | 0 | 81 | 0 |
| Stage III | 0 | 0 | 0 | 23 |
| Grade of periodontitis status | ||||
| No evidence of progression | 31 | 0 | 0 | 0 |
| Grade A | 0 | 7 | 7 | 0 |
| Grade B | 0 | 7 | 70 | 13 |
| Grade C | 0 | 0 | 4 | 10 |
| CAL (mm) | 2.4 ± 0.5 | 2.3 ± 0.5 | 3.4 ± 0.8 | 4.8 ± 1.2 |
| PPD (mm) | 2.2 ± 0.3 | 2.2 ± 0.4 | 3.0 ± 0.7 | 3.9 ± 0.9 |
| Number of teeth present (No.) | 27 ± 2 | 25 ± 2 | 24 ± 3 | 22 ± 4 |
| VPI (%) | 43 ± 22 | 29 ± 20 | 48 ± 27 | 63 ± 28 |
| BOP (%) | 42 ± 25 | 37 ± 18 | 56 ± 23 | 62 ± 23 |
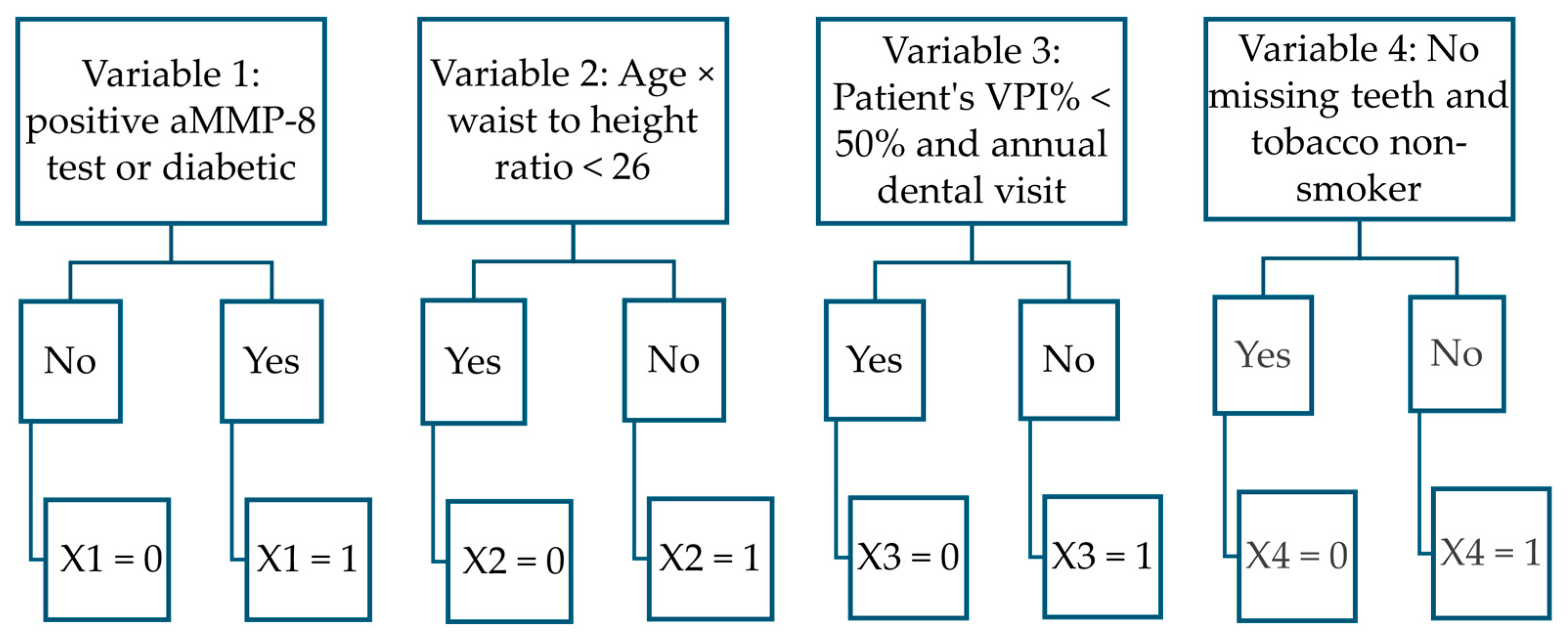
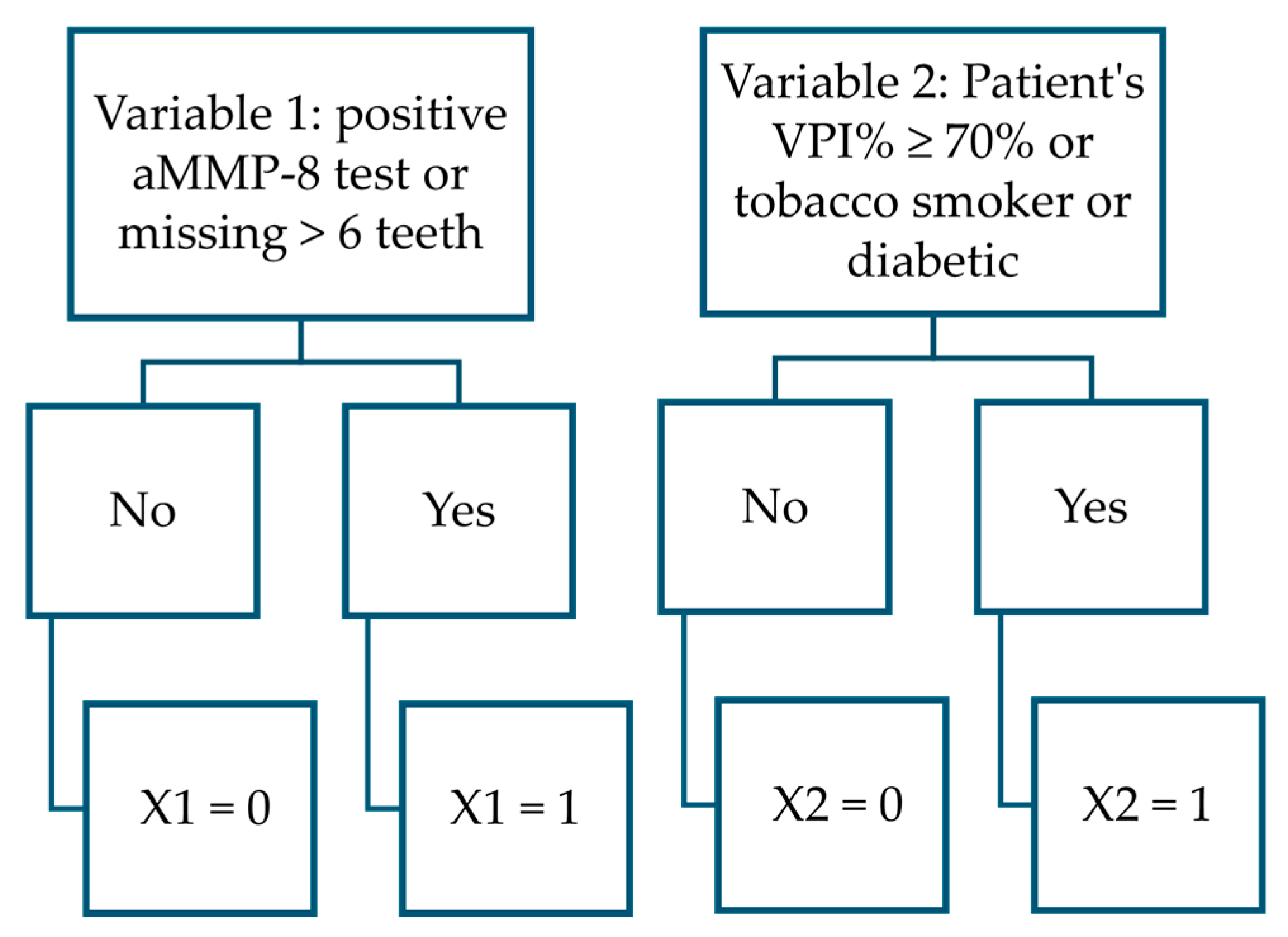
| Model and Variables | B | S.E. | Wald | df | Sig | Exp(B) |
|---|---|---|---|---|---|---|
| Function separating healthy and stage I patients from stage II and III patients | ||||||
| aMMP-8 test (cut-off: 20 ng/mL) or diabetic | 1.997 | 0.598 | 11.150 | 1 | <0.001 | 7.365 |
| Age × waist-to-height ratio cut-off 26 yrs. × cm/cm | 1.615 | 0.522 | 9.566 | 1 | 0.002 | 5.029 |
| Patient’s VPI% < 50% and annual dental visit | 1.653 | 0.483 | 11.717 | 1 | <0.001 | 5.221 |
| No missing teeth and tobacco non-smoker | 1.180 | 0.526 | 5.045 | 1 | 0.025 | 3.256 |
| Constant | −2.649 | 0.675 | 15.403 | 1 | <0.001 | 0.071 |
| Function separating stage II and III patients | ||||||
| aMMP-8 test (cut-off: 20 ng/mL) or >6 teeth missing | 1.719 | 0.624 | 7.592 | 1 | 0.006 | 5.581 |
| Patient’s VPI% ≥ 70% or is a tobacco smoker or diabetic | 1.988 | 0.617 | 10.362 | 1 | 0.001 | 7.297 |
| Constant | −3.706 | 0.755 | 24.107 | 1 | <0.001 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penttala, M.; Sorsa, T.; Thomas, J.T.; Grigoriadis, A.; Sakellari, D.; Sahni, V.; Gupta, S.; Pärnänen, P.; Pätilä, T.; Räisänen, I.T. Determination of the Stage of Periodontitis with 20 ng/mL Cut-Off aMMP-8 Mouth Rinse Test and Polynomial Functions in a Mobile Application. Diagnostics 2025, 15, 1411. https://doi.org/10.3390/diagnostics15111411
Penttala M, Sorsa T, Thomas JT, Grigoriadis A, Sakellari D, Sahni V, Gupta S, Pärnänen P, Pätilä T, Räisänen IT. Determination of the Stage of Periodontitis with 20 ng/mL Cut-Off aMMP-8 Mouth Rinse Test and Polynomial Functions in a Mobile Application. Diagnostics. 2025; 15(11):1411. https://doi.org/10.3390/diagnostics15111411
Chicago/Turabian StylePenttala, Miika, Timo Sorsa, Julie Toby Thomas, Andreas Grigoriadis, Dimitra Sakellari, Vaibhav Sahni, Shipra Gupta, Pirjo Pärnänen, Tommi Pätilä, and Ismo T. Räisänen. 2025. "Determination of the Stage of Periodontitis with 20 ng/mL Cut-Off aMMP-8 Mouth Rinse Test and Polynomial Functions in a Mobile Application" Diagnostics 15, no. 11: 1411. https://doi.org/10.3390/diagnostics15111411
APA StylePenttala, M., Sorsa, T., Thomas, J. T., Grigoriadis, A., Sakellari, D., Sahni, V., Gupta, S., Pärnänen, P., Pätilä, T., & Räisänen, I. T. (2025). Determination of the Stage of Periodontitis with 20 ng/mL Cut-Off aMMP-8 Mouth Rinse Test and Polynomial Functions in a Mobile Application. Diagnostics, 15(11), 1411. https://doi.org/10.3390/diagnostics15111411






