Home Spirometry for Post-COVID Recovery: A Clinical Validation Study of an Ultrasonic Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Spirometry
2.3. Home Spirometry
2.4. Quality Control and Spirometry Parameters
2.5. Patient Satisfaction
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Compliance with Home Spirometry
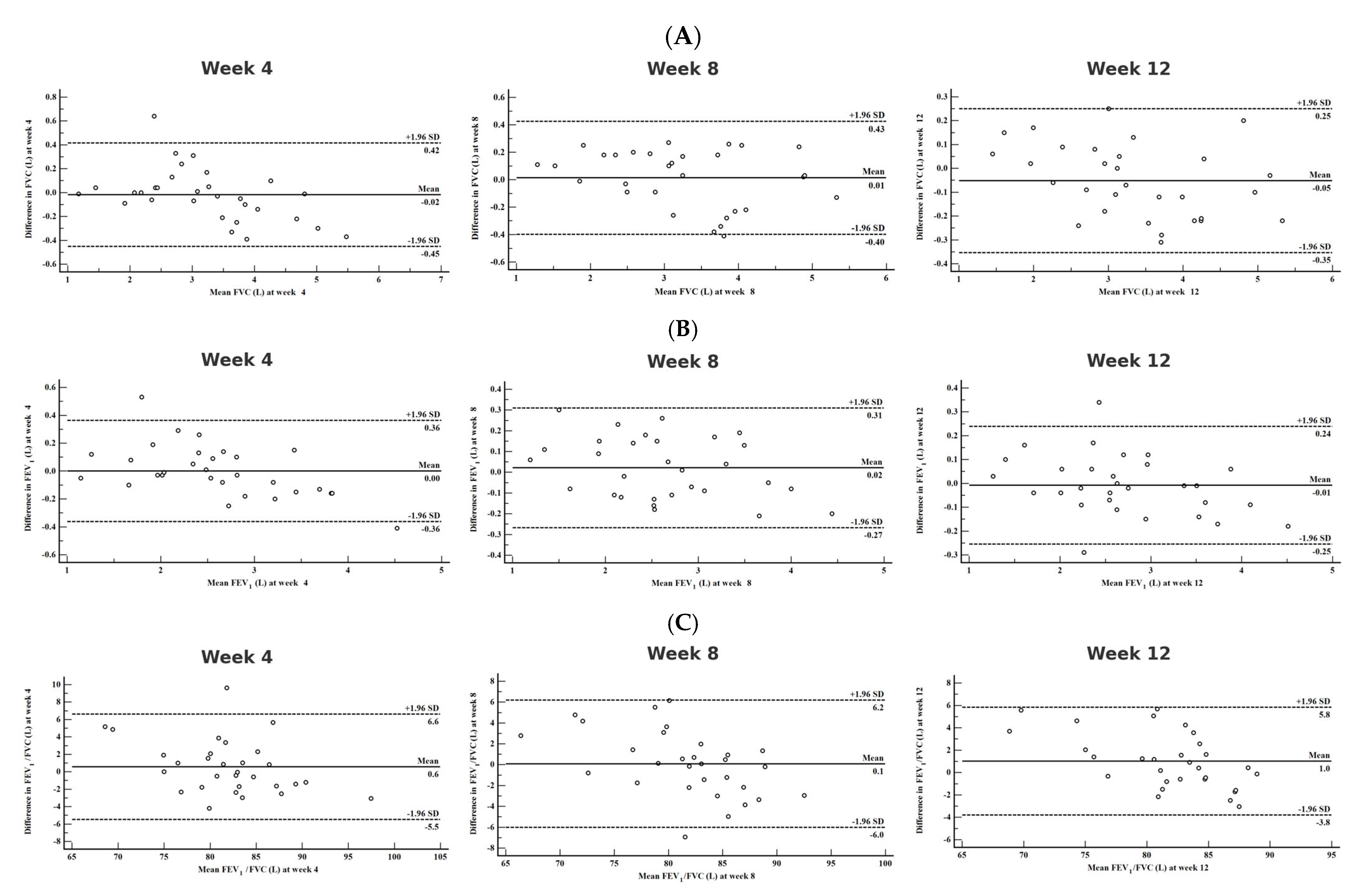
3.3. Agreement Between Clinical and Home Spirometry
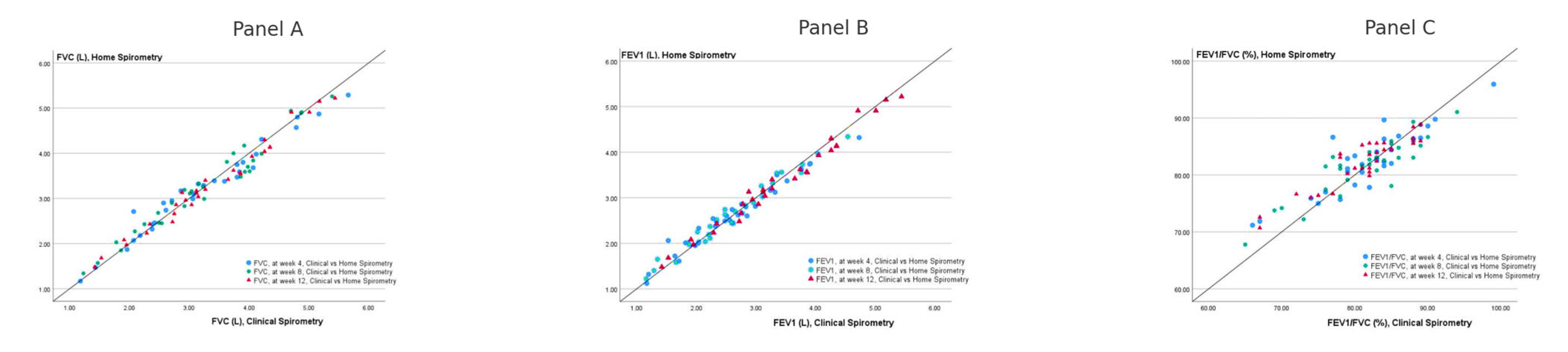
3.4. Correlations Between Clinic and Home Spirometry Measurements
3.5. Validity of Home Spirometry
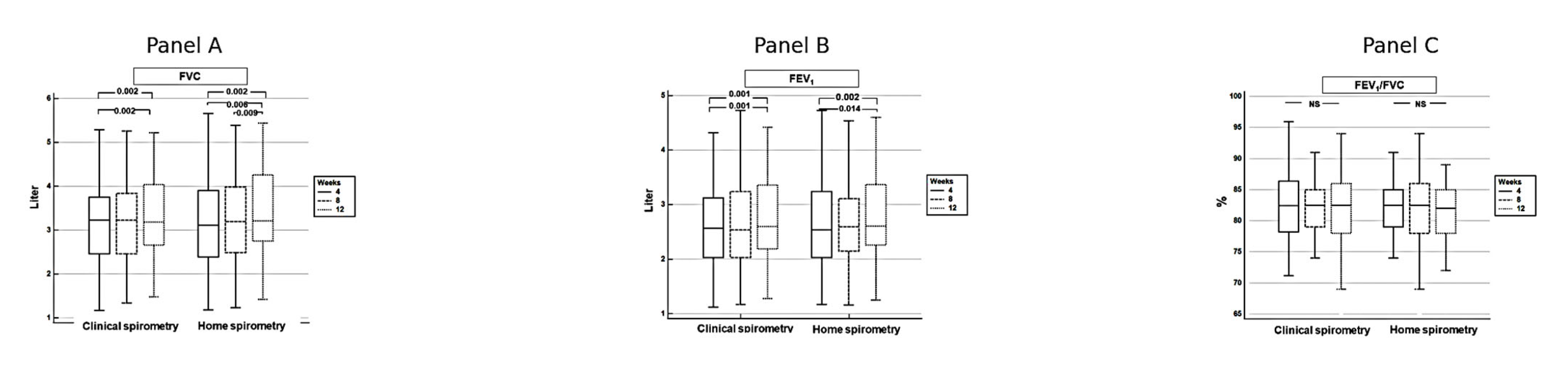
3.6. Improvement in Pulmonary Functions over Time
3.7. Patient Satisfaction with Home vs. Clinical Spirometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FVC | forced vital capacity |
| FEV1 | forced expiratory volume at 1st second |
| COVID-19 | Coronavirus disease 2019 |
| PFT | pulmonary function test |
| DLCO | carbon monoxide diffusing capacity |
| COPD | chronic obstructive pulmonary disease |
| ATS | American Thoracic Society |
| ERS | European Respiratory Society |
| PCR | polymerase chain reaction |
| GLI | Global Lung Initiative |
| SD | standard deviation |
| CI | confidence interval |
| IQR | interquartile range |
| HS | home spirometry |
| CS | clinical spirometry |
| LoA | limits of agreement |
| ICC | intraclass correlation coefficient |
| Z | z-score |
Appendix A
Appendix A.1. Quality Grades of Spirometry Performed with Clinical and Home Spirometry
| Quality Grading of The Test Session | |||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| Clinical spirometry, week 4, n = 29 | FVC | 16 | 12 | 1 | |||
| FEV1 | 19 | 9 | 1 | ||||
| Clinical spirometry, week 8, n = 39 | FVC | 21 | 7 | 1 | |||
| FEV1 | 23 | 6 | |||||
| Clinical spirometry, week 12, n = 29 | FVC | 24 | 4 | 1 | |||
| FEV1 | 23 | 5 | 1 | ||||
| Home spirometry, week 4, n = 30 | FVC | 21 | 7 | 2 | |||
| FEV1 | 20 | 8 | 1 | 1 | |||
| Home spirometry, week 8, n = 30 | FVC | 19 | 8 | 1 | 2 | ||
| FEV1 | 25 | 2 | 1 | 2 | |||
| Home spirometry, week 12, n = 30 | FVC | 23 | 6 | 1 | |||
| FEV1 | 19 | 10 | 1 | ||||
References
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542-2020. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J.Ž. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Ramani, C.; Davis, E.M.; Kim, J.S.; Provencio, J.J.; Enfield, K.B.; Kadl, A. Post-ICU COVID-19 outcomes: A case series. Chest 2020, 159, 215. [Google Scholar] [CrossRef]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef]
- Stockley, J.A.; Alhuthail, E.A.; Coney, A.M.; Parekh, D.; Geberhiwot, T.; Gautum, N.; Madathil, S.C.; Cooper, B.G. Lung function and breathing patterns in hospitalised COVID-19 survivors: A review of post-COVID-19 Clinics. Respir. Res. 2021, 22, 255. [Google Scholar] [CrossRef]
- Pietranis, K.A.; Izdebska, W.M.; Kuryliszyn-Moskal, A.; Dakowicz, A.; Ciołkiewicz, M.; Kaniewska, K.; Dzięcioł-Anikiej, Z.; Wojciuk, M. Effects of pulmonary rehabilitation on respiratory function and thickness of the diaphragm in patients with post-COVID-19 syndrome: A randomized clinical trial. J. Clin. Med. 2024, 13, 425. [Google Scholar] [CrossRef]
- Valenzuela, C.; Nigro, M.; Chalmers, J.D.; Wagers, S.; Aujayeb, A.; Hellemons, M.E.; Löffler-Ragg, J.; Brightling, C.E.; Aliberti, S. COVID-19 follow-up programmes across Europe: An ERS END-COVID CRC survey. Eur. Respir. J. 2022, 60, 2200923. [Google Scholar] [CrossRef]
- Li, J.; Jing, G.; Fink, J.B.; Porszasz, J.; Moran, E.M.; Kiourkas, R.D.; McLaughlin, R.; Vines, D.L.; Dhand, R. Airborne particulate concentrations during and after pulmonary function testing. Chest 2021, 159, 1570–1574. [Google Scholar] [CrossRef]
- Gemicioğlu, B.; Börekçi, Ş.; Dilektaşlı, A.G.; Ulubay, G.; Azap, Ö.; Saryal, S. Turkish thoracic society experts consensus report: Recommendations for pulmonary function tests during and after COVID 19 pandemic. Turk. Thorac. J. 2020, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.H.; Lloyd, J.K.; Cooper, B.G. Lung function testing in the COVID-19 endemic. Lancet Respir. Med. 2020, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Beaucage, F.; Comondore, V.; Faughnan, M.; Kovesi, T.; McCoy, C.; McParland, C.; Pawluski, D.; Refahi, F.; Road, J. Resumption of pulmonary function testing during the COVID-19 pandemic: A position statement from the Canadian Thoracic Society and the Canadian Society of Respiratory Therapists. Can. J. Respir. Crit. Care Sleep Med. 2022, 6, 78–81. [Google Scholar] [CrossRef]
- Cortés-Telles, A.; López-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021, 288, 103644. [Google Scholar] [CrossRef]
- Stewart, I.; Jacob, J.; George, P.M.; Molyneaux, P.L.; Porter, J.C.; Allen, R.J.; Aslani, S.; Baillie, J.K.; Barratt, S.L.; Beirne, P. Residual lung abnormalities after COVID-19 hospitalization: Interim analysis of the UKILD post–COVID-19 study. Am. J. Respir. Crit. Care Med. 2023, 207, 693–703. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Shang, Y.-M.; Song, W.-B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Nakshbandi, G.; Moor, C.; Wijsenbeek, M. Home monitoring for patients with ILD and the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 1172–1174. [Google Scholar] [CrossRef]
- Rockwell, K.L.; Gilroy, A.S. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am. J. Manag. Care 2020, 26, 147–148. [Google Scholar]
- Wilson, C.L.; McLaughlin, C.; Cairncross, A.; Gabbay, E.; Noble, P.B.; Blakey, J.D.; Crawford, A.L. Home spirometry appears accurate and feasible for monitoring chronic respiratory disease. ERJ Open Res. 2024, 10, 00937-2023. [Google Scholar] [CrossRef]
- Anand, R.; McLeese, R.; Busby, J.; Stewart, J.; Clarke, M.; Man, W.D.; Bradley, J. Unsupervised home spirometry versus supervised clinic spirometry for respiratory disease: A systematic methodology review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220248. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Hanania, N.A.; Chaudhuri, R.; Sagara, H.; Bailes, Z.; Fowler, A.; Peachey, G.; Pizzichini, E.; Slade, D. Clinic vs home spirometry for monitoring lung function in patients with asthma. Chest 2023, 164, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Sekerel, B.E.; Unsal, H.; Sahiner, U.M.; Uysal Soyer, O.; Damadoglu, E.; Karakaya, G.; Kalyoncu, A.F. Clinical Validation of the Spirohome Clinic Ultrasonic Spirometer in Child and Adolescent Patients. J. Asthma Allergy 2022, 15, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ilić, M.; Javorac, J.; Milenković, A.; Živanović, D.; Miljković, D.; Kašiković Lečić, S.; Savić, N.; Tot Vereš, K.; Kovačević, D.; Vujičić, E. Home-Based Spirometry in Patients with Interstitial Lung Diseases: A Real-Life Pilot “FACT” Study from Serbia. J. Pers. Med. 2023, 13, 793. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.M.; Sivam, S.; Dentice, R.L.; Dwyer, T.J.; Jo, H.E.; Lau, E.M.; Munoz, P.A.; Nolan, S.A.; Taylor, N.A.; Visser, S.K.; et al. Quality of home spirometry performance amongst adults with cystic fibrosis. J Cyst. Fibros. 2022, 21, 84–87. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Johnson, B.; Steenbruggen, I.; Graham, B.L.; Coleman, C. Improving spirometry testing by understanding patient preferences. ERJ Open Res. 2021, 7, 00712-2020. [Google Scholar] [CrossRef]
- Bland, J. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Noth, I.; Cottin, V.; Chaudhuri, N.; Corte, T.J.; Johannson, K.A.; Wijsenbeek, M.; Jouneau, S.; Michael, A.; Quaresma, M.; Rohr, K.B.; et al. Home spirometry in patients with idiopathic pulmonary fibrosis: Data from the INMARK trial. Eur. Respir. J. 2021, 58, 2001518. [Google Scholar] [CrossRef]
- Kocks, J.; le Rütte, T.; Kerkhof, M.; Gerritsma, Y.; Driessen-Roelfszema, M.; van den Bemt, L.; Muris, J.; Riemersma, R.; Sandelowsky, H.; Stridh, B. Feasibility, quality and added value of unsupervised at-home spirometry in primary care. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Avdimiretz, N.; Wilson, D.; Grasemann, H. Comparison of a handheld turbine spirometer to conventional spirometry in children with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Paynter, A.; Khan, U.; Heltshe, S.L.; Goss, C.H.; Lechtzin, N.; Hamblett, N.M. A comparison of clinic and home spirometry as longtudinal outcomes in cystic fibrosis. J. Cyst. Fibros. 2022, 21, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Curley, R.; Campbell, M.J.; Walters, S.J.; Hoo, Z.H.; Wildman, M.J. Regarding the articles on home spirometry. J. Cyst. Fibros. 2022, 21, e212–e214. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Izmailova, E.S.; Jackson, N.; Ellis, R.; Bhatia, G.; Ruddy, M.; Singh, D. Remote FEV1 Monitoring in Asthma Patients: A Pilot Study. Clin. Transl. Sci. 2021, 14, 529–535. [Google Scholar] [CrossRef]
- Gerbase, M.; Dupuis-Lozeron, E.; Schindler, C.; Keidel, D.; Bridevaux, P.; Kriemler, S.; Probst-Hensch, N.; Rochat, T.; Künzli, N. Agreement between spirometers: A challenge in the follow-up of patients and populations? Respiration 2013, 85, 505–514. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Rodriguez-Roisin, R.; Tetzlaff, K.; Watz, H.; Wouters, E.F.; Disse, B.; Finnigan, H.; Magnussen, H.; Calverley, P.M. Daily home-based spirometry during withdrawal of inhaled corticosteroid in severe to very severe chronic obstructive pulmonary disease. Int. J. Chron Obs. Pulmon Dis. 2016, 11, 1973–1981. [Google Scholar] [CrossRef]
| n = 30 | |
|---|---|
| Age, years, mean (SD) | 48.64 ± 10.49 |
| Male, n (%) | 21 (70) |
| Height, m, mean (SD) | 167.50 ± 10.41 |
| Body mass index, kg/m2, mean (SD) | 27.60 ± 8.16 |
| FVC, L, mean (SD) | 3.20 ± 0.99 |
| FVC, predicted %, mean (SD) | 83.93 ± 22.11 |
| FVC, z-score, mean (SD) | −1.62 ± 1.86 |
| FEV1, L, mean (SD) | 2.60 ± 0.75 |
| FEV1, predicted%, mean (SD) | 82.79 ± 18.02 |
| FEV1, z-score, mean (SD) | −1.46 ± 1.55 |
| FEV1/FVC, %, mean (SD) | 104.36 ± 7.49 |
| FEV1/FVC, z-score, mean (SD) | 0.35 ± 0.96 |
| The Mean Difference Between Clinic–Home Spirometry 95% CI | LoA Mean ± 2 SD | ICC 95% CI | |
|---|---|---|---|
| FVC, best measurement, L, week 4 | −0.02 −0.10–0.06 | −0.45–0.42 | 0.989 0.976–0.995 |
| FVC, best measurement, L, week 8 | 0.01 −0.06–0.09 | −0.40–0.42 | 0.989 0.978–0.995 |
| FVC, best measurement, L, week 12 | −0.05 −0.11–0.01 | −0.35–0.25 | 0.994 0.988–0.997 |
| FEV1, best measurement, L, week 4 | 0.00 −0.07–0.07 | −0.36–0.36 | 0.987 0.972–0.994 |
| FEV1, best measurement, L, week 8 | 0.02 −0.03–0.07 | −0.27–0.31 | 0.991 0.982–0.996 |
| FEV1, best measurement, L, week 12 | −0.01 −0.05–0.03 | −0.25–0.24 | 0.963 0.923–0.983 |
| FEV1/FVC, best measurement, %,week 4 | 0.58 −0.58–1.72 | −5.48–6.63 | 0.932 0.856–0.967 |
| FEV1/FVC, best measurement, week 8 | 0.09 −1.07–1.26 | −6.00–6.20 | 0.928 0.848–0.966 |
| FEV1/FVC, best measurement, week 12 | 1.02 0.10–1.94 | −3.80–5.84 | 0.941 0.876–0.972 |
| The Mean Difference Between Clinic–Home Spirometry 95% CI | LoA Mean ± 2 SD | ICC 95% CI | |
|---|---|---|---|
| z-FVC, week 4 | −0.04 −0.32–0.23 | −1.43–1.34 | 0.866 0.796–0.936 |
| z-FVC, week 8 | −0.04 −0.28–0.20 | −1.25–1.16 | 0.90 0.85–0.96 |
| z-FVC, week 12 | −0.24 −0.45–−0.04 | −1.28–0.79 | 0.914 0.87–0.96 |
| z-FEV1, week 4 | −0.015 −0.29–0.26 | −1.38–1.35 | 0.81 0.71–0.91 |
| z-FEV1, week 8 | −0.033 −0.26–0.20 | −1.2–1.13 | 0.861 0.790–0.933 |
| z-FEV1, week 12 | −0.18 −0.38–0.03 | −6.32–−1.64 | 0.446 0.156–0.736 |
| z-FEV1/FVC, %, week 4 | 0.24 −0.15–0.62 | −1.71–2.19 | 0.472 0.195–0.748 |
| z-FEV1/FVC, week 8 | −0.02 −0.27 −0.27 | −1.37–1.365 | 0.700 0.533–0.854 |
| z-FEV1/FVC, week 12 | 0,11 −0.05–0.27 | −0.68–0.9 | 0.831 0.741–0.921 |
| CS—FVC vs HS—FVC | CS—FVC-z vs HS—FVC- z | CS—FEV1 vs HS—FEV1 | CS—FEV1-z vs HS—FEV1- z | CS—FEV1/FVC vs HS—FEV1/FVC | CS—FEV1/FVC-z vs HS—FEV1/FVC- z | |
|---|---|---|---|---|---|---|
| Week 4 | 0.983 * | 0.931 * | 0.981 * | 0.900 * | 0.887 * | 0.814 * |
| Week 8 | 0.981 * | 0.948 * | 0.985 * | 0.928 * | 0.895 * | 0.833 * |
| Week 12 | 0.990 * | 0.956 * | 0.929 * | 0.943 * | 0.919 * | 0.912 * |
| Week 4 | Week 8 | Week 12 | |
|---|---|---|---|
| FVC, mL, mean ± SD | |||
| Clinical spirometry | 3.20 ± 0.99 | 3.27 ± 0.99 | 3.32 ± 0.99 |
| Home spirometry | 3.22 ± 1.09 | 3.25 ± 1.05 | 3.37 ± 1.05 |
| Difference, absolute, mean ± SD | −0.18 ± 0.22 | 0.14 ± 0.21 | −0.05 ± 0.15 |
| p | 0.66 | 0.72 | 0.08 |
| FEV1, mL, mean ± SD | |||
| Clinical spirometry | 2.60 ± 0.75 | 2.65 ± 0.77 | 2.71 ± 0.74 |
| Home spirometry | 2.60 ± 0.85 | 2.62 ± 0.82 | 2.72 ± 0.82 |
| Difference, absolute, mean ± SD | 0.00 ± 0.18 | 0.02 ± 0.15 | −0.00 ± 0.13 |
| p | 0.97 | 0.43 | 0.75 |
| FEV1/FVC, %, mean ± SD | |||
| Clinical spirometry | 82.31 ± 5.50 | 81.72 ± 5.18 | 82.22± 4.48 |
| Home spirometry | 81.73 ± 6.64 | 81.63 ± 6.72 | 81.20 ± 5.83 |
| Difference, absolute, mean ± SD | 0.58 ± 3.08 | 0.09 ± 3.12 | 1.02 ± 2.46 |
| p | 0.31 | 0.72 | 0.03 |
| Week 4 | Week 8 | Week 12 | |
|---|---|---|---|
| FVC, z-score | |||
| Clinical spirometry | −1.62 ± 1.86 | −1.52 ± 1.81 | −1.41 ± 1.76 |
| Home spirometry | −1.58 ± 1.92 | −1.48 ± 1.93 | −1.17 ± 1.79 |
| Difference, absolute, mean ± SD | −0.44 ± 0.71 | −0.04 ± 0.61 | −0.24 ± 0.53 |
| p | 0.743 | 0.723 | 0.021 |
| FEV1, z-score | |||
| Clinical spirometry | −1.46 ± 1.55 | −1.39 ± 1.56 | −1.25 ± 1.58 |
| Home spirometry | −1.45 ± 1.57 | −1.36 ± 1.58 | −1.08 ± 1.46 |
| Difference, absolute, mean ± SD | −0.01 ± 0.69 | −0.33 ± 0.59 | −0.17 ± 0.52 |
| p | 0.909 | 0.772 | 0.085 |
| FEV1/FVC, z-score | |||
| Clinical spirometry | 0.35 ± 0.96 | 0.24 ± 0.89 | 0.26 ± 0.84 |
| Home spirometry | 0.12 ± 1.37 | 0.25 ± 1.24 | 0.14 ± 0.98 |
| Difference, absolute, mean ± SD | 0.24 ± 0.99 | −0.00 ± 0.69 | 0.11 ± 0.40 |
| p | 0.214 | 0.986 | 0.167 |
| Questions | Home Spirometry | Clinical Spirometry | p |
|---|---|---|---|
| Experienced anxiety when performing spirometry due to uncertainty about executing the maneuver accurately | 15 (51.7%) | 9 (31.0%) | 0.070 |
| To keep blowing even though you do not feel anything is coming out | 10 (34.5%) | 11 (37.9%) | 0.375 |
| Coughing during the maneuver | 12 (41.4%) | 10 (34.5%) | 0.500 |
| Feeling tired after the test | 12 (41.4%) | 10 (34.5%) | 0.500 |
| Concerns about shortness of breath due to the test | 6 (20.7%) | 5 (17.2%) | 1.000 |
| Feeling dizzy | 8 (27.6%) | 5 (17.2%) | 0.250 |
| Nose clip uncomfortable | 8 (27.6%) | 9 (31.0%) | 1.000 |
| Mouthpiece uncomfortable | 5 (17.2%) | 7 (24.1%) | 0.625 |
| Not given enough information about why the test is performed | 2 (6.9%) | 3 (10.3%) | 1.000 |
| Dryness in mouth | 8 (27.6%) | 8 (27.6%) | 1.000 |
| Not given enough information about how to perform the test | 1 (3.4%) | 0 (0%) | 1.000 |
| Lack of support from healthcare professionals running the test | 1 (3.4%) | 0 (0%) | 0.453 |
| Feel embarrassed during the test (e.g., being shouted at to blow) | 2 (6.9%) | 1 (3.4%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilektasli, A.G.; Odabas, A.; Polat, I.; Dogan, A.; Ozkaya, G.; Guclu, O.A.; Acet Ozturk, N.A.; Coskun, F.; Karadag, M. Home Spirometry for Post-COVID Recovery: A Clinical Validation Study of an Ultrasonic Device. Diagnostics 2025, 15, 1396. https://doi.org/10.3390/diagnostics15111396
Dilektasli AG, Odabas A, Polat I, Dogan A, Ozkaya G, Guclu OA, Acet Ozturk NA, Coskun F, Karadag M. Home Spirometry for Post-COVID Recovery: A Clinical Validation Study of an Ultrasonic Device. Diagnostics. 2025; 15(11):1396. https://doi.org/10.3390/diagnostics15111396
Chicago/Turabian StyleDilektasli, Asli Gorek, Ayten Odabas, Ismet Polat, Abdurrahman Dogan, Guven Ozkaya, Ozge Aydin Guclu, Nilufer Aylin Acet Ozturk, Funda Coskun, and Mehmet Karadag. 2025. "Home Spirometry for Post-COVID Recovery: A Clinical Validation Study of an Ultrasonic Device" Diagnostics 15, no. 11: 1396. https://doi.org/10.3390/diagnostics15111396
APA StyleDilektasli, A. G., Odabas, A., Polat, I., Dogan, A., Ozkaya, G., Guclu, O. A., Acet Ozturk, N. A., Coskun, F., & Karadag, M. (2025). Home Spirometry for Post-COVID Recovery: A Clinical Validation Study of an Ultrasonic Device. Diagnostics, 15(11), 1396. https://doi.org/10.3390/diagnostics15111396






