Design and Evaluation of a Portable Pinhole SPECT System for 177Lu Imaging: Monte Carlo Simulations and Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. MC Simulations
2.2. Portable Gamma Camera
- i.
- Tungsten shielding box and pinhole collimator, Weldstone Components GmbH, Germany [34];
- ii.
- CsI(Na) pixelated scintillator, EPIC Crystals (type: GAGG: Gadolinium Aluminium Gallium Garnet), China [35];
- iii.
- Position sensitive photomultiplier (PSPMT), Hamamatsu, Japan [36];
- iv.
- Data acquisition system/electronics (FPGA) [37].
2.3. System’s Evaluation
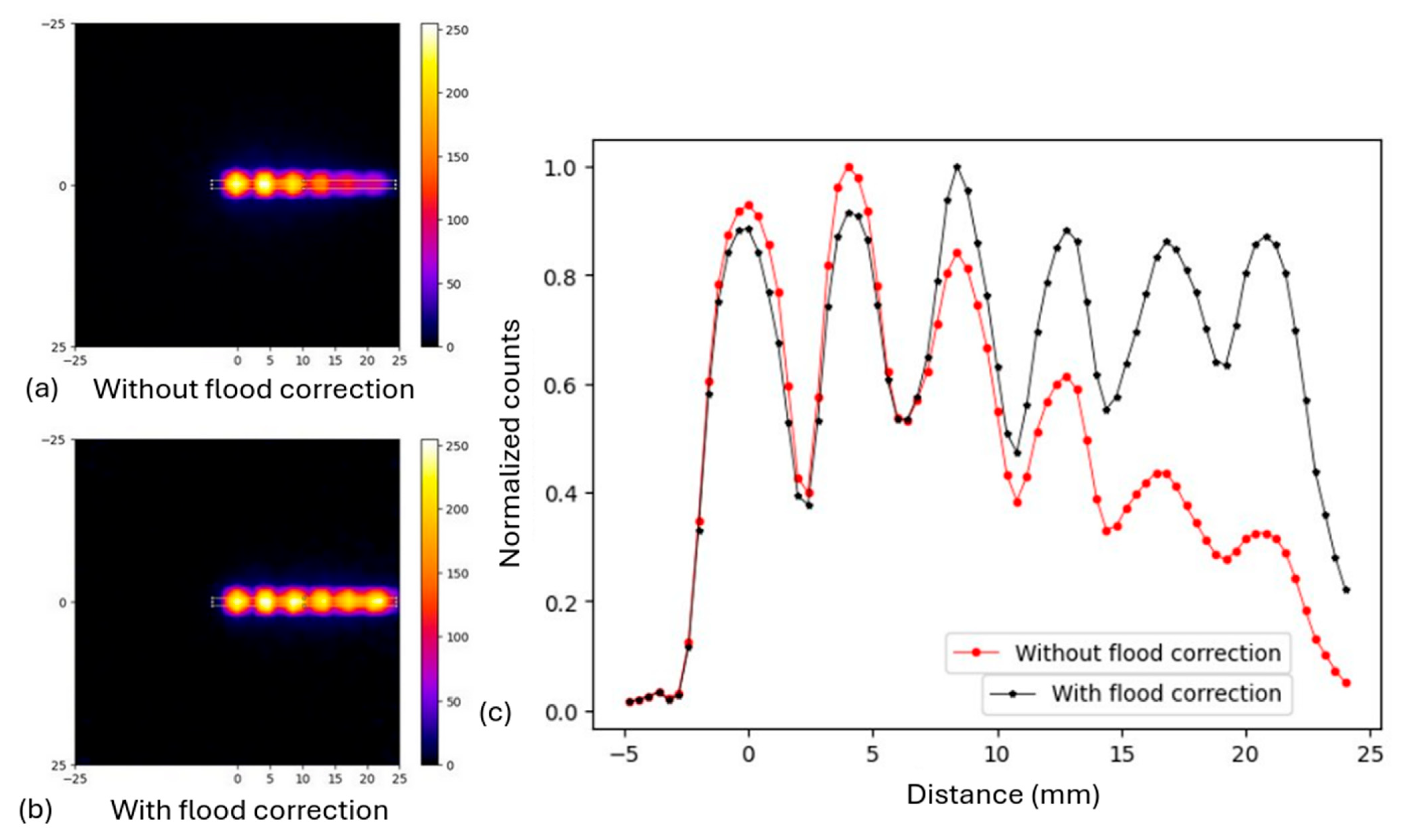
2.3.1. Non-Uniformity Correction
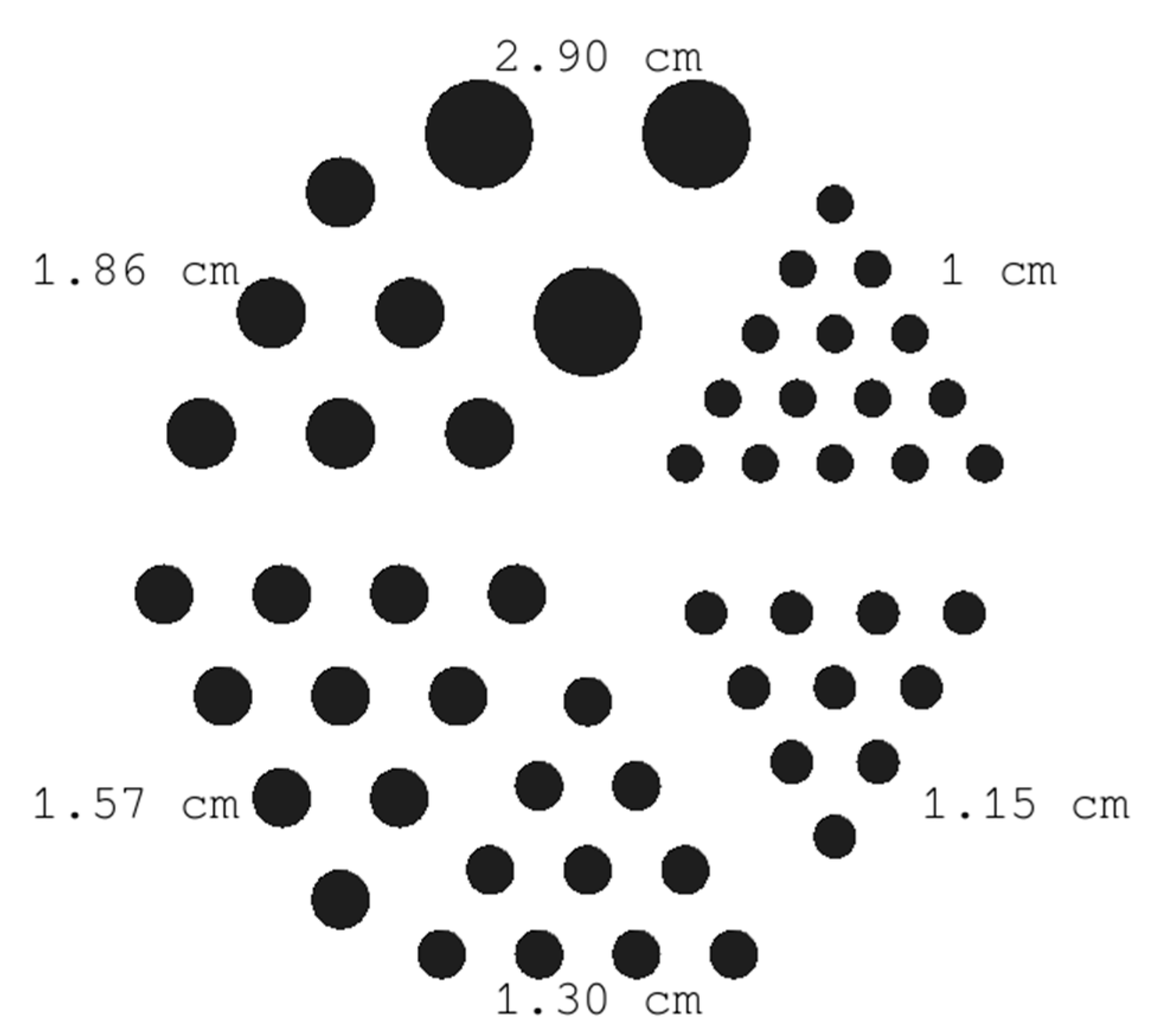
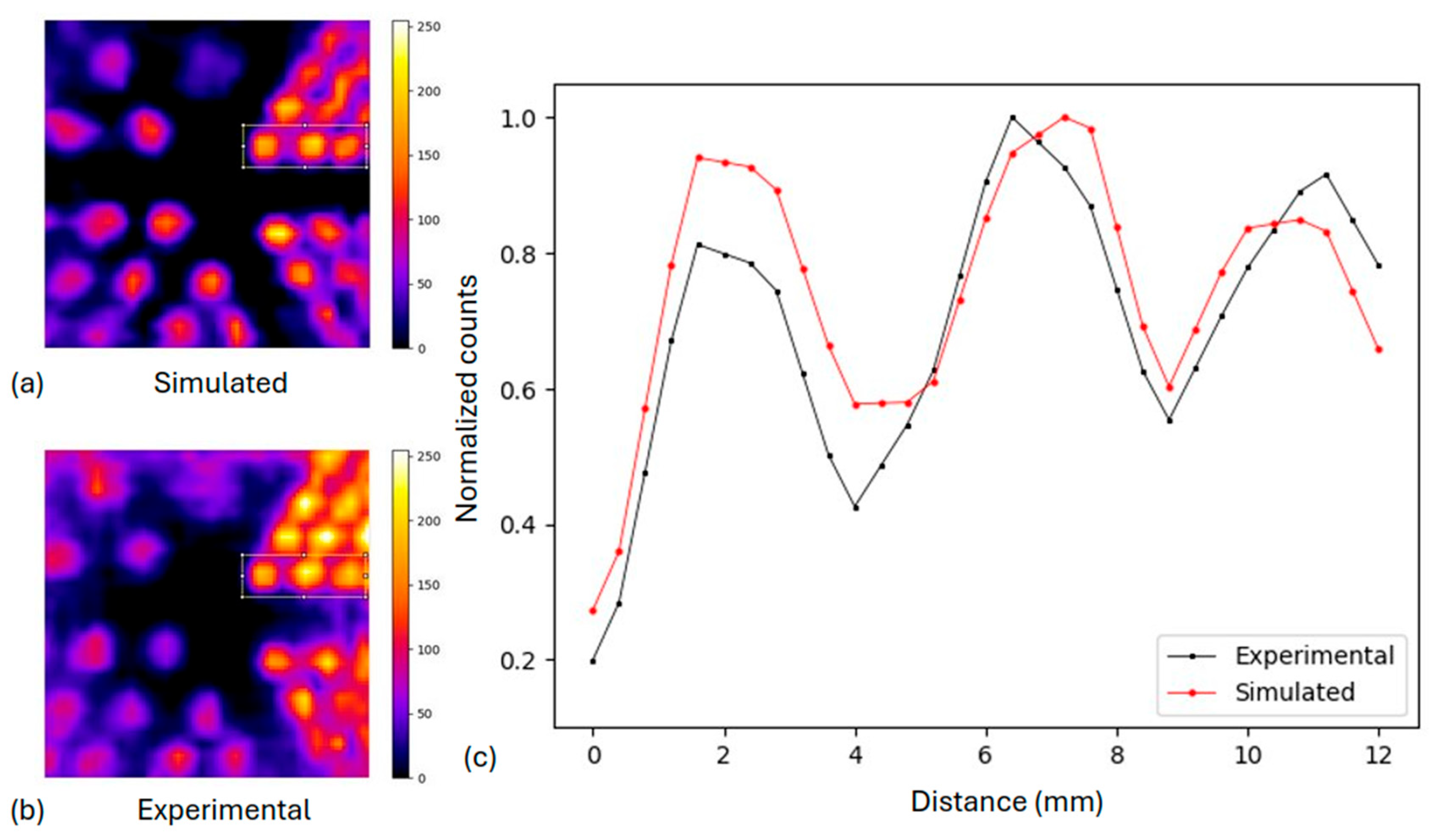
2.3.2. Derenzo-like Phantom
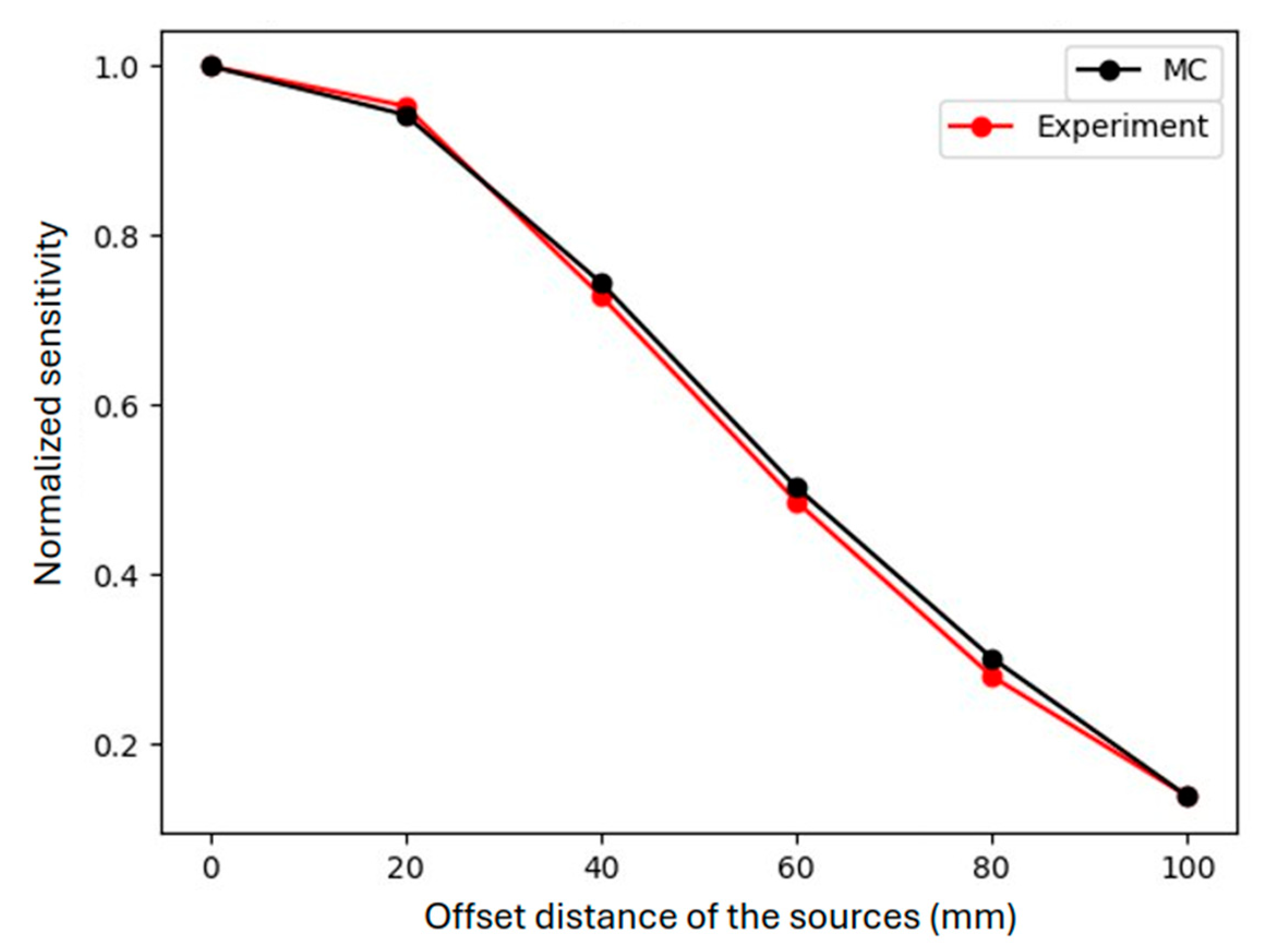
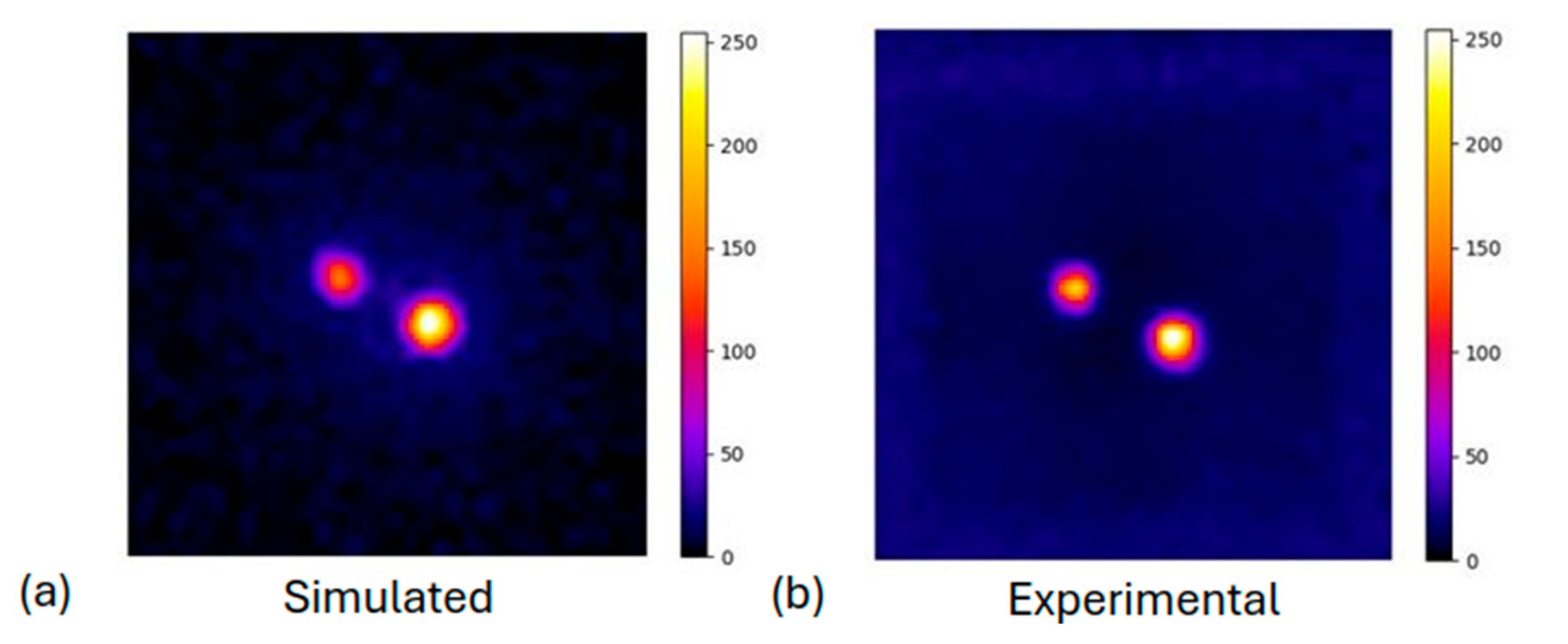
2.3.3. Off-Center Point Sources and Spheres at Different Depths
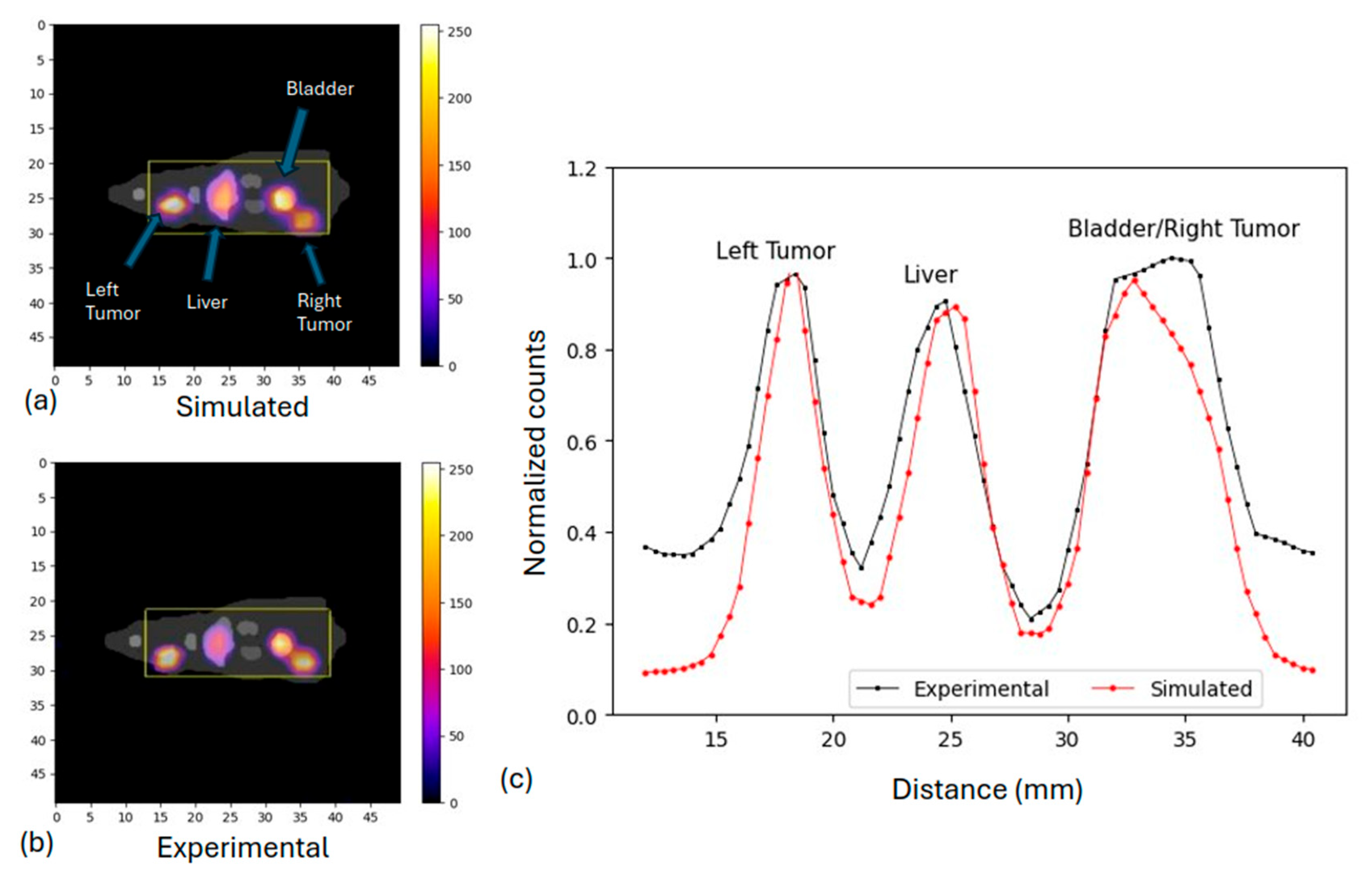
2.3.4. Preclinical Phantom Study
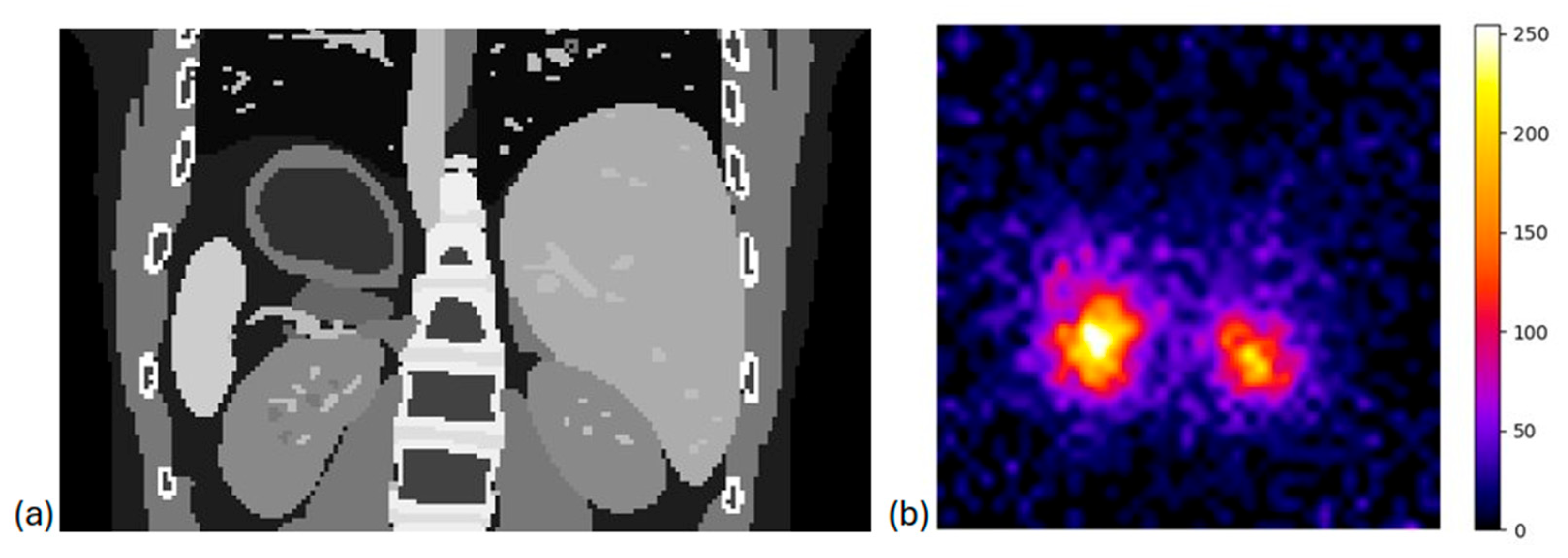
2.3.5. Clinical Simulations
3. Results
3.1. In Silico and Experimental Investigation of the System
3.1.1. Non-Uniformity Correction with Flood Source
3.1.2. Derenzo-like Phantom
3.1.3. Off-Center Point Sources and Spheres Sources at Different Depths
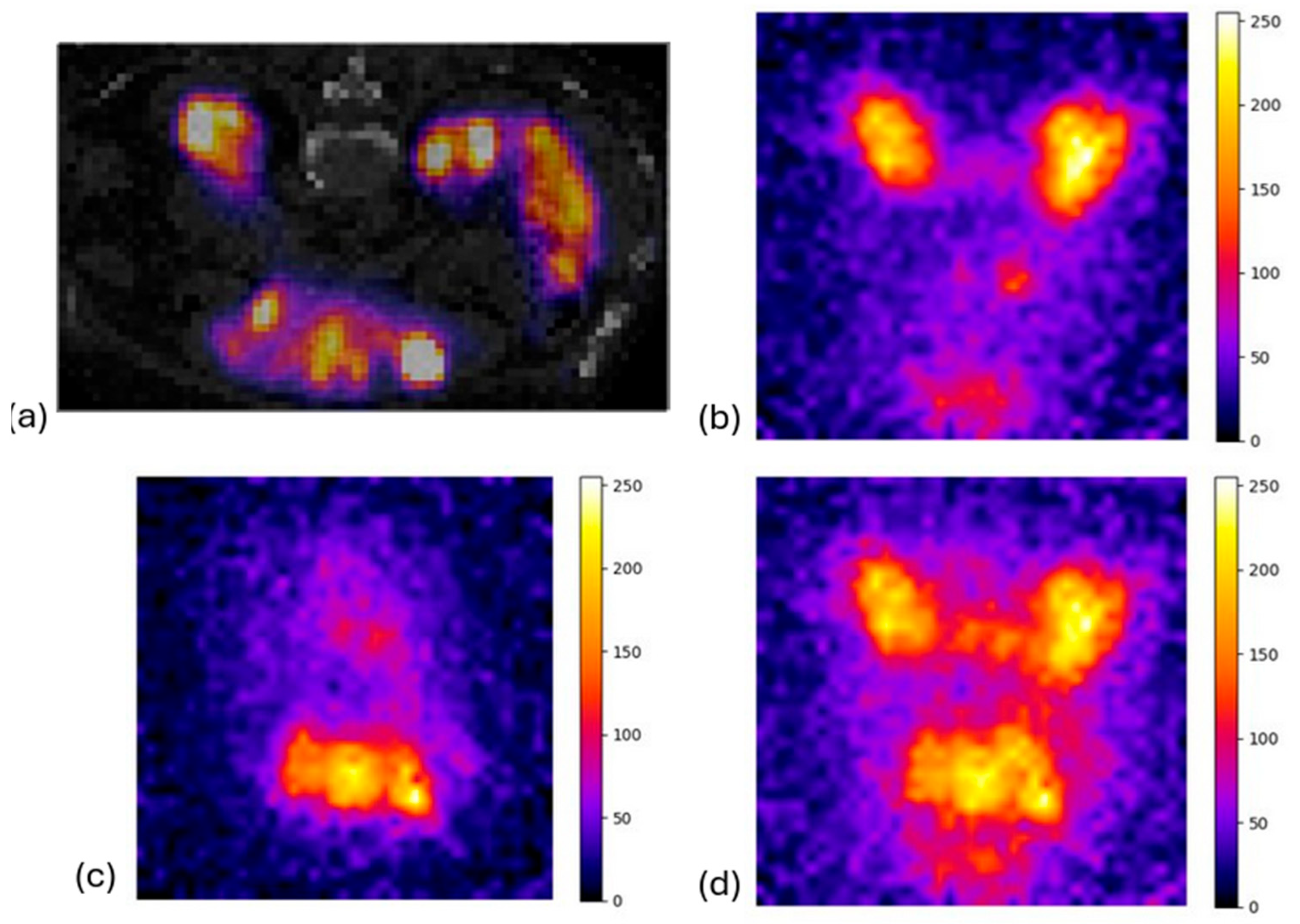
3.2. Preclinical Study: Rat Phantom
3.3. System Evaluation in Clinical Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Paluri, R.K.; Killeen, R.B. Neuroendocrine Tumor Lu-177-Dotatate Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fu, J.; Qiu, F.; Stolniceanu, C.R.; Yu, F.; Zang, S.; Xiang, Y.; Huang, Y.; Matovic, M.; Stefanescu, C.; Tang, Q.; et al. Combined use of 177Lu-DOTATATE peptide receptor radionuclide therapy and fluzoparib for treatment of well-differentiated neuroendocrine tumors: A preclinical study. J. Neuroendocrinol. 2022, 34, e13109. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Garje, R.; Rumble, R.B.; Parikh, R.A. Systemic Therapy Update on 177Lutetium-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer: ASCO Rapid Recommendation. J. Clin. Oncol. 2022, 40, 3664–3666. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; Assenat, E.; Tacher, V.; Terroir-Cassou-Mounat, M.; Mariano-Goulart, D.; Amaddeo, G.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Danieli, R.; Milano, A.; Gallo, S.; Veronese, I.; Lascialfari, A.; Indovina, L.; Botta, F.; Ferrari, M.; Cicchetti, A.; Raspanti, D.; et al. Personalized dosimetry in targeted radiation therapy: A look to methods, tools and critical aspects. J. Pers. Med. 2022, 12, 205. [Google Scholar] [CrossRef]
- Danieli, R.; Stella, M.; Leube, J.; Tran-Gia, J.; Marin, C.; Uribe, C.F.; Vanderlinden, B.; Reynaert, N.; Flamen, P.; Levillain, H. Quantitative 177Lu SPECT/CT imaging for personalized dosimetry using a ring-shaped CZT-based camera. EJNMMI Phys. 2023, 10, 64. [Google Scholar] [CrossRef]
- Mu’minah, I.A.S.; Hidayati, N.R.; Widodo, P.; Shintawati, R.; Soejoko, D.S. Investigation of Image Quality for Quantitative Lu-177 in SPECT imaging: A Phantom Study. J. Phys. Conf. Ser. 2020, 1505, 012048. [Google Scholar] [CrossRef]
- Sagisaka, Y.; Takahashi, Y.; Hosokawa, S.; Kanazawa, N.; Yamamoto, H.; Takai, G.; Nagano, K. Acquisition Conditions for Lu-177 DOTATATE Imaging. Radiation 2024, 4, 17–25. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; Sinaasappel, M.; Dekker, M.C.; Stokkel, M.P.M.; de Wit-van der Veen, B.J. 177 Lutetium SPECT/CT: Evaluation of collimator, photopeak and scatter correction. J. Appl. Clin. Med. Phys. 2020, 21, 272–277. [Google Scholar] [CrossRef]
- Toderas, L.; Chen, D.; Iravani, A.; Scherzer, Z.; Erickson, M.; Miyaoka, R. Clinical Implementation of Lu-177 PSMA therapy, including SPECT/CT imaging post therapy. J. Nucl. Med. 2023, 64 (Suppl. S1), T56. [Google Scholar]
- Peters, S.M.B.; Mink, M.C.T.; Privé, B.M.; de Bakker, M.; de Lange, F.; Muselaers, C.H.J.; Mehra, N.; Witjes, J.A.; Gotthardt, M.; Nagarajah, J.; et al. Optimization of the radiation dosimetry protocol in Lutetium-177-PSMA therapy: Toward clinical implementation. EJNMMI Res. 2023, 13, 6. [Google Scholar] [CrossRef]
- Roth, D.; Larsson, E.; Sundlöv, A.; Sjögreen Gleisner, K. Characterisation of a hand-held CZT-based gamma camera for 177Lu imaging. EJNMMI Phys. 2020, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.; Silva-Rodríguez, J.; Herranz, M.; Ruibal, A. Preliminary experience with small animal SPECT imaging on clinical gamma cameras. Biomed. Res. Int. 2014, 2014, 369509. [Google Scholar] [CrossRef]
- Bugby, S.L.; Farnworth, A.L.; Brooks, W.R.; Perkins, A.C. Seracam: Characterisation of a new small field of view hybrid gamma camera for nuclear medicine. EJNMMI Phys. 2024, 11, 57. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Cozzella, M.; Fazio, A.; Ungania, S.; Cazzato, M.; Iaccarino, G.; D’Andrea, M.; Strigari, L.; Fenwick, A.; Cox, M.; et al. Absolute gamma camera calibration for quantitative SPECT imaging with 177Lu. Phys. Medica 2016, 32, 114. [Google Scholar] [CrossRef]
- Raskin, S.; Gamliel, D.; Abookasis, D.; Ben-Haim, S.; Chicheportiche, A. Towards accurate 177Lu SPECT activity quantification and standardization using lesion-to-background voxel ratio. EJNMMI Phys. 2023, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.A.; Ivanovic, M.; Franceschi, D.; Strand, S.E.; Erlandsson, K.; Franceschi, M.; Atkins, H.L.; Coderre, J.A.; Susskind, H.; Button, T. Pinhole SPECT: An approach to in vivo high resolution SPECT imaging in small laboratory animals. J. Nucl. Med. 1994, 35, 342–348. [Google Scholar]
- Van Audenhaege, K.; Van Holen, R.; Vandenberghe, S.; Vanhove, C.; Metzler, S.D.; Moore, S.C. Review of SPECT collimator selection, optimization, and fabrication for clinical and preclinical imaging. Med. Phys. 2015, 42, 4796–4813. [Google Scholar] [CrossRef]
- Vastenhouw, B.; Beekman, F. Submillimeter total-body murine imaging with U-SPECT-I. J. Nucl. Med. 2007, 48, 487–493. [Google Scholar]
- Huang, W.; Mok, G.S.P. Multi-pinhole collimator design in different numbers of projections for brain SPECT. Front. Med. 2023, 10, 1211726. [Google Scholar] [CrossRef] [PubMed]
- Ilisie, V.; Moliner, L.; Morera, C.; Nuyts, J.; Benlloch, J.M. Gamma Camera Imaging with Rotating Multi-Pinhole Collimator. A Monte Carlo Feasibility Study. Sensors 2021, 21, 3367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Liu, H.; Xue, M.; Li, C.; Gao, L.; Liu, F.; Wu, J.; Liu, Y. Performance evaluation of a novel multi-pinhole SPECT system. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2024, 1059, 168976. [Google Scholar] [CrossRef]
- Islamian, J.P.; Azazrm, A.; Mahmoudian, B.; Gharapapagh, E. Advances in pinhole and multi-pinhole collimators for single photon emission computed tomography imaging. World J. Nucl. Med. 2015, 14, 3–9. [Google Scholar] [CrossRef]
- Cha, H.; Cho, K.; Jung, Y.-J.; Bae, S.; Kim, K.M.; Kim, M.; Lee, H.; Lee, K. Development of organ-specific dual-head single-photon emission computed tomography using variable pinhole collimator. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 987, 164822. [Google Scholar] [CrossRef]
- Gonzalez-Montoro, A.; Vera-Donoso, C.D.; Konstantinou, G.; Sopena, P.; Martinez, M.; Ortiz, J.B.; Carles, M.; Benlloch, J.M.; Gonzalez, A.J. Nuclear-medicine probes: Where we are and where we are going. Med. Phys. 2022, 49, 4372–4390. [Google Scholar] [CrossRef]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardiès, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Benoit, D.; Becheva, E.; Carlier, T.; Cassol, F.; Descourt, P.; Frisson, T.; Grevillot, L.; Guigues, L.; Maigne, L.; et al. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys. Med. Biol. 2011, 56, 881–901. [Google Scholar] [CrossRef]
- Sarrut, D.; Arbor, N.; Baudier, T.; Borys, D.; Etxebeste, A.; Fuchs, H.; Gajewski, J.; Grevillot, L.; Jan, S.; Kagadis, G.C.; et al. The OpenGATE ecosystem for Monte Carlo simulation in medical physics. Phys. Med. Biol. 2022, 67, 184001. [Google Scholar] [CrossRef]
- Sarrut, D.; Bardiès, M.; Boussion, N.; Freud, N.; Jan, S.; Létang, J.-M.; Loudos, G.; Maigne, L.; Marcatili, S.; Mauxion, T.; et al. A review of the use and potential of the GATE Monte Carlo simulation code for radiation therapy and dosimetry applications. Med. Phys. 2014, 41, 064301. [Google Scholar] [CrossRef]
- Sarrut, D.; Bała, M.; Bardiès, M.; Bert, J.; Chauvin, M.; Chatzipapas, K.; Dupont, M.; Etxebeste, A.; Fanchon, L.M.; Jan, S.; et al. Advanced Monte Carlo simulations of emission tomography imaging systems with GATE. Phys. Med. Biol. 2021, 66, 10TR03. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.; Amako, K.; Apostolakis, J.; Arce, P.; Asai, M.; Aso, T.; Bagli, E.; Bagulya, A.; Banerjee, S.; Barrand, G.; et al. Recent Developments in Geant4. Nucl. Instrum. Meth. 2016, 835, 186–225. [Google Scholar] [CrossRef]
- Weldstone Advanced Solutions. Available online: https://www.weldstone.com/en (accessed on 1 February 2025).
- Epic-Crystals. Available online: https://www.epic-crystal.com/ (accessed on 1 February 2025).
- Hamamatsu. Available online: https://www.hamamatsu.com (accessed on 1 February 2025).
- Fysikopoulos, E.; Loudos, G.; Georgiou, M.; David, S.; Matsopoulos, G. A Spartan 6 FPGA-based data acquisition system for dedicated imagers in nuclear medicine. Meas. Sci. Technol. 2012, 23, 125403. [Google Scholar] [CrossRef]
- Smith, M.F.; Jaszczak, R.J. The effect of gamma ray penetration on angle-dependent sensitivity for pinhole collimation in nuclear medicine. Med. Phys. 1997, 24, 1701–1709. [Google Scholar] [CrossRef]
- BIOEMTECH. Available online: https://bioemtech.com/product/phantom/ (accessed on 1 February 2025).
- Segars, W.P.; Sturgeon, G.; Mendonca, S.; Grimes, J.; Tsui, B.M. 4D XCAT phantom for multimodality imaging research. Med. Phys. 2010, 37, 4902–4915. [Google Scholar] [CrossRef]
- Plachouris, D.; Eleftheriadis, V.; Nanos, T.; Papathanasiou, N.; Sarrut, D.; Papadimitroulas, P.; Savvidis, G.; Vergnaud, L.; Salvadori, J.; Imperiale, A.; et al. A radiomic- and dosiomic-based machine learning regression model for pretreatment planning in 177Lu-DOTATATE therapy. Med. Phys. 2023, 50, 7222–7235. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.L.; Graves, S.A.; Farhoud, M.; Barnhart, T.E.; Jeffery, J.J.; Eliceiri, K.W.; Nickles, R.J. Development of a novel linearly-filled Derenzo microPET phantom. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 199–204. [Google Scholar]
- Hoffmann, J.V.; Janssen, J.P.; Kanno, T.; Shibutani, T.; Onoguchi, M.; Lapa, C.; Grunz, J.-P.; Buck, A.K.; Higuchi, T. Performance evaluation of fifth-generation ultra-high-resolution SPECT system with two stationary detectors and multi-pinhole imaging. EJNMMI Phys. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Higaki, Y.; Kobayashi, M.; Uehara, T.; Hanaoka, H.; Arano, Y.; Kawai, K. Appropriate collimators in a small animal SPECT scanner with CZT detector. Ann. Nucl. Med. 2013, 27, 271–278. [Google Scholar] [CrossRef]
- Bhatia, B.S.; Bugby, S.L.; Lees, J.E.; Perkins, A.C. A scheme for assessing the performance characteristics of small field-of-view gamma cameras. Phys. Medica Eur. J. Med. Phys. 2015, 31, 98–103. [Google Scholar] [CrossRef][Green Version]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F., Jr. 177Lu radiopharmaceuticals for therapy and diagnosis: A review. J. Radioanal. Nucl. Chem. 2015, 305, 127–139. [Google Scholar]
- Banerjee, S.; Pillai, M.R.A.; Ramamoorthy, N. Therapeutic applications of 177Lu and radio-lanthanides in nuclear medicine. Appl. Radiat. Isot. 2005, 62, 157–163. [Google Scholar] [CrossRef]
- Lamprou, E.; Sanchez, F.; Benlloch, J.; Gonzalez, A. In-depth evaluation of TOF-PET detectors based on crystal arrays and the TOFPET2 ASIC. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 977, 164295. [Google Scholar] [CrossRef]
- van der Have, F.; Vastenhouw, B.; Ramakers, R.M.; Branderhorst, W.; Krah, J.O.; Ji, C.; Staelens, S.G.; Beekman, F.J. U-SPECT-II: An ultra-high-resolution device for molecular small-animal imaging. J. Nucl. Med. 2009, 50, 599–605. [Google Scholar] [CrossRef]
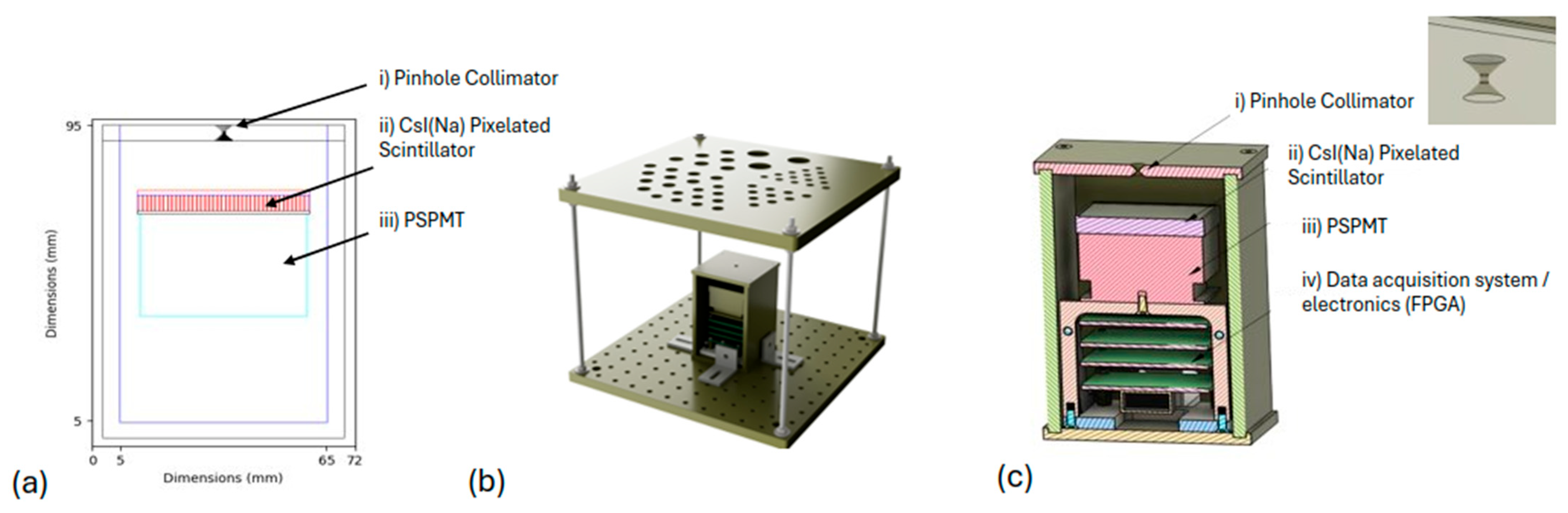



| Tungsten shielding box | |
| Thickness | 5 mm |
| Outer dimensions | 70 × 70 × 100 mm3 |
| Pinhole collimator | |
| Diameter (d) | 1.2 mm |
| Acceptance angle (a) | 94 degrees |
| Channel height | 1 mm |
| Scintillator | |
| Ce pixelated array | 50 × 50 mm2 GAGG |
| Crystal dimensions | 1 × 1 × 5 mm3 (0.2 mm septa) |
| Optical guide (glass) thickness | 1 mm |
| PSPMTs | |
| Dimensions | 48.5 × 48.5 × 32.5 mm3 each |
| Peak QE (@ 380 nm) | 10% |
| Model | H12700A Hamamatsu |
| FPGA-based data acquisition system | |
| Organ at SPECT Image | Ratio on Simulated Data | Ratio on Clinical Data |
|---|---|---|
| Left kidney/right kidney | 0.81 | 0.77 |
| Background/right kidney | 0.09 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvidis, G.; Eleftheriadis, V.; Paneta, V.; Fysikopoulos, E.; Georgiou, M.; Lamprou, E.; Lagoumtzi, S.; Loudos, G.; Katsakiori, P.; Kagadis, G.C.; et al. Design and Evaluation of a Portable Pinhole SPECT System for 177Lu Imaging: Monte Carlo Simulations and Experimental Study. Diagnostics 2025, 15, 1387. https://doi.org/10.3390/diagnostics15111387
Savvidis G, Eleftheriadis V, Paneta V, Fysikopoulos E, Georgiou M, Lamprou E, Lagoumtzi S, Loudos G, Katsakiori P, Kagadis GC, et al. Design and Evaluation of a Portable Pinhole SPECT System for 177Lu Imaging: Monte Carlo Simulations and Experimental Study. Diagnostics. 2025; 15(11):1387. https://doi.org/10.3390/diagnostics15111387
Chicago/Turabian StyleSavvidis, Georgios, Vasileios Eleftheriadis, Valentina Paneta, Eleftherios Fysikopoulos, Maria Georgiou, Efthimis Lamprou, Sofia Lagoumtzi, George Loudos, Paraskevi Katsakiori, George C. Kagadis, and et al. 2025. "Design and Evaluation of a Portable Pinhole SPECT System for 177Lu Imaging: Monte Carlo Simulations and Experimental Study" Diagnostics 15, no. 11: 1387. https://doi.org/10.3390/diagnostics15111387
APA StyleSavvidis, G., Eleftheriadis, V., Paneta, V., Fysikopoulos, E., Georgiou, M., Lamprou, E., Lagoumtzi, S., Loudos, G., Katsakiori, P., Kagadis, G. C., & Papadimitroulas, P. (2025). Design and Evaluation of a Portable Pinhole SPECT System for 177Lu Imaging: Monte Carlo Simulations and Experimental Study. Diagnostics, 15(11), 1387. https://doi.org/10.3390/diagnostics15111387






