The Role of [18F]FDG PET Imaging for the Assessment of Vegetative State or Unresponsive Wakefulness Syndrome: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
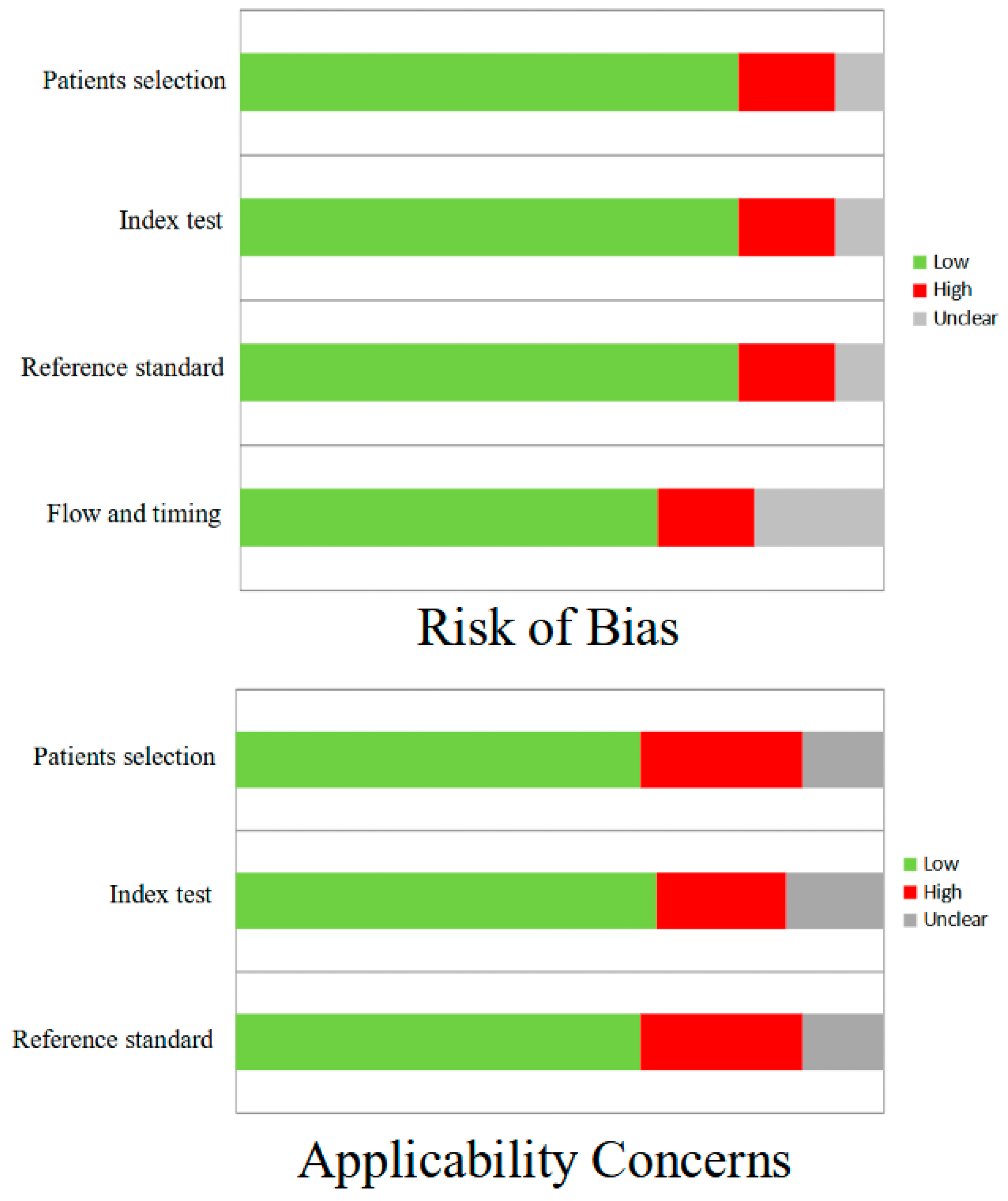
2.3. Quality Assessment
2.4. Data Extraction
3. Results
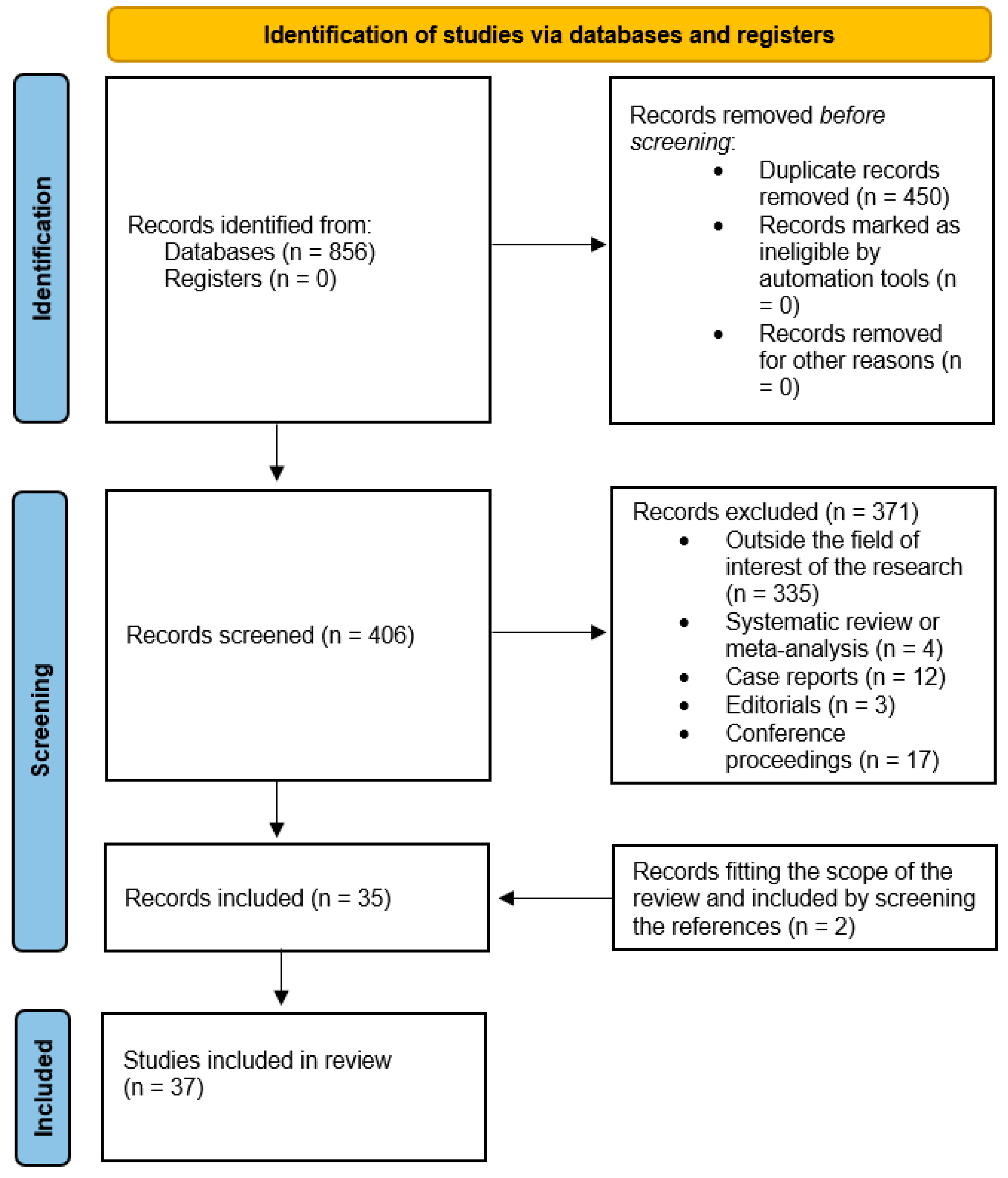
3.1. Literature Search
3.2. The Role of [18F]FDG PET Imaging for the Evaluation of VS/UWS
3.3. Studies with Mixed Tracers
4. Discussion
4.1. Limitations
4.2. Ethical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eapen, B.C.; Georgekutty, J.; Subbarao, B.; Bavishi, S.; Cifu, D.X. Disorders of Consciousness. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Stevens, R.D. Classifying Disorders of Consciousness: Past, Present, and Future. Semin. Neurol. 2022, 42, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, S.; Cranford, R.; Bernat, J. Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1). N. Engl. J. Med. 1994, 330, 1499–1508. [Google Scholar] [CrossRef]
- Kondziella, D.; Friberg, C.K.; Frokjaer, V.G.; Fabricius, M.; Møller, K. Preserved consciousness in vegetative and minimal conscious states: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 485–492. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Shah, S.; Eddy, C.M.; Williams, A.; Rickards, H. Consciousness: A neurological perspective. Behav. Neurol. 2011, 24, 107–116. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Jox, R.J.; Grill, E.; Straube, A.; Lulé, D. Persistent vegetative state and minimally conscious state: A systematic review and meta-analysis of diagnostic procedures. Dtsch. Arztebl. Int. 2015, 112, 235–242. [Google Scholar] [CrossRef]
- Farg, H.; Elnakib, A.; Gebreil, A.; Alksas, A.; van Bogaert, E.; Mahmoud, A.; Khalil, A.; Ghazal, M.; El-Ghar, M.A.; El-Baz, A.; et al. Diagnostic value of PET imaging in clinically unresponsive patients. Br. J. Radiol. 2024, 97, 283–291. [Google Scholar] [CrossRef]
- Guedj, E.; Varrone, A.; Boellaard, R.; Albert, N.L.; Barthel, H.; van Berckel, B.; Brendel, M.; Cecchin, D.; Ekmekcioglu, O.; Garibotto, V.; et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur. J. Nucl Med. Mol. Imaging 2022, 49, 632–651. [Google Scholar] [CrossRef]
- Hanania, J.U.; Reimers, E.; Bevington, C.W.J.; Sossi, V. PET-based brain molecular connectivity in neurodegenerative disease. Curr. Opin. Neurol. 2024, 37, 353–360. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Ramezannezhad, E.; Sardaripour, A.; Seyedi, S.A.; Salehi, N.; Rezaeimanesh, N.; Naser Moghadasi, A. [18F]FDG brain PET and clinical symptoms in different autoantibodies of autoimmune encephalitis: A systematic review. Neurol. Sci. 2022, 43, 4701–4718. [Google Scholar] [CrossRef] [PubMed]
- Dondi, F.; Bertagna, F. Applications of 18F-Fluorodesoxyglucose PET Imaging in Leukemia. PET Clin. 2024, 19, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, A.M.; Bonfioli, G.B.; Dondi, F.; Riccardi, M.; Bertagna, F.; Metra, M.; Vizzardi, E. Contemporary Role of Positron Emission Tomography (PET) in Endocarditis: A Narrative Review. J. Clin. Med. 2024, 13, 4124. [Google Scholar] [CrossRef]
- Sammartino, A.M.; Falco, R.; Drera, A.; Dondi, F.; Bellini, P.; Bertagna, F.; Vizzardi, E. Vascular inflammation and cardiovascular disease: Review about the role of PET imaging. Int. J. Cardiovasc. Imaging 2023, 39, 433–440. [Google Scholar] [CrossRef]
- Dondi, F.; Bertoli, M.; Lucchini, S.; Cerudelli, E.; Albano, D.; Bertagna, F. PET imaging for the evaluation of cerebral amyloid angiopathy: A systematic review. Clin. Transl. Imaging 2022, 10, 391–401. [Google Scholar] [CrossRef]
- Dondi, F.; Gazzilli, M.; Viganò, G.L.; Pisani, A.R.; Ferrari, C.; Rubini, G.; Bertagna, F. The Role of 11C-Methionine PET Imaging for the Evaluation of Lymphomas: A Systematic Review. Hematol. Rep. 2024, 16, 752–768. [Google Scholar] [CrossRef]
- Borja, A.J.; Hancin, E.C.; Raynor, W.Y.; Ayubcha, C.; Detchou, D.K.; Werner, T.J.; Revheim, M.E.; Alavi, A. A Critical Review of PET Tracers Used for Brain Tumor Imaging. PET Clin. 2021, 16, 219–231. [Google Scholar] [CrossRef]
- Barthel, H.; Schroeter, M.L.; Hoffmann, K.T.; Sabri, O. PET/MR in dementia and other neurodegenerative diseases. Semin. Nucl. Med. 2015, 45, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Levy, D.E.; Sidtis, J.J.; Rottenberg, D.A.; Jarden, J.O.; Strother, S.C.; Dhawan, V.; Ginos, J.Z.; Tramo, M.J.; Evans, A.C.; Plum, F. Differences in cerebral blood flow and glucose utilization in vegetative versus locked-in patients. Ann. Neurol. 1987, 22, 673–682. [Google Scholar] [CrossRef]
- De Volder, A.G.; Michel, C.; Guérit, J.M.; Bol, A.; Georges, B.; de Barsy, T.; Laterre, C. Brain glucose metabolism in postanoxic syndrome due to cardiac arrest. Acta Neurol. Belg. 1994, 94, 183–189. [Google Scholar] [PubMed]
- Tommasino, C.; Grana, C.; Lucignani, G.; Torri, G.; Fazio, F. Regional cerebral metabolism of glucose in comatose and vegetative state patients. J. Neurosurg. Anesthesiol. 1995, 7, 109–116. [Google Scholar] [CrossRef]
- Rudolf, J.; Ghaemi, M.; Ghaemi, M.; Haupt, W.F.; Szelies, B.; Heiss, W.D. Cerebral glucose metabolism in acute and persistent vegetative state. J. Neurosurg. Anesthesiol. 1999, 11, 17–24. [Google Scholar] [CrossRef]
- Laureys, S.; Goldman, S.; Phillips, C.; Van Bogaert, P.; Aerts, J.; Luxen, A.; Franck, G.; Maquet, P. Impaired effective cortical connectivity in vegetative state: Preliminary investigation using PET. Neuroimage 1999, 9, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Faymonville, M.-E.; Degueldre, C.; Fiore, G.D.; Damas, P.; Lambermont, B.; Janssens, N.; Aerts, J.; Franck, G.; Luxen, A.; et al. Auditory processing in the vegetative state. Brain 2000, 123, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Faymonville, M.E.; Peigneux, P.; Damas, P.; Lambermont, B.; Del Fiore, G.; Degueldre, C.; Aerts, J.; Luxen, A.; Franck, G.; et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage 2002, 17, 732–741. [Google Scholar] [CrossRef]
- Rudolf, J.; Sobesky, J.; Ghaemi, M.; Heiss, W.D. The correlation between cerebral glucose metabolism and benzodiazepine receptor density in the acute vegetative state. Eur. J. Neurol. 2002, 9, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Beuthien-Baumann, B.; Handrick, W.; Schmidt, T.; Burchert, W.; Oehme, L.; Kropp, J.; Schackert, G.; Pinkert, J.; Franke, W.-G. Persistent vegetative state: Evaluation of brain metabolism and brain perfusion with PET and SPECT. Nucl. Med. Commun. 2003, 24, 643–649. [Google Scholar] [CrossRef]
- Kassubek, J.; Juengling, F.D.; Els, T.; Spreer, J.; Herpers, M.; Krause, T.; Moser, E.; Lücking, C.H. Activation of a residual cortical network during painful stimulation in long-term postanoxic vegetative state: A 15O-H2O PET study. J. Neurol. Sci. 2003, 212, 85–91. [Google Scholar] [CrossRef]
- Coleman, M.R.; Menon, D.K.; Fryer, T.D.; Pickard, J.D. Neurometabolic coupling in the vegetative and minimally conscious states: Preliminary findings. J. Neurol. Neurosurg. Psychiatry 2005, 76, 432–434. [Google Scholar] [CrossRef]
- Juengling, F.D.; Kassubek, J.; Huppertz, H.J.; Krause, T.; Els, T. Separating functional and structural damage in persistent vegetative state using combined voxel-based analysis of 3-D MRI and FDG-PET. J. Neurol. Sci. 2005, 228, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Okumura, A.; Shinoda, J.; Nakashima, T.; Iwama, T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: An FDG-PET study with statistical parametric mapping analysis. J. Neurol. Neurosurg. Psychiatry 2006, 77, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.-A.; Vanhaudenhuyse, A.; Schnakers, C.; Boly, M.; Gosseries, O.; Demertzi, A.; Majerus, S.; Moonen, G.; Hustinx, R.; Laureys, S. Visual fixation in the vegetative state: An.; observational case series PET study. BMC Neurol. 2010, 10, 35. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, H.S.; An, Y.S.; Im, S.H. Voxel-based statistical analysis of cerebral glucose metabolism in patients with permanent vegetative state after acquired brain injury. Chin. Med. J. 2010, 123, 2853–2857. [Google Scholar]
- Snyman, N.; Egan, J.R.; London, K.; Howman-Giles, R.; Gill, D.; Gillis, J.; Scheinberg, A. Zolpidem for persistent vegetative state--a placebo-controlled trial in pediatrics. Neuropediatrics 2010, 41, 223–227. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bruno, M.-A.; Maquet, P.; Boly, M.; Noirhomme, Q.; Schnakers, C.; Vanhaudenhuyse, A.; Bonjean, M.; Hustinx, R.; Moonen, G.; et al. “Relevance vector machine” consciousness classifier applied to cerebral metabolism of vegetative and locked-in patients. Neuroimage 2011, 56, 797–808. [Google Scholar] [CrossRef]
- Thibaut, A.; Bruno, M.A.; Chatelle, C.; Gosseries, O.; Vanhaudenhuyse, A.; Demertzi, A.; Schnakers, C.; Thonnard, M.; Charland-Verville, V.; Bernard, C.; et al. Metabolic activity in external and internal awareness networks in severely brain-damaged patients. J. Rehabil. Med. 2012, 44, 487–494. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, H.S.; An, Y.S. Brain metabolism in patients with vegetative state after post-resuscitated hypoxic-ischemic brain injury: Statistical parametric mapping analysis of F-18 fluorodeoxyglucose positron emission tomography. Chin. Med. J. (Engl.) 2013, 126, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Stender, J.; Kupers, R.; Rodell, A.; Thibaut, A.; Chatelle, C.; Bruno, M.-A.; Gejl, M.; Bernard, C.; Hustinx, R.; Laureys, S.; et al. Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J. Cereb. Blood Flow Metab. 2015, 35, 58–65. [Google Scholar] [CrossRef]
- Stender, J.; Gosseries, O.; Bruno, M.-A.; Charland-Verville, V.; Vanhaudenhuyse, A.; Demertzi, A.; Chatelle, C.; Thonnard, M.; Thibaut, A.; Heine, L.; et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet 2014, 384, 514–522. [Google Scholar] [CrossRef]
- Steinhoff, N.; Heine, A.M.; Vogl, J.; Weiss, K.; Aschraf, A.; Hajek, P.; Schnider, P.; Tucek, G. A pilot study into the effects of music therapy on different areas of the brain of individuals with unresponsive wakefulness syndrome. Front. Neurosci. 2015, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Bodart, O.; Gosseries, O.; Wannez, S.; Thibaut, A.; Annen, J.; Boly, M.; Rosanova, M.; Casali, A.G.; Casarotto, S.; Tononi, G.; et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. Neuroimage Clin. 2017, 14, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Golkowski, D.; Merz, K.; Mlynarcik, C.; Kiel, T.; Schorr, B.; Lopez-Rolon, A.; Lukas, M.; Jordan, D.; Bender, A.; Ilg, R. Simultaneous EEG-PET-fMRI measurements in disorders of consciousness: An exploratory study on diagnosis and prognosis. J. Neurol. 2017, 264, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.; Du, J.; Huo, S.; Li, R.; Song, W. Neural correlates of different behavioral response to transcranial direct current stimulation between patients in the unresponsive wakefulness syndrome and minimally conscious state. Neurol. Sci. 2020, 41, 75–82. [Google Scholar] [CrossRef]
- Hermann, B.; Salah, A.B.; Perlbarg, V.; Valente, M.; Pyatigorskaya, N.; Habert, M.-O.; Raimondo, F.; Stender, J.; Galanaud, D.; Kas, A.; et al. Habituation of auditory startle reflex is a new sign of minimally conscious state. Brain 2020, 143, 2154–2172. [Google Scholar] [CrossRef]
- Carrière, M.; Cassol, H.; Aubinet, C.; Panda, R.; Thibaut, A.; Larroque, S.K.; Simon, J.; Martial, C.; A Bahri, M.; Chatelle, C.; et al. Auditory localization should be considered as a sign of minimally conscious state based on multimodal findings. Brain Commun. 2020, 2, fcaa195. [Google Scholar] [CrossRef]
- Sattin, D.; Rossi Sebastiano, D.; D’incerti, L.; Guido, D.; Marotta, G.; Benti, R.; Tirelli, S.; Magnani, F.G.; Bersano, A.; Duran, D.; et al. Visual behaviors in disorders of consciousness: Disentangling conscious visual processing by a multimodal approach. Eur. J. Neurosci. 2020, 52, 4345–4355. [Google Scholar] [CrossRef]
- Hermann, B.; Stender, J.; Habert, M.-O.; Kas, A.; Denis-Valente, M.; Raimondo, F.; Pérez, P.; Rohaut, B.; Sitt, J.D.; Naccache, L. Multimodal FDG-PET and EEG assessment improves diagnosis and prognostication of disorders of consciousness. Neuroimage Clin. 2021, 30, 102601. [Google Scholar] [CrossRef]
- Sattin, D.; Rossi Sebastiano, D.; Magnani, F.G.; D’Incerti, L.; Marotta, G.; Benti, R.; Tirelli, S.; Bersano, A.; Duran, D.; Visani, E.; et al. Visual fixation in disorders of consciousness: Development of predictive models to support differential diagnosis. Physiol. Behav. 2021, 230, 113310. [Google Scholar] [CrossRef]
- Curatola, A.; Graglia, B.; Granata, G.; Conti, G.; Capossela, L.; Manni, L.; Ferretti, S.; Di Giuda, D.; Romeo, D.M.; Calcagni, M.L.; et al. Combined treatment of nerve growth factor and transcranical direct current stimulations to improve outcome in children with vegetative state after out-of-hospital cardiac arrest. Biol. Direct. 2023, 18, 24. [Google Scholar] [CrossRef]
- Gatto, A.; Capossela, L.; Conti, G.; Eftimiadi, G.; Ferretti, S.; Manni, L.; Curatola, A.; Graglia, B.; Di Sarno, L.; Calcagni, M.L.; et al. Intranasal human-recombinant NGF administration improves outcome in children with post-traumatic unresponsive wakefulness syndrome. Biol. Direct. 2023, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Fan, S.; Xu, Z.; Wan, X.; Yang, Q.; Yin, Y.; Wu, X.; Wu, S.; Zhang, H.; Ma, C. Trigeminal nerve stimulation for prolonged disorders of consciousness: A randomized double-blind sham-controlled study. Brain Stimul. 2023, 16, 819–827. [Google Scholar] [CrossRef]
- Usami, N.; Asano, Y.; Ikegame, Y.; Takei, H.; Yamada, Y.; Yano, H.; Shinoda, J. Cerebral Glucose Metabolism in Patients with Chronic Disorders of Consciousness. Can. J. Neurol. Sci. 2023, 50, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Zhang, Q.; Quan, Z.; Wang, Y.; Ma, T.; Jiang, J.; Kang, F.; Wang, J. Whole-brain glucose metabolic pattern differentiates minimally conscious state from unresponsive wakefulness syndrome. CNS Neurosci. Ther. 2024, 30, e14787. [Google Scholar] [CrossRef]
- He, Z.; Lu, R.; Ge, J.; Guan, Y.; Chen, Y.; Liu, G.; Xie, H.; Bai, Y.; Wu, Y.; Wu, J.; et al. Disorder of consciousness related pattern could distinguish minimally conscious state from unresponsive wakefulness syndrome: A F-18-FDG-PET study. Brain Res. Bull. 2024, 215, 111023. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, N.; Song, M.; Chai, X.; He, Q.; Cao, T.; Kong, D.; Song, Z.; Zhang, G.; Liu, L.; et al. Global glucose metabolism rate as diagnostic marker for disorder of consciousness of patients: Quantitative FDG-PET study. Front. Neurol. 2025, 15, 1425271. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef]
- Luce, J.M. Chronic disorders of consciousness following coma: Part two: Ethical, legal, and social issues. Chest 2013, 144, 1388–1393. [Google Scholar] [CrossRef]
- Tung, J.; Speechley, K.N.; Gofton, T.; Gonzalez-Lara, L.E.; Graham, M.; Naci, L.; Peterson, A.H.; Owen, A.M.; Weijer, C. Towards the assessment of quality of life in patients with disorders of consciousness. Qual. Life Res. 2020, 29, 1217–1227. [Google Scholar] [CrossRef]
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef] [PubMed]
| Authors | N. Ref | Year | Country | Study Design | N. Pts. | VS/UWS (%) | Aetiology |
|---|---|---|---|---|---|---|---|
| Levy D.E. et al. | [20] | 1987 | USA, Canada | Prospective | 10 | 7 (70) | CPA, trauma, anesthesia-hypoxia and asphyxia |
| De Volder A.G. et al. | [21] | 1994 | Belgium | Prospective | 12 | 7 (58) | CPA |
| Tommasino C. et al. | [22] | 1995 | Italy | Prospective | 25 | 10 (40) | Cerebrovascular accident, trauma and CPA |
| Rudolf J. et al. | [23] | 1999 | Germany | Prospective | 24 | 24 (100) | CPA, primary ipo-anoxia due to myasthenic crisis, attempted suicide, anesthesia |
| Laureys S. et al. | [55] | 1999 | Belgium | Retrospective | 82 | 4 (5) | intoxication, CPA, trauma |
| Laureys S. et al. | [24] | 2000 | Belgium | Prospective | 58 | 5 (9) | CPA |
| Laureys S. et al. | [25] | 2002 | Belgium | Prospective | 30 | 15 (50) | CPA, anoxia, drug abuse, encephalitis, and intoxication |
| Rudolf J. et al. | [26] | 2002 | Germany | Prospective | 18 | 9 (50) | CPA |
| Beuthien-Baumann B. et al. | [27] | 2003 | Germany | Prospective | 23 | 16 (69) | Trauma, hemorrhage |
| Kassubek J. et al. | [28] | 2003 | Germany | Prospective | 7 | 7 (100) | CPA, intoxication, |
| Coleman M.R. et al. | [29] | 2005 | UK | Prospective | 10 | 6 (60) | Trauma, hemorrhage |
| Juengling F.D. et al. | [30] | 2005 | Switzerland, Germany | Prospective | 5 | 5 (100) | CPA, intoxication |
| Nakayama N. et al. | [31] | 2006 | Japan | Retrospective | 82 | 17 (21) | Trauma |
| Bruno M.A. et al. | [56] | 2010 | Belgium | Prospective | 49 | 10 (20) | Anoxia |
| Kim Y.W. et al. | [32] | 2010 | Republic of Korea | Prospective | 24 | 12 (50) | Trauma, hypoxia, cerebrovascular injury |
| Snyman N. et al. | [33] | 2010 | Australia | Prospective | 3 | 3 (100) | Trauma, drowning |
| Phillips C.L. et al. | [34] | 2011 | Belgium | Retrospective | 82 | 13 (16) | Anoxia, CPA, trauma, thrombosis, hemorrhage |
| Thibaut A. et al. | [35] | 2012 | Belgium | Prospective | 70 | 24 (34) | CPA, anoxia, trauma, stroke, meningitis, hemorrhage |
| Kim Y.W. et al. | [36] | 2013 | Republic of Korea | Prospective | 32 | 17 (53) | CPA |
| Stender J. et al. | [37] | 2014 | Denmark, Belgium, Australia, USA, Canada | Retrospective | 41 | 14 (34) | Trauma, CPA, infection, stroke, hemorrhage |
| Stender J. et al. | [38] | 2014 | Belgium, Canada, Denmark | Retrospective | 12 | 41 (33) | Trauma, cardiac arrest, stroke, hemorrhage, infection and metabolic disorders |
| Steinhoff N. et al. | [39] | 2015 | Austria | Prospective | 4 | 4 (100) | ns |
| Bodart O. et al. | [40] | 2017 | Belgium, USA, Italy, Brazil | Retrospective | 24 | 9 (38) | Trauma and non-traumatic injuries |
| Golkowski D. et al. | [41] | 2017 | Germany | Prospective | 20 | 14 (70) | Trauma, stroke, anoxia, metabolic |
| Zhang Y. et al. | [42] | 2019 | China | Prospective | 19 | 8 (42) | Anoxia, trauma, hemorrhage |
| Hermann B. et al. | [43] | 2020 | France, Belgium, USA | Prospective | 96 | 48 (50) | Anoxia, trauma, hemorrhage |
| Carrière M. et al. | [44] | 2020 | Belgium, UK | Retrospective | 186 | 64 (34) | Trauma and non-traumatic injuries |
| Sattin D. et al. | [45] | 2020 | Italy | Retrospective | 54 | 42 (78) | Trauma, anoxia, ischemia and hemorrhage |
| Hermann B. et al. | [46] | 2021 | France, Belgium | Prospective | 52 | 21 (40) | Trauma, anoxia, ischemia and hemorrhage, intoxication, encephalomielitis |
| Sattin D. et al. | [47] | 2021 | Italy | Retrospective | 58 | 42 (72) | Trauma, anoxia, ischemia and hemorrhage |
| Curatola A. et al. | [48] | 2023 | Italy | Prospective | 3 | 3 (100) | PCA |
| Gatto A. et al. | [49] | 2023 | Italy | Prospective | 3 | 3 (100) | Trauma |
| Ma H. et al. | [50] | 2023 | China | Prospective | 60 | 53 (88) | Anoxia, trauma, and stroke |
| Usami N. et al. | [51] | 2023 | Japan | Retrospective | 50 | 15 (30) | Trauma |
| Guo K. et al. | [52] | 2024 | China | Prospective | 57 | 21 (37) | Trauma, hemorrhage, ischemia, toxic encephalopathy. |
| He Z. et al. | [53] | 2024 | China | Prospective | 15 | 7 (47) | Trauma, hypoxia, ischemia |
| Liu D. et al. | [54] | 2025 | China, UK | Retrospective | 56 | 21 (38) | Trauma, hypoxia–ischemia, hemorrhage |
| Authors | Ref. | Device and Tracers | Reported Mean Activity (MBq) | DOCs Comparison | Cerebral Regions | Main Findings |
|---|---|---|---|---|---|---|
| Levy D.E.et al. | [20] | PET; [15O]CO2, [18F]FDG | 555/min for 8 min for [15O]CO2, 185–370 for [18F]FDG | VS vs. LIS | All | Cortical glucose metabolism is more reduced in VS than in LIS patients. In VS, this reduction is present also in basal nuclei and cerebellum. Cerebral blood flow exhibits more variable reduction. |
| DeVolder A.G. et al. | [21] | PET; [18F]FDG | ns | na | Parieto-occipital cortex, frontomesial junction, striatum, and visual cortex | Cerebral glucose metabolism is decreased in VS. The most consistent regional alterations are in the parieto-occipital cortex. |
| Tommasino C. et al. | [22] | PET; [18F]FDG | 3.7/Kg | VS vs. coma, VS vs. PVS | All | Coma and VS patients have reduced brain glucose metabolism with overlapping patterns. Higher metabolic activity is found in the primary visual cortex of VS subjects compared to coma patients. PVS subjects have a more reduced metabolism compared to VS. |
| Rudolf J. et al. | [23] | PET; [18F]FDG | 370 | PVS vs. AVS | All | Glucose metabolism is reduced in VS patients, with a significantly lower metabolism in PVS compared to AVS. |
| Laureys S. et al. | [55] | PET; [18F]FDG | 185–370 | na | Prefrontal, premotor, and parietotemporal association areas and posterior cingulate cortex/precuneus | Impaired glucose metabolism in the prefrontal, premotor, and parietotemporal association areas and posterior cingulate cortex/precuneus is present in VS. Various prefrontal and premotor areas are less tightly connected with the posterior cingulate cortex than in HC. |
| Laureys S. et al. | [24] | PET; [18F]FDG and [15O]H2O | 185–370 for [18F]FDG, 222–296 for [15O]H2O | na | The study focuses on Brodmann areas 41 and 42 | VS patients have lower resting metabolism but maintain the activation of bilateral auditory areas after stimulation. Temporo-parietal junction fails to activate. |
| Laureys S. et al. | [25] | PET; [18F]FDG and [15O]H2O | 185–370 for [18F]FDG, 222 for [15O]H2O | na | All | VS patients have reduced brain glucose metabolism. However, somatosensory stimulation activates the midbrain, contralateral thalamus, and primary somatosensory cortex, even in the absence of detectable cortical activation. Secondary somatosensory, bilateral insular, posterior parietal, and anterior cingulate cortices did not show activation. The activated primary somatosensory cortex is functionally disconnected from secondary somatosensory, bilateral posterior parietal, premotor, polysensory superior temporal, and prefrontal cortices. |
| Rudolf J. et al. | [26] | PET; [18F]FDG and [11C]flumazenil | Ns for [18F]FDG, 740 for [11C]flumazenil | na | All supratentorial cortical regions | VS patients have overall reduced glucose metabolism. Benzodiazepine receptor binding sites are also reduced, with a gross correspondence between the two alterations. |
| Beuthien-Baumann B. et al. | [27] | PET; [18F]FDG | 300 | na | Frontal cortex, temporal cortex, less frequently occipital cortex, parietal cortex, and cerebellum | VS patients demonstrate a reduction in glucose metabolism, with less grade in the vermis cerebelli, similarl to the reduction in perfusion at SPECT images. |
| Kassubek J. et al. | [28] | PET; [15O]H2O and [18F]FDG | 1000 for [15O]H2O, 200 ± 20 for [18F]FDG | na | Parietal and frontotemporal cortices, cuneus and precuneus, cingulum, frontal medial gyrus, the precentral gyrus, transverse temporal gyrus and thalamus, | VS subjects have widespread hypometabolism. Pain-induced hyperperfusion is present in the posterior insula/secondary somatosensory cortex, postcentral gyrus/primary somatosensory cortex, the cingulate cortex contralateral to the stimulus, and in the posterior insula ipsilateral to the stimulus. |
| Coleman M.R. et al. | [29] | PET; [18F]FDG | 74 | MCS vs. VS | All | The coupling between neuronal electrical activity and regional glucose metabolism is preserved in MCS patients but absent in VS subjects. |
| Juengling F.D. et al. | [30] | PET; [18F]FDG | 200 ± 20 | na | Parietal, parietooccipital, and frontotemporal cortices, cingulum, frontal medial and precentral gyrus, and bilateral thalamus. | VS subjects demonstrate widespread hypometabolism in the parietal, parietooccipital, and frontotemporal cortices, cingulum, frontal medial and precentral gyrus, and within the bilateral thalamus. |
| Nakayama N. et al. | [31] | PET; [18F]FDG | 4.44/Kg | VS vs. MCS | Medial prefrontal regions, the medial frontobasal regions, the cingulate gyrus, and the thalamus. | Bilateral hypometabolism in the medial prefrontal regions, the medial frontobasal regions, the cingulate gyrus, and the thalamus is most widespread and prominent in VS patients compared to MCS and higher brain dysfunction subjects. |
| Bruno M.A. et al. | [56] | PET; [18F]FDG | ns | na | Fronto-parietal cortical network | Patients without fixation show metabolic dysfunction in fronto-parietal cortical network, which is not different from the brain function in patients with visual fixation. |
| Kim Y.W. et al. | [32] | PET; [18F]FDG | 555 | na | Left precuneus, both posterior cingulate cortices, left superior parietal lobule, cerebellum, and right supramarginal cortex. | Patients in PVS have decreased glucose metabolism in the left precuneus, both posterior cingulate cortices, and the left superior parietal lobule and increased metabolism in both the cerebellum and the right supramarginal cortices. Decreased level of consciousness is correlated with decreased metabolism in both posterior cingulate cortices. |
| Snyman N.. et al. | [33] | PET/CT; [18F]FDG | 370 scaled to weight | na | na | No objective changes in PET after zolpidem therapy are demonstrated in pediatric PVS patients. |
| Phillips C.L et al. | [34] | PET; [18F]FDG | 185–370 | VS vs. LIS | Brainstem, bilateral lower temporal lobe and cerebellum | A classifier able to distinguish LIS and VS with good performances is built. |
| Thibaut A. et al. | [35] | PET; [18F]FDG | 185–370 | VS vs. MCS, VS vs. LIS | Thalami and cortical network encompassing the extrinsic/lateral network and the intrinsic/medial network. | VS/UWS patients show metabolic dysfunction in extrinsic and intrinsic networks and thalami. |
| Kim Y.W. et al. | [36] | PET; [18F]FDG | 555 | na | Decreased metabolism in bilateral precuneus, bilateral posterior cingulate gyrus, bilateral middle frontal gyri, bilateral superior parietal gyri, bilateral middle occipital gyri, bilateral precentral gyri, and increased metabolism in bilateral insula, bilateral cerebella, and the brainstem | VS demonstrates decreased metabolism in bilateral precuneus, bilateral posterior cingulate gyrus, bilateral middle frontal gyri, bilateral superior parietal gyri, bilateral middle occipital gyri, bilateral precentral gyri, and increased brain metabolism in bilateral insula, bilateral cerebella, and the brainstem. Level of consciousness is correlated with brain metabolism in bilateral fusiform and superior temporal gyri. |
| Stender J. et al. | [37] | PET/CT; [18F]FDG | 185–370 | VS vs. MCS | Frontoparietal cortex, brainstem, and thalamus | Differences in glucose metabolism between VS/UWS and MCS are most pronounced in the frontoparietal cortex. In the brainstem and thalamus, metabolism declines equally. |
| Stender J. et al. | [38] | ns; [18F]FDG | ns | na | ns | PET could be used to complement bedside examinations and predict the long-term recovery of patients with UWS. MRI seems to be less accurate. |
| Steinhoff N. et al. | [39] | PET/CT; [18F]FDG | 230 | na | Frontal, hippocampal, and cerebellar | Tracer uptake in frontal, hippocampal, and cerebellar regions is higher in VS patients after music therapy. |
| Bodart O. et al. | [40] | PET/CT; [18F]FDG | 185–370 | na | Preservation of the metabolism only in the brainstem or the cerebellum | PET and EEG provide congruent results, revealing preserved metabolic rates and high complexity levels in patients who are behaviourally unresponsive. |
| Golkowski D. et al. | [41] | PET/MR; [18F]FDG | 185 | VS vs. MCS | Occipital cortex | Glucose metabolism in the occipital lobe is significantly higher in MCS than in VS patients. |
| Zhang Y. et al. | [42] | PET/CT; [18F]FDG | 3.7–7.4 Kg | VS vs. MCS | Left caudate, right caudate, left precentral, left mid-temporal gyrus, left Heschl gyrus, left rolandic operculum, right Heschl gyrus, right superior temporal gyrus, left insula, left midfrontal gyrus and orbital part of inferior frontal gyrus | Reduced metabolism is present in VS patients compared to MCS and HC after transcranial direct current stimulation. A residual brain activity in stimulated areas is necessary for behavioral response to transcranial direct current stimulation. |
| Hermann B. et al. | [43] | PET; [18F]FDG | ns | VS vs. MCS | Posterior and anterior cingulate, premotor area and anterior prefrontal cortex | The presence of auditory startle reflex is suggestive of an MCS or conscious state and correlates with the functional preservation of large-scale cortical networks related to conscious processing and voluntary inhibitory control of behaviour. |
| Carrière M. et al. | [44] | PET; [18F]FDG | 185–370 | VS vs. MCS | fronto-parieto-occipital | Patients without auditory localization show decreased metabolism in a large bilateral fronto-parieto-occipital network. Patients with localization show regional decreased metabolism with the hotspots located in the ventral anterior and posterior cingulate cortex left premotor cortex, right frontal eye fields, right angular gyrus, right visual secondary and associative areas, and right fusiform gyrus. |
| Sattin D. et al. | [45] | PET; [18F]FDG | 140 ± 30 | na | Right calcarine cortex and neighboring right lingual gyrus. | PET underlines differences in the right calcarine cortex and neighboring right lingual gyrus between patients with reflexive behavior and those manifesting a cognitively and cortically mediated behavior after visual stimuli. |
| Hermann B. et al. | [46] | PET/CT; [18F]FDG | 2/Kg | VS vs. MCS | Best preserved hemisphere | [18F]FDG metabolic index of the best-preserved hemisphere has good diagnostic performances in distinguishing between MCS and VS subjects. |
| Sattin D. et al. | [47] | PET; [18F]FDG | 140 ± 30 | VS vs. MCS | na | PET data could be included in a model with good performances to predict visual fixation to support differential diagnosis in DOC. |
| Curatola A. et al. | [48] | PET; [18F]FDG | 3/Kg | na | All | The improvement derived from treatment with NGF and transcranial direct current stimulations can be assessed with PET imaging. |
| Gatto A. et al. | [49] | PET; [18F]FDG | 3/Kg | na | All | The improvement of treatment with intranasal human-recombinant NGF can be assessed with PET imaging. |
| Ma H. et al. | [50] | PET/CT; [18F]FDG | 150 | na | Right parahippocampal cortex, right precuneus, and bilateral middle cingulate cortex | Glucose metabolism of the right parahippocampal cortex, right precuneus, and bilateral middle cingulate cortex are significantly increased in DOC patients treated with trigeminal nerve stimulation. |
| Usami N. et al. | [51] | PET; [18F]FDG | 3.5/Kg | VS vs. MCS | Left occipital region | Glucose metabolism reduces with consciousness deterioration in cerebral regions. Metabolic activity is higher in the left occipital region in MCS—patients than in VS subjects. |
| Guo K. et al. | [52] | PET/CT; [18F]FDG | 3.7/Kg | VS vs. MCS | Hypometabolism in the frontal–parietal cortex, hypermetabolism in the unilateral lentiform nucleus, putamen, and ante- rior cingulate gyrus | A PET-based score was able to distinguish between UWS and MCS with good performances and to predict the outcome is built. |
| He Z. et al. | [53] | PET/CT; [18F]FDG | 185 | VS vs. MCS | Hypometabolism in the medial and lateral frontal lobes, parieto-temporal lobes, cingulate gyrus, and caudate increased metabolism in the cerebellum and brainstem | A DOC-related pattern of decreased metabolism is present in the medial and lateral frontal lobes, parieto-temporal lobes, cingulate gyrus, and caudate, associated with relatively increased metabolism in the cerebellum and brainstem. This pattern has good accuracy in differentiating between MCS and UWS. |
| Liu D. et al. | [54] | PET/CT; [18F]FDG | 3.7/Kg | VS vs. MCS | Left occipital lobe and the left precuneus | There is a significant difference in cortical metabolism index between MCS and UWS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dondi, F.; Latronico, N.; Bellini, P.; Lucchini, S.; Camoni, L.; Cossandi, M.; Viganò, G.L.; Santo, G.; Bertagna, F. The Role of [18F]FDG PET Imaging for the Assessment of Vegetative State or Unresponsive Wakefulness Syndrome: A Systematic Review. Diagnostics 2025, 15, 1406. https://doi.org/10.3390/diagnostics15111406
Dondi F, Latronico N, Bellini P, Lucchini S, Camoni L, Cossandi M, Viganò GL, Santo G, Bertagna F. The Role of [18F]FDG PET Imaging for the Assessment of Vegetative State or Unresponsive Wakefulness Syndrome: A Systematic Review. Diagnostics. 2025; 15(11):1406. https://doi.org/10.3390/diagnostics15111406
Chicago/Turabian StyleDondi, Francesco, Nicola Latronico, Pietro Bellini, Silvia Lucchini, Luca Camoni, Michela Cossandi, Gian Luca Viganò, Giulia Santo, and Francesco Bertagna. 2025. "The Role of [18F]FDG PET Imaging for the Assessment of Vegetative State or Unresponsive Wakefulness Syndrome: A Systematic Review" Diagnostics 15, no. 11: 1406. https://doi.org/10.3390/diagnostics15111406
APA StyleDondi, F., Latronico, N., Bellini, P., Lucchini, S., Camoni, L., Cossandi, M., Viganò, G. L., Santo, G., & Bertagna, F. (2025). The Role of [18F]FDG PET Imaging for the Assessment of Vegetative State or Unresponsive Wakefulness Syndrome: A Systematic Review. Diagnostics, 15(11), 1406. https://doi.org/10.3390/diagnostics15111406





