Impact of Storage Conditions on Bronchoalveolar Lavage Fluid Analysis: A Human Study
Abstract
1. Introduction
2. Materials and Methods
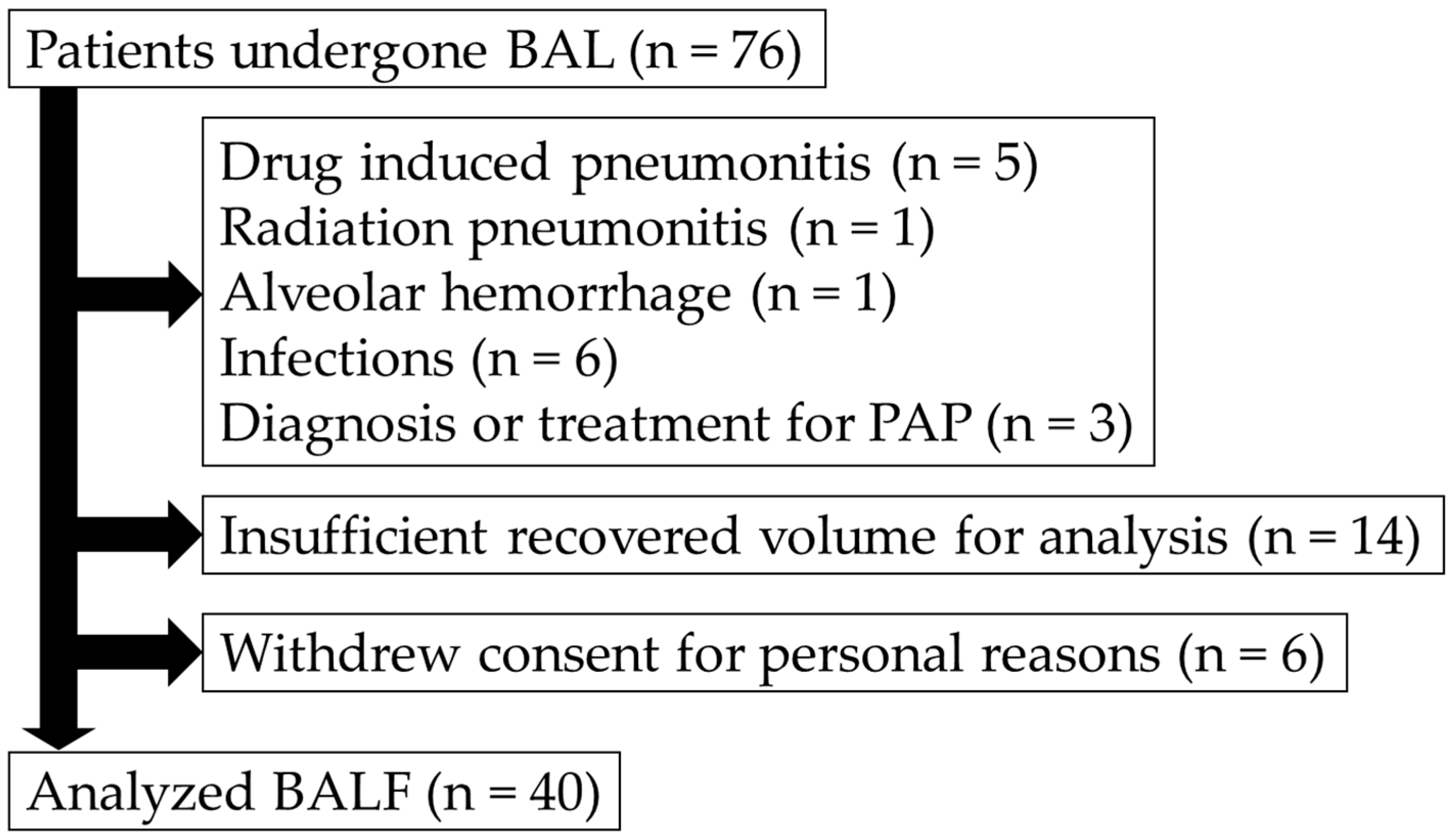
2.1. Study Design and Participants
2.2. BAL Procedure
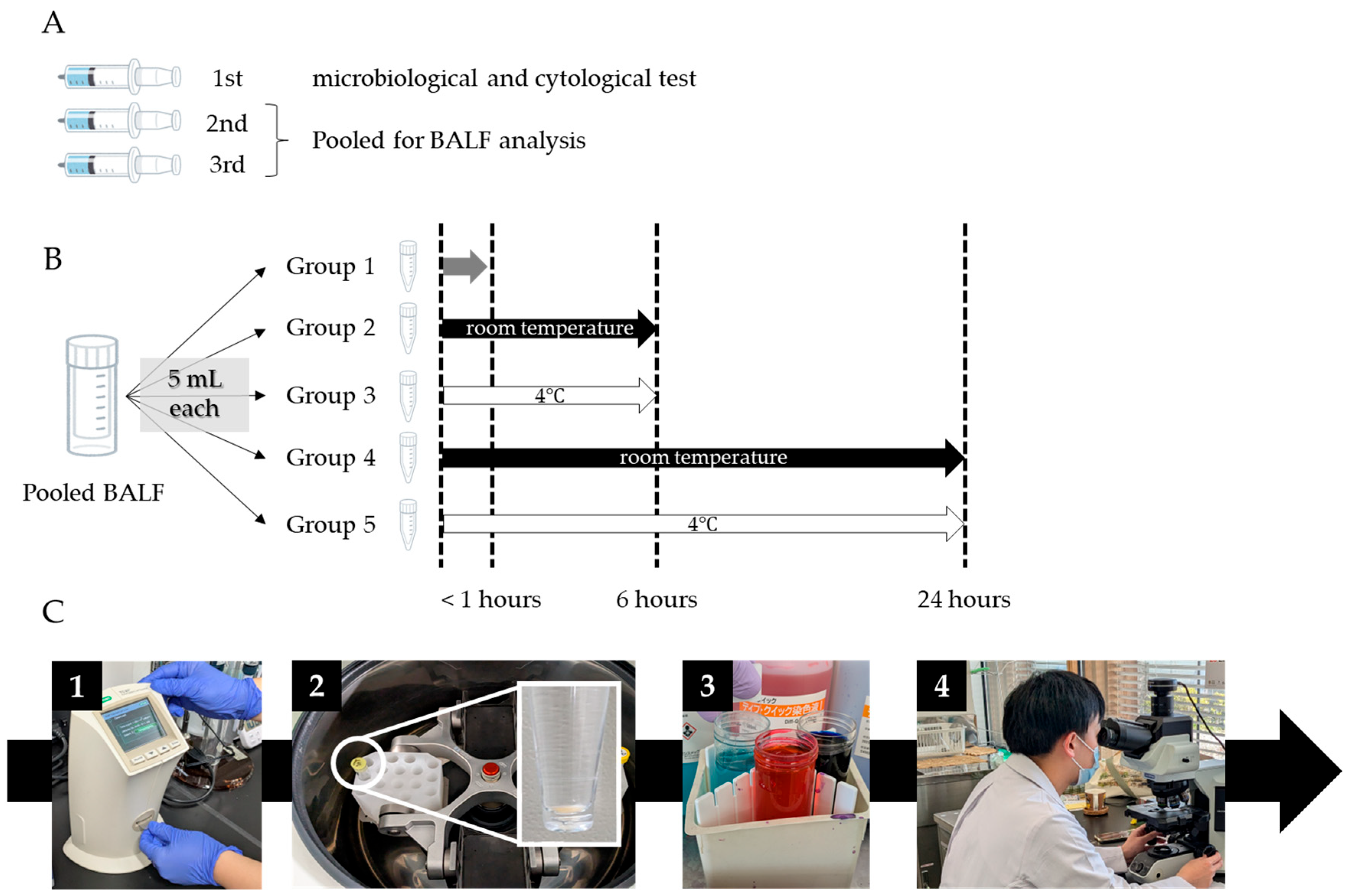
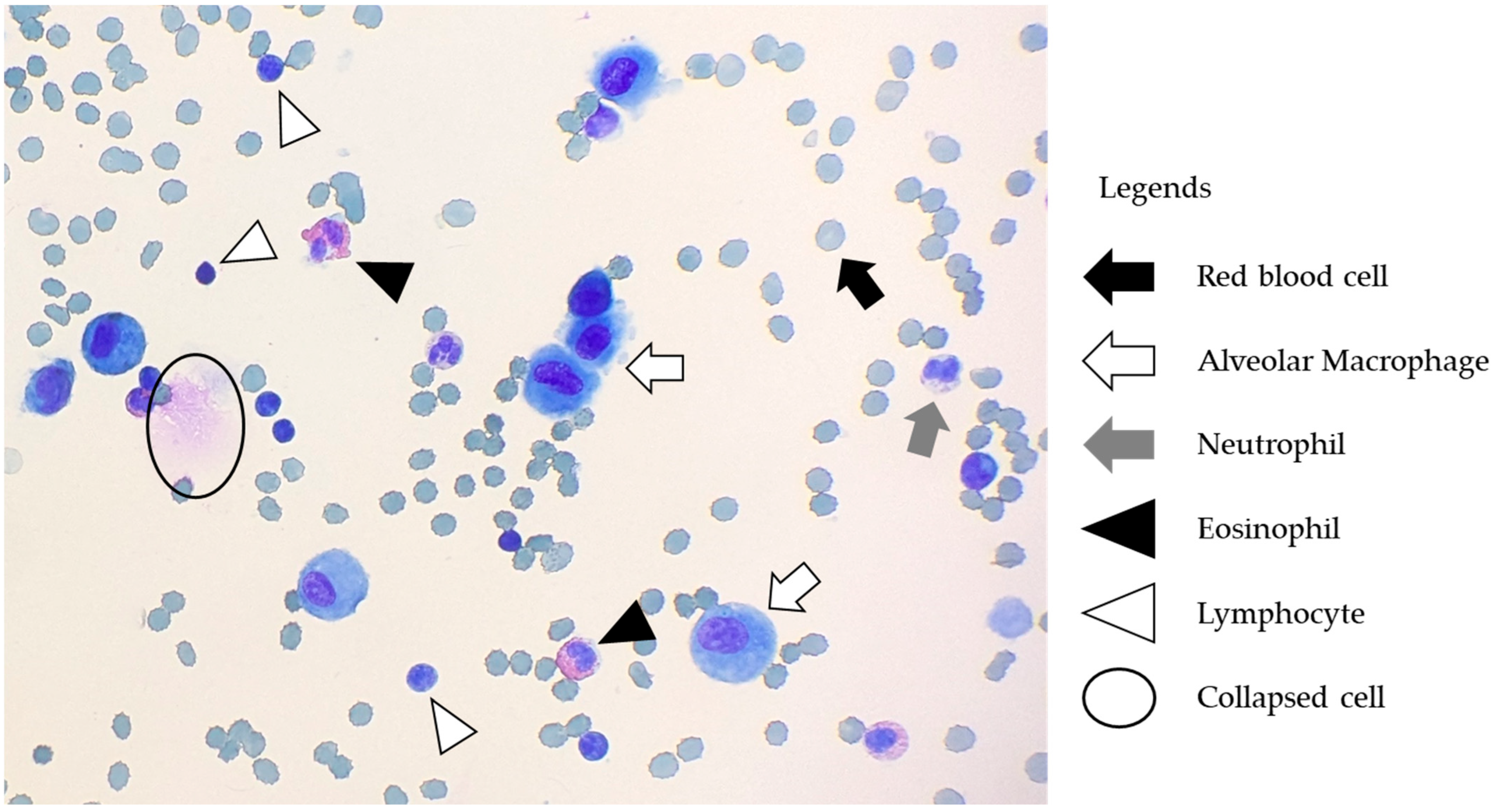
2.3. BALF Handling, Processing, and Analysis
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
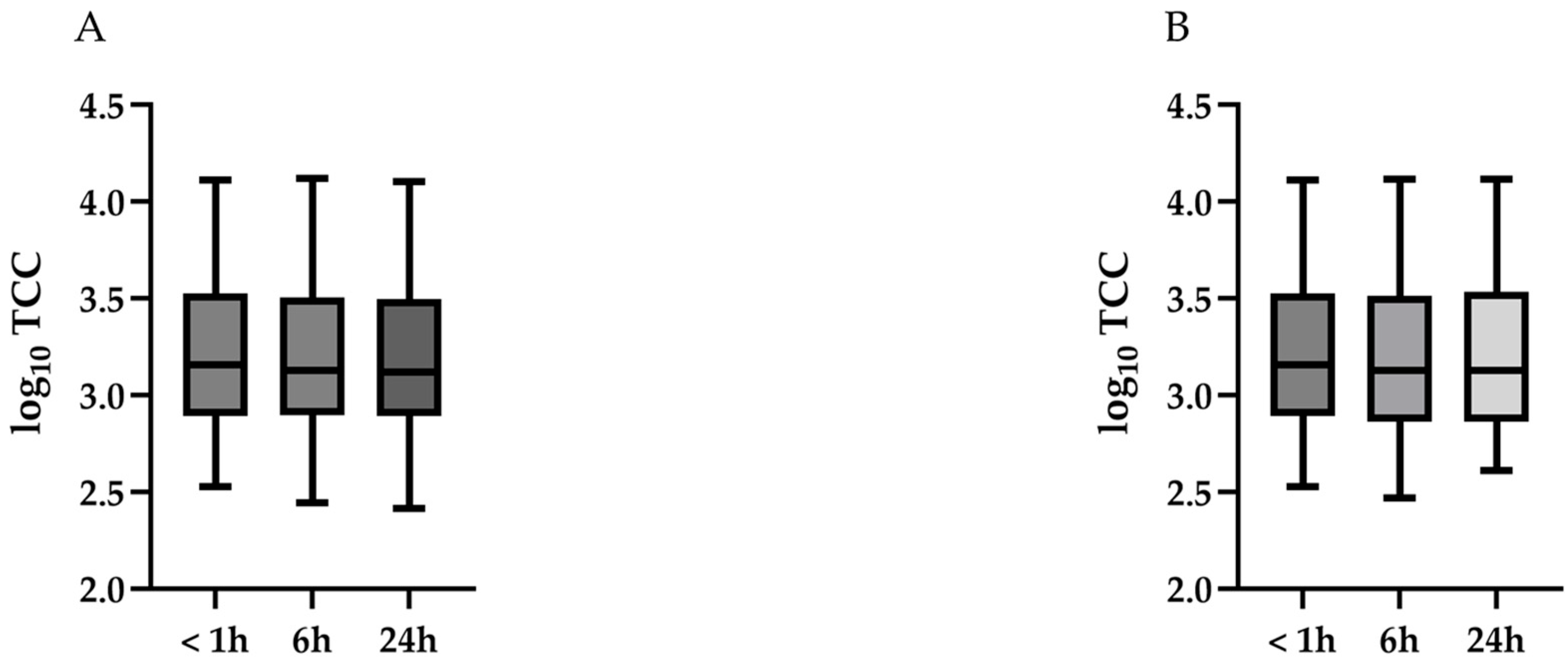
3.2. TCC
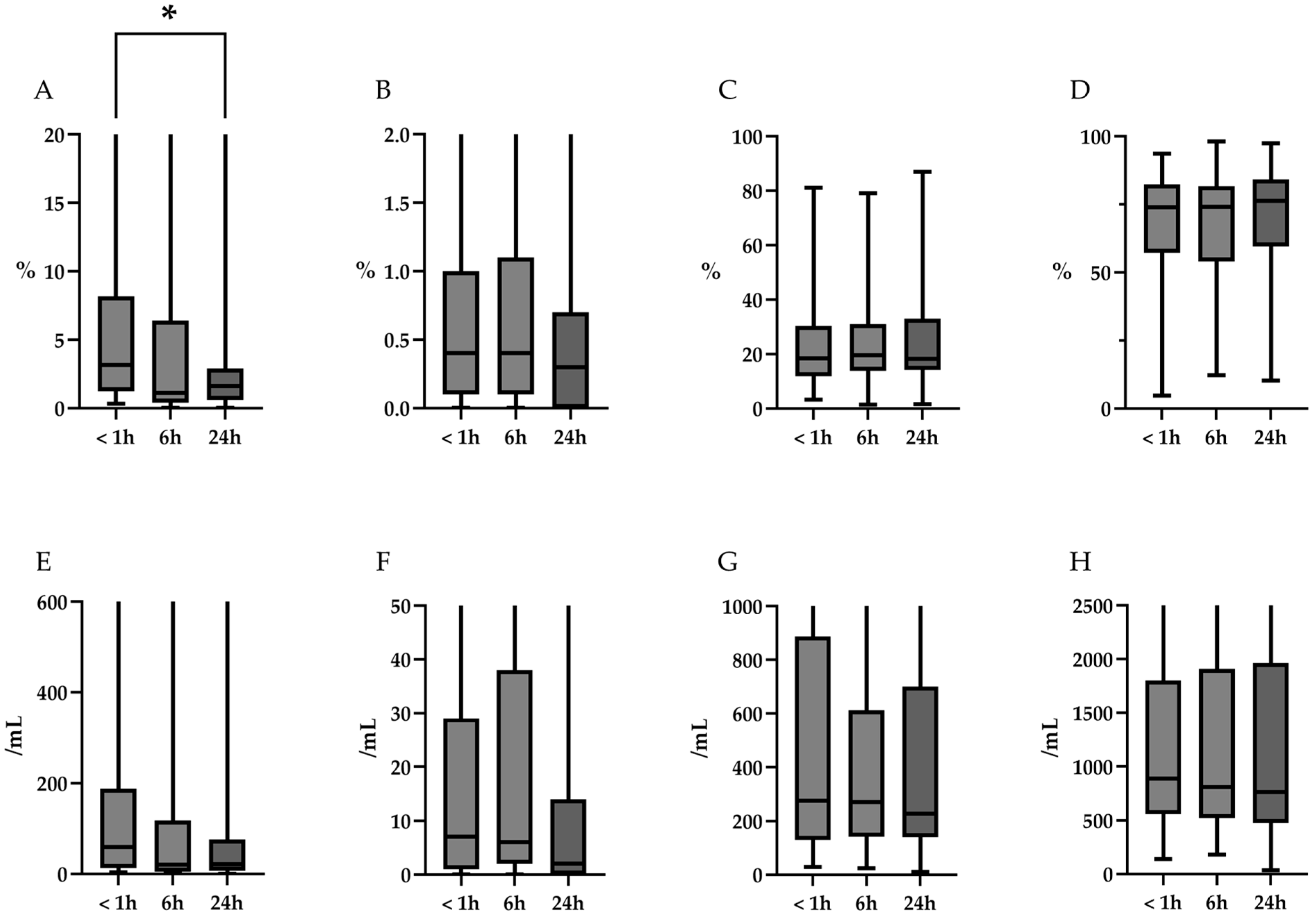
3.3. Cell Differentials
3.4. Inter-Observer Agreement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATS | American Thoracic Society |
| BAL | Bronchoalveolar lavage |
| BALF | Bronchoalveolar lavage fluid |
| HRCT | High-resolution computed tomography |
| ICC | Intraclass Correlation Coefficient |
| ILD | Interstitial lung disease |
| RT | Room temperature |
| TCC | Total cell counts |
References
- Reynolds, H.Y. Bronchoalveolar lavage. Am. Rev. Respir. Dis. 1987, 135, 250–263. [Google Scholar]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kinder, B.W.; Brown, K.K.; Schwarz, M.I.; Ix, J.H.; Kervitsky, A.; King, T.E., Jr. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008, 133, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Watase, M.; Mochimaru, T.; Kawase, H.; Shinohara, H.; Sagawa, S.; Ikeda, T.; Yagi, S.; Yamamura, H.; Matsuyama, E.; Kaji, M.; et al. Diagnostic and prognostic biomarkers for progressive fibrosing interstitial lung disease. PLoS ONE 2023, 18, e0283288. [Google Scholar] [CrossRef]
- Lewandowska, K.B.; Barańska, I.; Sobiecka, M.; Radwan-Rohrenschef, P.; Dybowska, M.; Franczuk, M.; Roży, A.; Skoczylas, A.; Bestry, I.; Kuś, J.; et al. Factors predictive for immunomodulatory therapy response and survival in patients with hypersensitivity pneumonitis-retrospective cohort analysis. Diagnostics 2022, 12, 2767. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic pulmonary fibrosis (an update) and Progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- The Japanese Respiratory Society (Ed.) A Clinical Guideline for Bronchial Alveolar Lavage, 3rd ed.; Kokuseido Co., Ltd.: Tokyo, Japan, 2017. (In Japanese) [Google Scholar]
- Rankin, J.A.; Naegel, G.P.; Reynolds, H.Y. Use of a central laboratory for analysis of bronchoalveolar lavage fluid. Am. Rev. Respir. Dis. 1986, 133, 186–190. [Google Scholar]
- Ishioka, S.; Shirotani, Y.; Takahashi, K.; Oyama, T.; Hozawa, S.; Saitoh, T.; Inyaku, K.; Yamakido, M. The Impact of Storage Conditions on Bronchoalveolar Lavage Fluid Analysis. Diagn. Treat. 1992, 80–87, 1225–1228. (In Japanese) [Google Scholar]
- Isobe, T.; Miyamoto, A.; Waseda, Y.; Tsubata, Y.; Otsuka, M.; Ishida, M.; Horimasu, Y.; Nureki, S.; Hamaguchi, S.; Kurimoto, N.; et al. Nationwide questionnaire-based survey on methods for performing bronchoalveolar lavage. Respir. Endosc. 2024, 2, 141–147. [Google Scholar] [CrossRef]
- Kelly, C.; Ward, C.; Bird, G.; Hendrick, D.; Walters, H. The effect of filtration on absolute and differential cell counts in fluid obtained at bronchoalveolar lavage. Respir. Med. 1989, 83, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Pickles, K.; Pirie, R.S.; Rhind, S.; Dixon, P.M.; McGorum, B.C. Cytological analysis of equine bronchoalveolar lavage fluid. Part 3: The effect of time, temperature and fixatives. Equine Vet. J. 2002, 34, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Nafe, L.A.; DeClue, A.E.; Reinero, C.R. Storage alters feline bronchoalveolar lavage fluid cytological analysis. J. Feline Med. Surg. 2011, 13, 94–100. [Google Scholar] [CrossRef]
- Bronchoalveolar Lavage Constituents in Healthy Individuals, Idiopathic Pulmonary Fibrosis, and Selected Comparison Groups. The BAL Cooperative Group Steering Committee. Am. Rev. Respir. Dis. 1990, 141, S169–S202. [Google Scholar]
- Nagai, S.; Kitaichi, M.; Itoh, H.; Nishimura, K.; Izumi, T.; Colby, T.V. Idiopathic nonspecific interstitial pneumonia/fibrosis: Comparison with idiopathic pulmonary fibrosis and BOOP. Eur. Respir. J. 1998, 12, 1010–1019. [Google Scholar] [CrossRef]
- Veeraraghavan, S.; Latsi, P.I.; Wells, A.U.; Pantelidis, P.; Nicholson, A.G.; Colby, T.V.; Haslam, P.L.; Renzoni, E.A.; du Bois, R.M. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur. Respir. J. 2003, 22, 239–244. [Google Scholar] [CrossRef]
- Yoshioka, S.; Mukae, H.; Sugiyama, K.; Kakugawa, T.; Sakamoto, N.; Nakayama, S.; Abe, K.; Fujii, T.; Kadota, J.; Kohno, S. High-BAL fluid concentrations of RANTES in nonspecific interstitial pneumonia compared with usual interstitial pneumonia. Respir. Med. 2004, 98, 945–951. [Google Scholar] [CrossRef]
- Park, I.N.; Jegal, Y.; Kim, D.S.; Do, K.-H.; Yoo, B.; Shim, T.S.; Lim, C.M.; Lee, S.D.; Koh, Y.; Kim, W.S.; et al. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur. Respir. J. 2009, 33, 68–76. [Google Scholar] [CrossRef]
- Suda, T.; Kono, M.; Nakamura, Y.; Enomoto, N.; Kaida, Y.; Fujisawa, T.; Imokawa, S.; Yasuda, K.; Hashizume, H.; Yokomura, K.; et al. Distinct prognosis of idiopathic nonspecific interstitial pneumonia (NSIP) fulfilling criteria for undifferentiated connective tissue disease (UCTD). Respir. Med. 2010, 104, 1527–1534. [Google Scholar] [CrossRef]
- Murphy, J.; Summer, R.; Wilson, A.A.; Kotton, D.N.; Fine, A. The prolonged life-span of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2008, 38, 380–385. [Google Scholar] [CrossRef]
- Savill, J.S.; Wyllie, A.H.; Henson, J.E.; Walport, M.J.; Henson, P.M.; Haslett, C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Investig. 1989, 83, 865–875. [Google Scholar] [PubMed]
- Hébert, M.J.; Takano, T.; Holthöfer, H.; Brady, H.R. Sequential morphologic events during apoptosis of human neutrophils. Modulation by lipoxygenase-derived eicosanoids. J. Immunol. 1996, 157, 3105–3115. [Google Scholar] [CrossRef]
- Park, Y.M.; Bochner, B.S. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol. Res. 2010, 2, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Tabuena, R.P.; Nagai, S.; Tsutsumi, T.; Handa, T.; Minoru, T.; Mikuniya, T.; Shigematsu, M.; Hamada, K.; Izumi, T.; Mishima, M. Cell profiles of bronchoalveolar lavage fluid as prognosticators of idiopathic pulmonary fibrosis/usual interstitial pneumonia among Japanese Patients. Respiration 2005, 72, 490–498. [Google Scholar] [CrossRef]
- Soares Pires, F.; Caetano Mota, P.; Melo, N.; Costa, D.; Jesus, J.M.; Cunha, R.; Guimarães, S.; Souto-Moura, C.; Morais, A. Fibrose pulmonar idiopática: Apresentação clínica, evolução e fatores de prognóstico basais numa coorte portuguesa. Rev. Port. Pneumol. 2013, 19, 19–27. [Google Scholar] [CrossRef]
- Lee, J.-U.; Kim, M.K.; Kim, M.-S.; Lee, S.J.; Park, S.-L.; Chang, H.S.; Park, J.S.; Park, C.S. S100 calcium-binding protein A9, a potential novel diagnostic biomarker for idiopathic pulmonary fibrosis. J. Korean Med. Sci. 2024, 39, e13. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Bolivar, E.M.; Ros, J.A.; Pérez-Fernández, S.; Campillo, J.A.; López-Hernández, R.; González-López, R.; Otalora-Alcaraz, A.; Ortuño-Hernández, C.; Gimeno, L.; Ruiz-Lorente, I.; et al. Neutrophils and lymphocytes: Yin and yang of lung fibrosis and patient outcome in diffuse interstitial lung diseases. Biomedicines 2024, 12, 2439. [Google Scholar] [CrossRef]
- Goh, N.S.L.; Veeraraghavan, S.; Desai, S.R.; Cramer, D.; Hansell, D.M.; Denton, C.P.; Black, C.M.; du Bois, R.M.; Wells, A.U. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 2007, 56, 2005–2012. [Google Scholar] [CrossRef]
- Kono, M.; Miyashita, K.; Hirama, R.; Oshima, Y.; Takeda, K.; Mochizuka, Y.; Tsutsumi, A.; Miwa, H.; Miki, Y.; Hashimoto, D.; et al. Prognostic significance of bronchoalveolar lavage cellular analysis in patients with acute exacerbation of interstitial lung disease. Respir. Med. 2021, 186, 106534. [Google Scholar] [CrossRef]
| Parameter | All (n = 40) |
| Age, years, median (range) | 69 (31–88) |
| Sex (male/female), n | 18/22 |
| Smoking history (never/past/current), n | 18/21/1 |
| Usage of immunosuppressive drug, n | 5 |
| Diagnosis of pre-bronchoscopy test | |
| IIPs (IPF/NSIP/COP/DIP), n | 14 (7/4/2/1) |
| CTD-ILD, n | 9 |
| HP, n | 9 |
| Sarcoidosis, n | 4 |
| PPFE, n | 3 |
| Familial interstitial pneumonitis, n | 1 |
| BAL targeted segment, n | |
| right B4 | 7 |
| right B5 | 18 |
| right B4+5 | 1 |
| left B4 | 8 |
| left B5 | 5 |
| left B4 + 5 | 1 |
| Usage of loose gauze for removing gross mucus, n | 5 |
| Total installed volume, ml (range) | 150 (150–150) |
| Total recovered volume, ml (range) | 81.5 (48–118) |
| Recovered volume of the 2nd and 3rd aliquots, ml (range) | 65 (36–85) |
| Time interval from BAL completion to processing, minutes (range) | |
| Group 1 | 20 (6–60) |
| Groups 2 and 3 | 370 (300–488) |
| Groups 4 and 5 | 1463.5 (1221–1650) |
| Parameter | <1 h | 6 h | 24 h | p Value | |
|---|---|---|---|---|---|
| 4 °C | log10 TCC, median (range) | 3.16 (2.54–4.11) | 3.13 (2.44–4.12) | 3.12 (2.41–4.10) | 0.86 |
| RT | log10 TCC, median (range) | 3.16 (2.54–4.11) | 3.13 (2.47–4.11) | 3.13 (2.61–4.12) | 0.90 |
| Parameter | <1 h | 6 h | 24 h | p Value |
|---|---|---|---|---|
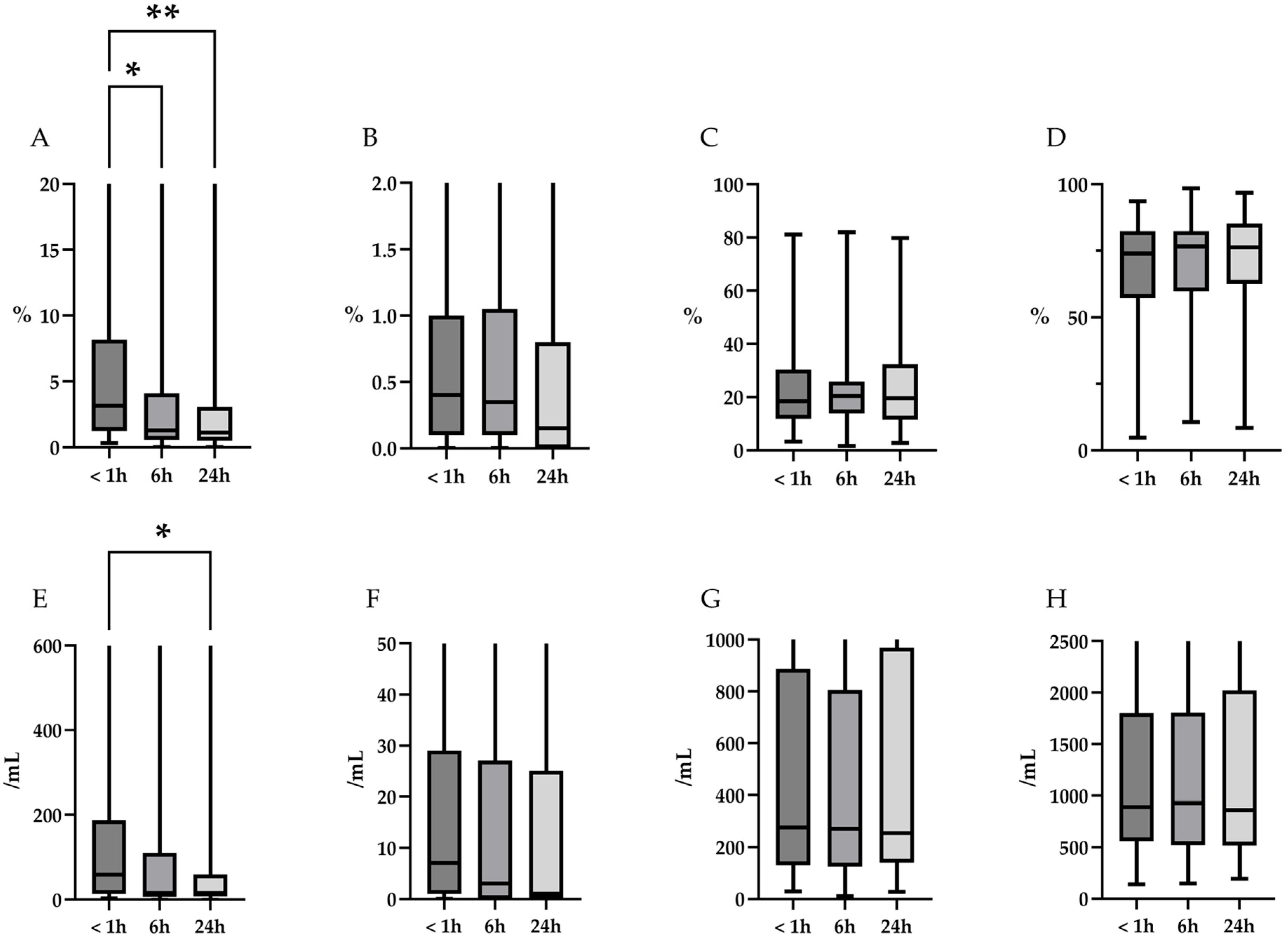
| Neutrophils, % median (range) | 3.3 (0.3–92.0) | 1.1 (0.0–82.5) | 1.6 (0.0–81.8) | 0.02 |
| Post hoc Steel test, 6 h/24 h | - | - | - | 0.09/0.02 |
| Cell counts of neutrophils, /mL, median (range) | 59 (3–6385) | 20 (0–5247) | 22 (0–3751) | 0.09 |
| Eosinophils, % median (range) | 0.4 (0.0–5.7) | 0.4 (0.0–8.9) | 0.3 (0.0–8.5) | 0.31 |
| Cell counts of eosinophils, /mL, median (range) | 7 (0–180) | 6 (0–269) | 2 (0–139) | 0.35 |
| Lymphocytes, %, median (range) | 18.4 (3.2–81.0) | 19.6 (1.4–79.1) | 18.2 (1.6–86.9) | 0.79 |
| Cell counts of lymphocytes, /mL, median (range) | 276 (29–6102) | 270 (24–6351) | 228 (11–7416) | 0.94 |
| Alveolar macrophages (AM), %, median (range) | 73.9 (4.8–93.6) | 74.1 (12.3–98.0) | 76.2 (10.3–97.4) | 0.74 |
| Cell counts of AM, /mL, median (range) | 890 (139–9765) | 812 (182–8705) | 765 (34–9634) | 0.90 |
| Parameter | <1 h | 6 h | 24 h | p Value |
|---|---|---|---|---|
| Neutrophils, % median (range) | 3.3 (0.3–92.0) | 1.3 (0.0–81.8) | 1.1 (0.0–81.6) | <0.01 |
| Post hoc Steel test, 6 h/24 h | - | - | - | 0.01/<0.01 |
| Cell counts of neutrophils, /mL, median (range) | 59 (3–6385) | 16 (0–4986) | 15 (0–4957) | 0.03 |
| Post hoc Steel test, 6 h/24 h | - | - | - | 0.054/0.04 |
| Eosinophils, % median (range) | 0.4 (0.0–5.7) | 0.4 (0.0–6.4) | 0.2 (0.0–5.5) | 0.32 |
| Cell counts of eosinophils, /mL, median (range) | 7 (0–180) | 3 (0–209) | 25 (0–161) | 0.38 |
| Lymphocytes, %, median (range) | 18.4 (3.2–81.0) | 20.4 (1.6–81.9) | 19.7 (2.7–79.8) | 0.80 |
| Cell counts of lymphocytes, /mL, median (range) | 276 (29–6102) | 270 (11–6708) | 255 (27–7282) | 0.97 |
| Alveolar macrophages (AM), %, median (range) | 73.9 (4.8–93.6) | 76.6 (10.5–98.4) | 76.3 (8.4–96.8) | 0.81 |
| Cell counts of AM, /mL, median (range) | 890 (139–9765) | 928 (147–10777) | 862 (193–4798) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shionoya, Y.; Maruyama, K.; Kawasaki, T.; Ono, M.; Murai, Y.; Hirama, R.; Horiuchi, D.; Sakuma, N.; Kitahara, S.; Sato, S.; et al. Impact of Storage Conditions on Bronchoalveolar Lavage Fluid Analysis: A Human Study. Diagnostics 2025, 15, 1386. https://doi.org/10.3390/diagnostics15111386
Shionoya Y, Maruyama K, Kawasaki T, Ono M, Murai Y, Hirama R, Horiuchi D, Sakuma N, Kitahara S, Sato S, et al. Impact of Storage Conditions on Bronchoalveolar Lavage Fluid Analysis: A Human Study. Diagnostics. 2025; 15(11):1386. https://doi.org/10.3390/diagnostics15111386
Chicago/Turabian StyleShionoya, Yu, Kanae Maruyama, Takeshi Kawasaki, Mayumi Ono, Yushi Murai, Ryutaro Hirama, Dai Horiuchi, Noriko Sakuma, Shinsuke Kitahara, Shun Sato, and et al. 2025. "Impact of Storage Conditions on Bronchoalveolar Lavage Fluid Analysis: A Human Study" Diagnostics 15, no. 11: 1386. https://doi.org/10.3390/diagnostics15111386
APA StyleShionoya, Y., Maruyama, K., Kawasaki, T., Ono, M., Murai, Y., Hirama, R., Horiuchi, D., Sakuma, N., Kitahara, S., Sato, S., Takahashi, K., Ozawa, Y., & Suzuki, T. (2025). Impact of Storage Conditions on Bronchoalveolar Lavage Fluid Analysis: A Human Study. Diagnostics, 15(11), 1386. https://doi.org/10.3390/diagnostics15111386







