Abstract
Background: Prostate cancer (PCa) remains one of the most prevalent malignancies in men, with diagnostic challenges arising from the limited specificity of current biomarkers, like PSA. Improved stratification tools are essential to reduce overdiagnosis and guide personalized patient management. Objective: This study aimed to identify and validate clinical and hematological biomarkers capable of differentiating PCa from benign prostatic hyperplasia (BPH) and precancerous lesions (PL) using univariate and multivariate statistical methods. Methods: In a cohort of 514 patients with suspected PCa, we performed a univariate analysis (Kruskal–Wallis and ANOVA) with preprocessing via adaptive Box–Cox transformation and missing value imputation through probabilistic principal component analysis (PPCA). LASSO regression was used for variable selection and classification. An ROC curve analysis assessed diagnostic performance. Results: Five variables—age, PSA, Index %, hemoglobin (HGB), and the International Index of Erectile Function (IIEF)—were consistently significant across univariate and multivariate analyses. The LASSO regression achieved a classification accuracy of 70% and an AUC of 0.74. Biplot and post-hoc analyses confirmed partial separation between PCa and benign conditions. Conclusions: The integration of multivariate modeling with reconstructed clinical data enabled the identification of blood-based biomarkers with strong diagnostic potential. These routinely available, cost-effective indicators may support early PCa diagnosis and patient stratification, reducing unnecessary invasive procedures.
1. Introduction
Prostate cancer (PCa) is among the most prevalent cancers in men, accounting for approximately 15% of all cancers globally [1]. Its high incidence and associated mortality rates pose a significant public health challenge, particularly as the global population ages. By 2040, the global incidence of new PCa cases is projected to nearly triple, potentially reaching three million annually. The Lancet Commission has recently published projections indicating a significant increase in PCa cases from 1.4 million in 2020 to 2.9 million by 2040, with the greatest rise anticipated in low- and middle-income countries, where healthcare access and early detection programs remain limited [2].
This alarming trend underscores the growing burden of PCa, which not only affects patients and their families but also exerts significant pressure on healthcare systems worldwide.
Unlike some other cancers, this increase in PCa cases cannot be effectively mitigated through lifestyle changes or public health interventions, further emphasizing the urgent need for alternative strategies to address this issue [3,4].
Identifying effective biomarkers using advanced statistical methods is paramount among these strategies importance, as they hold promise for improving early diagnosis, risk stratification, and the development of personalized treatment strategies [5,6]. PCa predominantly affects older men, with over 60% of diagnoses occurring in individuals above the age of 65 [7]. The etiology of PCa is complex and multifactorial, involving a combination of genetic predispositions, such as BRCA1 and BRCA2 mutations, as well as environmental factors like diet, physical activity, and exposure to carcinogens [8,9,10]. Current diagnostic methods, including digital rectal examination (DRE) and prostate-specific antigen (PSA) tests, suffer from significant limitations in terms of specificity and sensitivity, resulting in the overdiagnosis of indolent cases and underdiagnosis of aggressive tumors [11,12]. While emerging imaging technologies, such as multiparametric magnetic resonance imaging (mpMRI), show potential to enhance diagnostic accuracy, their widespread adoption is constrained by the need for further validation and the high cost associated with implementation [13,14]. Consequently, treatment strategies for PCa have evolved to encompass a broad spectrum, ranging from active surveillance for low-risk tumors to radical prostatectomy, radiotherapy, and androgen deprivation therapy for high-risk cases [15,16] These approaches increasingly rely on personalized medicine, which takes into account factors such as individual metabolic pathways, pathological characteristics, patient preferences, comorbidities, and overall life expectancy [17]. Inflammation plays a critical role in cancer development and progression, including PCa [17,18,19,20]. This study aims to address these diagnostic gaps by investigating a panel of hematological and inflammatory biomarkers that could improve PCa stratification beyond PSA alone. The rationale for integrating these biomarkers stems from increasing evidence linking systemic inflammation and hematological changes to tumor progression and prognosis. While acute inflammation serves a protective role in fighting infections and promoting tissue repair, chronic inflammation is associated with DNA damage, immune evasion, and enhanced tumorigenesis [21,22,23]. Recent studies have highlighted the potential of inflammatory markers derived from routine blood tests, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), as valuable prognostic tools in PCa [24]. Furthermore, hematological parameters such as hemoglobin (HGB), red cell distribution width (RDW), and hemoglobin distribution width (HDW) have also been shown to correlate with cancer outcomes, with anemia and elevated RDW frequently linked to poorer prognosis [25,26,27]. The role of eosinophils in PCa, through their dual involvement in anti-tumor immunity and tumor-promoting mechanisms, adds another layer of complexity and offers additional insights into disease progression [28,29,30].
Thus, the aim of this study was to integrate hematological and inflammatory biomarkers into a clinical model to predict the risk of positive prostate biopsy in patients suspected of prostate cancer. This approach could also assist in identifying clinically significant prostate cancer, thereby enhancing diagnostic precision and supporting personalized management strategies.
2. Materials and Methods
2.1. Study Design
This study was conducted on a cohort of 514 patients enrolled with clinical suspicion of prostate cancer (PCa) at the University Hospital of Sassari. Biological samples were collected from all the participants as part of a standardized diagnostic workflow. The screening protocol strictly adhered to the guidelines established by the Italian Association of Medical Oncology (AIOM), the Italian Society of Urology (SIU), and the Ministry of Health, with prostate biopsies serving as a pivotal component of the diagnostic process. Other procedures included comprehensive clinical evaluation, advanced imaging assessments, and blood-based biomarker analysis, leading to a diagnosis of benign prostatic hyperplasia (BPH), precancerous lesions (PL), or PCa.
This study received ethical approval from the Independent Ethics Committee of the Azienda Ospedaliero Universitaria di Cagliari (Approval Code: Prot. PG/2022/4985, Approval Date: 30 March 2022), in compliance with the Declaration of Helsinki and international guidelines for good clinical practice. Ethical considerations were rigorously upheld to ensure participant safety, confidentiality, and informed consent. A flow diagram detailing the patient journey, from initial presentation to final diagnosis, is provided in the graphical abstract. The eligibility criteria included male patients over the age of 50 presenting with clinical or biochemical indications of PCa, such as elevated serum PSA concentrations, abnormal findings on DRE, or radiological abnormalities. Patients with a prior history of PCa, ongoing or completed treatment for any malignancy, severe comorbidities contraindicating biopsy, or incomplete clinical data were excluded from this study.
Diagnostic Workflow and Histological Confirmation
All the patients included in this study (n = 514) were enrolled based on clinical suspicion of prostate cancer (PCa), following international guidelines. The diagnostic protocol adhered to a standardized, multi-step approach, consistent with recommendations from the Italian Association of Medical Oncology (AIOM) and the Italian Society of Urology (SIU), and was applied uniformly to all the participants. The diagnostic process included the following:
- Initial clinical evaluation, including digital rectal examination (DRE);
- Serum prostate-specific antigen (PSA) measurement (performed in all patients);
- Multiparametric magnetic resonance imaging (mpMRI) in 378 out of 514 patients (73.5%), evaluated according to PI-RADS v2.0 criteria. A PI-RADS score ≥ 3 prompted further evaluation;
- Transrectal ultrasound (TRUS)-guided prostate biopsy, performed in all patients with a minimum of 12 cores sampled;
- Histopathological evaluation of biopsy specimens, based on Gleason scoring and assignment to International Society of Urological Pathology (ISUP) Grade Groups;
- In selected cases, radical prostatectomy was performed to confirm ISUP grading and assess pathological staging (pT, pN);
- Radiological staging (e.g., CT, bone scintigraphy, or PSMA-PET) was performed in patients with PSA > 20 ng/mL or ISUP grade ≥ 3 to evaluate potential metastatic disease.
The diagnostic outcomes were histologically confirmed PCa, benign prostatic hyperplasia (BPH), or precancerous lesions (PL). The endpoint for classification was defined by the result of the biopsy. For PCa patients, ISUP Grade Groups were recorded for stratification purposes. When available, radical prostatectomy findings were used to validate initial biopsy-based diagnoses. The primary focus of the present study was the diagnostic evaluation before the initiation of any treatment.
2.2. Clinical and Laboratory Parameters
The following clinical, hematological, and inflammatory parameters were assessed for association with positive prostate biopsy outcomes: age, serum total PSA, hemoglobin (HGB), red blood cell count (RBC), white blood cell count (WBC), neutrophil count, neutrophil-to-lymphocyte ratio (NLR), systemic inflammatory response index (SIRI), hemoglobin distribution width (HDW), and erectile function score (International Index of Erectile Function, IIEF-5). Laboratory parameters were measured using standard automated hematology analyzers following international protocols.
2.3. Statistical Analysis
The data analysis was conducted using both univariate and multivariate approaches to ensure robust identification of significant variables. Statistical analyses were performed using GraphPad Prism (version 10.0.2, GraphPad Software, San Diego, CA, USA) and R software (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria).The initial examination of the dataset revealed substantial deviations from Gaussian distributions, leading to highly skewed data. To address this, two univariate methods were applied. First, the Kruskal–Wallis (KW) test, a non-parametric approach, was used to identify variables with significant differences in median values across groups. Second, the adaptive Box–Cox (ABC) transformation was employed to reshape variables to approximate Gaussian distributions, enabling the application of parametric tests such as ANOVA [31,32,33,34]. The results of the Lilliefors normality test applied to the data before and after the ABC transformation are presented in Supplementary Materials, Section S1.
The dataset contained approximately 7% missing values. To tackle this issue, we employed a probabilistic principal component analysis (PPCA). This approach models the data within a Gaussian latent variable framework, extending traditional principal component analysis to handle noise and missing data through maximum likelihood estimation. It has been shown that PPCA effectively estimates missing data, even in validation scenarios [35]. This method was applied to the ABC-transformed data, as PPCA performs well under the normality assumption.
The number of probabilistic principal components was 14, as they allowed for achieving 95% of the cumulative explained variance. Before performing PPCA, autoscaling was applied as a preprocessing step due to the diverse orders of magnitude across the different ABC-transformed variables. After the missing data imputation, the dataset was mapped back to its original space. Imputing missing values is crucial for enhancing the effectiveness of the ANOVA test, as it enables the inclusion of more data points. The data resulting from this combined operation will be hereafter referred to as ABC-PPCA reconstructed data.
On the reconstructed dataset, ANOVA was conducted to evaluate group differences, followed by Tukey–Kramer post-hoc tests to identify significant pairwise comparisons. The significance level for all the hypothesis tests was set at p < 0.05. In order to avoid potential false discovery errors from multiple comparisons, the Benjamini–Hochberg correction was applied with a false discovery rate (FDR) threshold of 5% [36].
From a multivariate perspective, the least absolute shrinkage and selection operator (LASSO) regression was employed to perform variable selection and evaluate their discriminatory power [37]. LASSO regression allows for both variable selection and fitting by identifying the coefficient values of a multiple linear regression such that an L1-penalized least squares objective function is minimized [38]. The solution of the problem generates a quasi-sparse coefficients vector. However, only BPH and PCa classes were considered in this case, since no clear separation was observed when the PL group was contemplated in the dataset, as reported later in the results section. In this regression analysis, membership in the BPH and PCa groups was represented by the numbers 1 and 2, respectively. For the test set, if the predicted response exceeded 1.54, the model assigned the sample to the PC class; otherwise, it was attributed to the BPH class. The optimal value of the penalization parameter was determined through Monte Carlo cross-validation with 5000 iterations (for each iteration, 30% of samples were used as a test set). Therefore, the optimal combination of cutoff and penalization parameter values was selected based on the highest achieved accuracy. Standard normal variate (SNV) preprocessing was applied before performing the regression to analyze the absolute values of the regression coefficients and compare their relative importance in discriminating the different classes. The features commonly selected by the three methods (Kruskal–Wallis, Box–Cox ANOVA, and LASSO) were recognized as significant for this study.
3. Results
3.1. Univariate Analysis
Table 1 summarizes the significant clinical variables identified through univariate analysis, presenting the corresponding parameter values following ABC transformation. The effect size for the one-way ANOVA, as measured by Cohen’s f, is reported in the Supplementary Material, Section S2.

Table 1.
Kruskal–Wallis and ANOVA test results through p-values for various clinical parameters. ANOVA was conducted on PPCA reconstructed and ABC transformed data. Benjamini–Hochberg corrected p-values were corrected.
The Kruskal–Wallis test identified statistically significant differences across groups in 11 variables: age, PSA, Index %, red blood cells (RBC), HGB, neutrophils, LMR, NLR, systemic inflammatory response index (SIRI), International Index of Erectile Function (IIEF), and transrectal ultrasound scan (TRUSS). The preprocessing step confirmed deviations from normality in these variables, reinforcing the necessity of transformation before further comparisons.
The further assessment of the ABC-PPCA reconstructed data confirmed statistical significance for 10 variables: age, PSA, Index %, HGB, RBC, HDW, neutrophils, NLR, SIRI, and IIEF [39]. While LMR and TRUSS were initially identified as significant, their effect was not confirmed in subsequent analyses. Conversely, HDW, which was not initially highlighted, emerged as significant in this adjusted dataset, suggesting borderline significance for certain variables. To minimize the risk of false positives, FDR correction was applied. This adjustment confirmed age, PSA, Index %, HGB, and IIEF as the most robust biomarkers, consistently distinguishing between patient groups. These findings reinforce their potential relevance in clinical stratification and disease characterization. A sensitivity analysis was performed to evaluate the normality of the distributions across different λ values, using the p-values from the Lilliefors test. This analysis was conducted for each variable and within each class. The detailed results are available in the Supplementary Material, Section S3.
The clinical, hematological, and inflammatory parameters evaluated in patients with benign prostatic hyperplasia (BPH), precancerous lesions (PLs), and prostate cancer (PCa) are summarized in Table 2. Mean values and standard deviations (SDs) for each variable are reported. Statistical comparisons among groups were performed using a one-way ANOVA followed by post hoc Tukey tests. The same table also reports the class for which the variable is upregulated. As can be observed, most markers are upregulated in the PCa class. In contrast, higher values of other variables, such as IIEF, are reasonably associated with patients in the PBH class.

Table 2.
Clinical, hematological, and inflammatory parameters in patients with BPH, PL, and PCa.
In order to assess the potential bias introduced by data imputation, we calculated the R2 coefficient on the previously known (non-missing) data prior to probabilistic PCA, obtaining a value of 0.99992. Additionally, we generated violin plots of the residuals for all the investigated variables, provided in the Supplementary Materials. These results confirm the reliability of the imputation process, indicating that no artificial significance was introduced. A detailed ISUP-based subgroup analysis is provided in Supplementary Section S4, including the distribution of patients across ISUP grades and associated variations in clinical and inflammatory biomarkers (Table S4).
3.2. Distribution of ISUP Grades in Confirmed PCa Patients
Among the 275 patients with histologically confirmed prostate cancer (PCa), the distribution across ISUP Grade Groups is reported in Supplementary Table S3. Briefly, 42.2% of patients were classified as ISUP Grade 1, 13.1% as Grade 2, 12.4% as Grade 3, 20.0% as Grade 4, and 12.4% as Grade 5. This distribution indicates a predominance of lower-grade disease (Grades 1–2), with higher-grade cancers (Grades 4–5) accounting for a significant proportion of aggressive cases.
Radical prostatectomy was performed in 63 out of the 275 PCa patients (22.9%), allowing for the pathological confirmation of ISUP grading and tumor staging (pT and pN). Radiological staging was performed in 64 patients with PSA > 20 ng/mL or ISUP Grade ≥ 3, identifying metastatic disease in 19 cases (7.0%).
Further stratification of clinical and hematological parameters across ISUP grades, including PSA, hemoglobin, white blood cell count, neutrophil-to-lymphocyte ratio (NLR), and systemic inflammatory response index (SIRI), is detailed in Supplementary Table S4.
3.3. Post-Hoc Analysis
Pairwise comparisons using a post-hoc analysis identified significant differences primarily between the BPH and PCa groups, while no significant variations were observed between BPH and PL. However, comparisons between PL and PCa revealed significant differences for PSA, Index %, HGB, HDW, and IIEF, indicating their potential role in distinguishing these two conditions (Table 3).

Table 3.
Post-hoc analysis for clinical parameters. The features that resulted to significantly change from ANOVA test are highlighted.
The RBC parameter exhibited a borderline p-value slightly above 0.05, likely due to its marginal significance in the initial analysis. This result suggests a reduction in statistical power when transitioning from an overall comparison to pairwise testing. These findings highlight the importance of appropriate data preprocessing and statistical correction methods to ensure robust and reliable conclusions.
3.4. Multivariate Analysis
Because the most significant differences in the univariate analysis were observed between the BPH and PCa groups, the PL class was excluded from the subsequent multivariate analysis. In this analysis, LASSO regression was applied, serving both as a variable selection technique and a classification tool. The analysis identified 12 nonzero regression coefficients, confirming their contribution to classification. Age, Index %, PSA, NLR, and neutrophils emerged as the most influential biomarkers, consistently with univariate findings. Among these, age and Index% exhibited the highest regression coefficients, reinforcing their diagnostic relevance. Additionally, PSA, RBC, NLR, neutrophils, and IIEF, which were identified as significant in prior analyses, also demonstrated strong discriminatory power in the LASSO model (Figure 1a). These findings support the clinical utility of a selected biomarker panel for distinguishing between BPH and PCa, highlighting their potential role in refining diagnostic strategies and improving patient stratification.
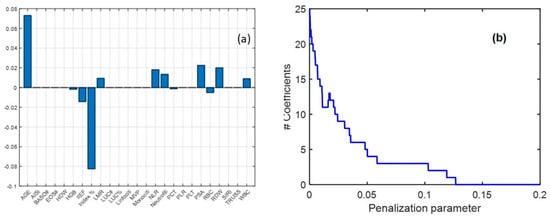
Figure 1.
Coefficient values obtained from the LASSO regression (a) and sensitivity analysis for LASSO coefficients by varying the penalization parameter (b). The plot illustrates the relationship between the penalization parameter (λ) and the number of nonzero coefficients retained in the model. As λ increases, the number of selected variables decreases, demonstrating the sparsity-inducing property of LASSO.
From the validation procedure, the optimal penalization parameter for the LASSO model was determined to be 0.02. The model achieved an average classification accuracy of 0.67 ± 0.04 during cross-validation and 0.70 when applied to the entire dataset. Classification performance is depicted in Figure 2a,b, showing the receiver operating characteristic (ROC) curve along with sensitivity and specificity trends as the threshold varied. The area under the ROC curve (AUC) was 0.74, demonstrating an acceptable classification performance. These results highlight the effectiveness of LASSO regression in identifying critical biomarkers and improving classification accuracy within a biochemical framework. When using PSA alone, an accuracy of 0.63 and an AUC of 0.66 were obtained, meaning that the consideration of multiple variables in a multivariate model can improve the classification performance (Figure 2c,d).
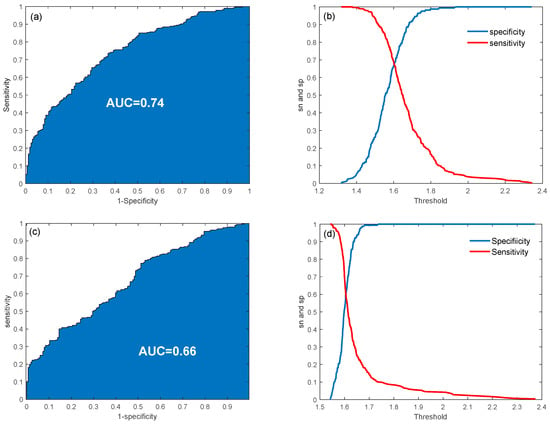
Figure 2.
ROC curves and sensitivity/specificity plot for the classification problem using multiple variables (a,b) and PSA only (c,d).
3.5. Biplot Analysis
The biplot presented in Figure 3 illustrates the first two principal components, PC1 (19% of variance) and PC2 (13% of variance), derived from the ABC-PPCA reconstruction. The plot reveals substantial overlap among the three clinical groups, underscoring the challenges of achieving clear classification between them. However, a partial separation is observed: several PCa observations predominantly exhibit negative values along PC1, while BPH observations tend to cluster more towards positive values, suggesting some degree of distinction.
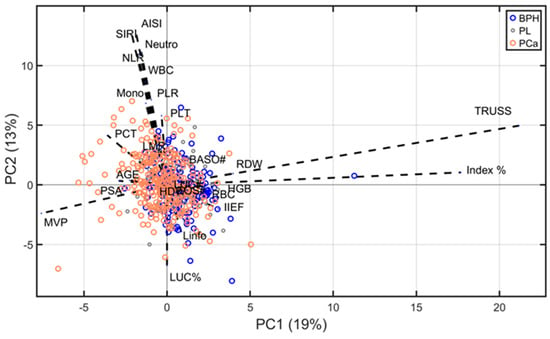
Figure 3.
Biplot of PPCA showing the distribution of observations from three groups (BPH, PL, and PCa) along the first two principal components. Blue arrows indicate the loading values. For a clearer identification of variables’ contribution, loading values were multiplied 20 times.
The direction and length of the arrows in the biplot represent the contribution of each variable to the principal components, reflecting the variables’ loadings. Notably, TRUSS and Index % exhibit strong influences on PC1, correlating positively with the BPH cluster. Conversely, mean platelet volume (MPV), PSA, and age display stronger projections aligned with the PCa cluster. Variables such as HDW, eosinophils, and basophils contribute less significantly, as indicated by their shorter arrows near the origin.
Importantly, most of the findings obtained through PPCA analysis align with the results from prior analyses, reinforcing the robustness and consistency of the observed trends. These observations highlight the potential of specific variables to discriminate between clinical groups, even in the context of overlapping distributions.
3.6. Joint Analysis
To identify key variables, both univariate and multivariate methods were employed. The combined representation of results from these methods provides a robust assessment of the variables’ importance. The Euler–Venn diagram presented in Figure 4 illustrates the intersection of variables identified as significant by the Kruskal–Wallis test, ANOVA (both following FDR correction), and LASSO regression. Five variables, age, PSA, Index %, HGB, and IIEF, were consistently found to show significant changes across different diagnostic groups using all three methodologies.
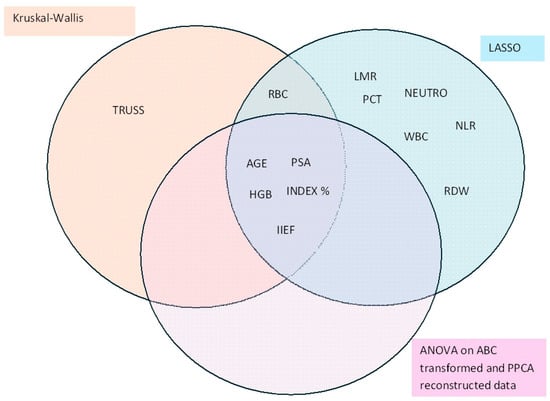
Figure 4.
Euler–Venn diagram displaying the features for which the diagnosis effect resulted to be significant using three different methods.
As shown in the diagram, the LASSO regression was less conservative in variable selection compared to FDR-corrected p-values. This discrepancy is likely attributable to the stringent selection criteria imposed by the univariate methods, which inherently focus on reducing false positives. The intersection of results across multiple methodologies highlights the robustness of the selected variables and their potential utility in distinguishing between diagnostic groups.
4. Discussion
This study employed a combination of univariate and multivariate statistical techniques to identify biomarkers with strong potential for distinguishing PCa from BPH and PL. The integration of these complementary approaches strengthens the reliability of the findings and highlights the clinical utility of these biomarkers in improving diagnostic accuracy and patient stratification. The distribution of ISUP grades observed in our cohort, predominantly favoring lower-grade tumors (ISUP 1–2), reflects the typical heterogeneity of prostate cancer presentation at initial diagnosis. However, the significant proportion of patients with high-grade disease (ISUP 4–5) and the detection of metastatic disease in 7% of staged cases highlight the persistent clinical challenge of accurately stratifying risk at diagnosis. These findings underscore the importance of integrative biomarker models to more accurately distinguish indolent from aggressive forms of prostate cancer, thus optimizing treatment decisions and reducing overtreatment.
Several studies have confirmed that PSA is a widely used biomarker for prostate cancer (PCa) detection, although its specificity remains limited, leading to potential overdiagnosis and overtreatment [12,40]. Our study reinforces the role of PSA and identifies additional biomarkers such as age, Index %, and hemoglobin (HGB), which offer improved classification performance. The inclusion of inflammatory markers such as NLR and neutrophils aligns with emerging evidence supporting the role of systemic inflammation in PCa progression [41]. The observed associations between hematological parameters and PCa highlight the interplay between systemic inflammation and tumor development, as previously suggested by [21]. In contrast to previous studies that relied solely on univariate methods, our study employed a combined approach that incorporates multivariate techniques to refine biomarker selection. The adaptive Box–Cox transformation and probabilistic PCA used in this analysis effectively addressed data skewness and missingness, thereby enhancing the reliability of the results. The LASSO regression enabled the identification of the most relevant variables by integrating univariate results and mitigating potential data bias through advanced preprocessing techniques [42,43]. The classification accuracy of the model (70%) and the area under the curve (AUC = 0.74) indicate acceptable performance, supporting its potential for future clinical translation. These findings are consistent with current studies and clinical needs that emphasize the development of multimodal biomarker panels to improve specificity and reduce unnecessary biopsies [44]. By integrating these biomarkers into routine clinical practice, it may be possible to refine risk stratification models and reduce diagnostic uncertainty, while minimizing unnecessary interventions, in line with current recommendations for personalized medicine [45].
4.1. Biological Interpretation of Significant Biomarkers
Molecularly, elevated PSA reflects increased production by malignant prostate epithelial cells, often due to the disruption of tissue architecture and increased vascular permeability in cancerous lesions. A high Index % (percentage of free PSA) is typically associated with benign conditions, whereas a lower Index % supports malignancy, reflecting the higher proportion of complexed PSA in PCa. Reduced hemoglobin (HGB) levels may indicate cancer-associated anemia, which can result from chronic inflammation, bone marrow infiltration, or cytokine-mediated suppression of erythropoiesis. The IIEF score, reflecting sexual function, is often lower in PCa patients and may indirectly relate to tumor burden or hormonal changes affecting erectile physiology. These biomarkers together reflect systemic and local changes associated with PCa pathogenesis, offering a multidimensional diagnostic profile.
4.2. Strengths of the Study
This study employed a rigorous analytical framework combining univariate and multivariate approaches to identify and validate PCa biomarkers. The integration of statistical methods such as the ABC transformation, PPCA, and LASSO regression ensured robust data analysis, minimizing bias and improving reliability. A key strength of this study is the identification of biomarkers that are cost-effective and readily accessible in clinical settings. Age, PSA, Index %, HGB, and IIEF demonstrated consistent diagnostic value, reinforcing their relevance in differentiating PCa from BPH and PL. These findings align with previous reports suggesting that hematological and inflammatory markers could complement traditional PCa diagnostics [25]. Moreover, this study highlights the importance of multimodal biomarker integration, which has the potential to enhance diagnostic precision while reducing reliance on invasive procedures. The alignment of our findings with prior literature strengthens their potential applicability in clinical practice.
4.3. Limitations of the Study
Despite the strengths of this study, certain limitations must be acknowledged. While the cohort of 514 patients provides meaningful insights, larger multicenter studies are needed to validate the generalizability of these findings. The exclusion of the PL group from the multivariate analysis, due to its ambiguous separation, may have limited the ability to fully assess its diagnostic relevance. Future studies should incorporate larger PL cohorts to refine classification models. Another limitation is the absence of external validation. Although internal cross-validation confirmed the robustness of the model, future research should focus on validating these biomarkers in independent cohorts to confirm their clinical applicability. Additionally, this study employed a cross-sectional design, which provides a snapshot of biomarker performance but does not assess their prognostic value. Longitudinal studies will be necessary to evaluate how these markers change over time and their predictive power in disease progression. Moreover, this study recognizes that ethnic and geographic variability may significantly impact biomarker expression and diagnostic accuracy, underscoring the necessity for population-specific validation to enhance the broader applicability of our findings across diverse clinical settings. Addressing these limitations in future research will be crucial to strengthening the clinical utility of these biomarkers, ultimately refining PCa diagnosis and risk stratification. These findings contribute to the expanding body of evidence supporting the integration of advanced statistical methodologies in clinical biomarker discovery, with the potential to optimize patient outcomes and inform precision medicine strategies in prostate cancer management.
5. Conclusions
This study identified a robust panel of clinically accessible biomarkers—PSA, Index %, hemoglobin (HGB), age, neutrophils, NLR, and IIEF—with significant potential for stratifying patients with prostate cancer (PCa), benign prostatic hyperplasia (BPH), and precancerous lesions (PL). By integrating univariate and multivariate statistical methods, particularly LASSO regression and PPCA, we achieved improved classification performance (AUC = 0.74) compared to PSA alone. PSA, Index %, and age emerged as dominant contributors to diagnostic discrimination, while HGB and IIEF provided additional context related to systemic status and functional health. The inclusion of inflammatory markers such as NLR and neutrophil count supports the growing evidence of the role of systemic inflammation in PCa progression. These findings demonstrate that routinely collected hematological and inflammatory data can be effectively repurposed to support early diagnosis and risk stratification, thereby reducing unnecessary invasive procedures. Future validation in larger cohorts will be essential to confirm the clinical utility of this biomarker signature and its integration into personalized diagnostic workflows.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics15111385/s1: Section S1: Normality test; Section S2: Effect size analysis for the one-way ANOVA test; Section S3: Sensitivity analysis of data normality; and Section S4: Detailed Stratification of the Prostate Cancer Cohort According to ISUP Grade.
Author Contributions
Conceptualization, D.C. and C.C.; Methodology, D.C., M.R.D.M. and A.Z.; Software, L.S. and M.G.; Investigation, L.S. and S.M. (Serenella Medici); Resources, M.M.; Data curation, A.T., S.M. (Sonia Marra), M.G. and C.C.; Writing—original draft, D.C. and M.G.; Writing—review & editing, D.C., A.A.M. and M.G.; Supervision, D.C.; Funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Regione Autonoma della Sardegna, pursuant to Regional Law 7 August 2007, n.7 “Promotion of Scientific Research and Technological Innovation in Sardinia”—UGOV Project RAS_CRP2023 CARRU: “Role of Circulating Biomarkers in the management of patients with prostate cancer”. Lilt Nazionale—5 per mille Program for the year 2022, LILT 2023 scientific-health research call: “Study of prostate cancer heterogeneity as a key to dissecting the cellular subtypes that characterize it”. Number: LILT—Protocol number 2024U0001294 of 29 March 2024. Fondazione di Sardegna MEDICI 2017 Project “Nuclear Magnetic Resonance Studies: toxic and carcinogenic metals and their interactions with cellular targets”.
Institutional Review Board Statement
COMITATO ETICO INDIPENDENTE—Azienda Ospedaliero Universitaria di Cagliari P.O. San Giovanni di Dio: via Ospedale 54–09124 Cagliari, Approval Code: Prot. PG/2022/4985, Approval Date: 30 March 2022.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study. Our study involved human subjects and received approval from the Ethics Committee.
Data Availability Statement
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request. Researchers seeking access may be required to provide a brief proposal outlining the intended use and agree to confidentiality terms as per institutional policies.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABC | Adaptive Box–Cox transformation |
| AIOM | Italian Association of Medical Oncology |
| AISI | Acute inflammation systemic index |
| AUC | Area under the curve |
| BRCA1/BRCA2 | Breast cancer gene 1/breast cancer gene 2 |
| BPH | Benign prostatic hyperplasia |
| DRE | Digital rectal examination |
| FDR | False discovery rate |
| HGB | Hemoglobin |
| HDW | Hemoglobin distribution width |
| IIEF | International Index of Erectile Function |
| KW | Kruskal–Wallis |
| LASSO | Least absolute shrinkage and selection operator |
| MLR | Monocyte-to-lymphocyte ratio |
| mpMRI | Multiparametric magnetic resonance imaging |
| NLR | Neutrophil-to-lymphocyte ratio |
| PCa | Prostate cancer |
| PL | Precancerous lesions |
| PLR | Platelet-to-lymphocyte ratio |
| PPCA | Probabilistic principal component analysis |
| PSA | Prostate-specific antigen |
| RBC | Red blood cells |
| RDW | Red cell distribution width |
| ROC | Receiver operating characteristic |
| SIU | Italian Society of Urology |
| SIRI | Systemic inflammatory response index |
| SNV | Standard normal variate |
| TRUSS | Transrectal ultrasound scan |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Tesfai, A.; Norori, N.; Harding, T.A.; Wong, Y.H.; Hobbs, M.D. Variation in harms and benefits of prostate-specific antigen screening for prostate cancer by socio-clinical risk factors: A rapid review. BJUI Compass 2024, 5, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Solinas, T.; Azara, E.; Culeddu, N.; Cruciani, S.; Zinellu, A.; Medici, S.; Maioli, M.; Madonia, M.; Carru, C. Plasma polyamine biomarker panels: Agmatine in support of prostate cancer diagnosis. Biomolecules 2022, 12, 514. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K. Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K. Advanced prostate cancer: AUA/ASTRO/SUO guideline part II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Desai, M.M.; Cacciamani, G.E.; Gill, K.; Zhang, J.; Liu, L.; Abreu, A.; Gill, I.S. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw. Open 2022, 5, e222246. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A. Underlying features of prostate cancer—Statistics, risk factors, and emerging methods for its diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Ziglioli, F.; Patera, A.; Isgrò, G.; Campobasso, D.; Guarino, G.; Maestroni, U. Impact of modifiable lifestyle risk factors for prostate cancer prevention: A review of the literature. Front. Oncol. 2023, 13, 1203791. [Google Scholar] [CrossRef]
- Coradduzza, D.; Medici, S.; Chessa, C.; Zinellu, A.; Madonia, M.; Angius, A.; Carru, C.; De Miglio, M.R. Assessing the predictive power of the hemoglobin/red cell distribution width ratio in cancer: A systematic review and future directions. Medicina 2023, 59, 2124. [Google Scholar] [CrossRef]
- Matsukawa, A.; Yanagisawa, T.; Bekku, K.; Parizi, M.K.; Laukhtina, E.; Klemm, J.; Chiujdea, S.; Mori, K.; Kimura, S.; Fazekas, T. Comparing the performance of digital rectal examination and prostate-specific antigen as a screening test for prostate cancer: A systematic review and meta-analysis. Eur. Urol. Oncol. 2024, 7, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Albaladejo, M.D.; Allué, J.A.; Castaño, M.A.; Morell-Garcia, D.; Ruiz, M.À.; Santamaría, M.; Torrejón, M.J.; Giménez, N. Prostate cancer screening: Guidelines review and laboratory issues. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Gondoputro, W.; Thompson, J.E.; Reesink, D.J.; van Riel, L.A.; Zhang, D.; Blazevski, A.; Doan, P.; Agrawal, S.; Matthews, J. Diagnostic accuracy of multiparametric magnetic resonance imaging to detect residual prostate cancer following irreversible electroporation—A multicenter validation study. Eur. Urol. Focus 2022, 8, 1591–1598. [Google Scholar] [CrossRef]
- Hoffman, R.M. Screening for prostate cancer. N. Engl. J. Med. 2011, 365, 2013–2019. [Google Scholar] [CrossRef]
- Ho, M.D.; Ross, A.E.; Eggener, S.E. Risk stratification of low-risk prostate cancer: Individualizing care in the era of active surveillance. J. Urol. 2023, 210, 38–45. [Google Scholar] [CrossRef]
- Coradduzza, D.; Solinas, T.; Balzano, F.; Culeddu, N.; Rossi, N.; Cruciani, S.; Azara, E.; Maioli, M.; Zinellu, A.; De Miglio, M.R. miRNAs as molecular biomarkers for prostate cancer. J. Mol. Diagn. 2022, 24, 1171–1180. [Google Scholar] [CrossRef]
- Sharma, U.; Sahu, A.; Shekhar, H.; Sharma, B.; Haque, S.; Kaur, D.; Tuli, H.S.; Mishra, A.; Ahmad, F. The heat of the battle: Inflammation’s role in prostate cancer development and inflammation-targeted therapies. Discov. Oncol. 2025, 16, 108. [Google Scholar] [CrossRef]
- Wang, M.; Dong, H.; He, Z.-Y. A novel prognosis and drug-susceptibility predictor based inflammatory-related genes signature in prostate cancer. J. Men’s Health 2024, 20, 26–38. [Google Scholar]
- Liu, X.; Yin, L.; Shen, S.; Hou, Y. Inflammation and cancer: Paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2023, 10, 151–164. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef] [PubMed]
- Melichar, B. Biomarkers in the management of lung cancer: Changing the practice of thoracic oncology. Clin. Chem. Lab. Med. (CCLM) 2023, 61, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, X.; Shen, L.; Xu, K.; Wen, Z.; Gao, N.; Fan, T.; Xun, S.; Zhu, Q.; Qu, X. Amplification-free analysis of bladder cancer micrornas on wrinkled silica nanoparticles with DNA-functionalized quantum dots. Anal. Chem. 2024, 96, 4860–4867. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wu, J.; Kong, Y.; Xu, F.; Zhou, Y.; Sun, Q.; Gao, Q.; Cai, Z.; Yang, C.; Huang, Y. Associations of systemic immune-inflammation index with high risk for prostate cancer in middle-aged and older US males: A population-based study. Immun. Inflamm. Dis. 2024, 12, e1327. [Google Scholar] [CrossRef] [PubMed]
- Orabi, H.; Howard, L.; Amling, C.L.; Aronson, W.J.; Cooperberg, M.R.; Kane, C.J.; Terris, M.K.; Klaassen, Z.; Janes, J.L.; Freedland, S.J. Red blood cell distribution width is associated with all-cause mortality but not adverse cancer-specific outcomes in men with clinically localized prostate cancer treated with radical prostatectomy: Findings based on a multicenter shared equal access regional cancer hospital registry. Eur. Urol. Open Sci. 2022, 37, 106–112. [Google Scholar]
- Schumacher, O.; Galvao, D.A.; Taaffe, D.R.; Chee, R.; Spry, N.; Newton, R.U. Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Carlisi, M.; Presti, R.L.; Plano, F.; Mancuso, S.; Siragusa, S.; Caimi, G. Changes in RDW according to prognostic predictors in newly diagnosed multiple myeloma. Sci. Rep. 2024, 14, 2832. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Munitz, A. T cell-eosinophil crosstalk—A new road for effective immune checkpoint blockade in breast cancer? Cancer Cell 2023, 41, 9–11. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Grass, D.G.; Torres-Roca, J.; Eschrich, S.A.; Gordon, Y.; Dolitzky, A.; Hazut, I.; Avlas, S.; Jacobsen, E.A. Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations. Oncoimmunology 2021, 10, 1859732. [Google Scholar] [CrossRef]
- Jacobse, J.; Aziz, Z.; Sun, L.; Chaparro, J.; Pilat, J.M.; Kwag, A.; Buendia, M.; Wimbiscus, M.; Nasu, M.; Saito, T. Eosinophils exert antitumorigenic effects in the development of esophageal squamous cell carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 961–983. [Google Scholar] [CrossRef]
- Yu, H.; Sang, P.; Huan, T. Adaptive box–cox transformation: A highly flexible feature-specific data transformation to improve metabolomic data normality for better statistical analysis. Anal. Chem. 2022, 94, 8267–8276. [Google Scholar] [CrossRef]
- Atkinson, A.C.; Riani, M.; Corbellini, A. The box–cox transformation: Review and extensions. Statist. Sci. 2021, 36, 239–255. [Google Scholar] [CrossRef]
- Fanari, F.; Carboni, G.; Desogus, F.; Grosso, M.; Wilhelm, M. A chemometric approach to assess the rheological properties of durum wheat dough by indirect FTIR measurements. Food Bioprocess Technol. 2022, 15, 1040–1054. [Google Scholar] [CrossRef]
- Sibono, L.; Grosso, M.; Tejedor-Calvo, E.; Casula, M.; Marco-Montori, P.; Garcia-Barreda, S.; Manis, C.; Caboni, P. A critical analysis of adaptive box-cox transformation for skewed distributed data management: Metabolomics of spanish and argentinian truffles as a case study. Anal. Chim. Acta 2025, 1345, 343704. [Google Scholar] [CrossRef]
- Sibono, L.; Grosso, M.; Tronci, S.; Errico, M.; Addis, M.; Vacca, M.; Manis, C.; Caboni, P. Investigation of seasonal variation in fatty acid and mineral concentrations of pecorino romano pdo cheese: Imputation of missing values for enhanced classification and metabolic profile reconstruction. Metabolites 2023, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Jonas, R.; Cook, J. Lasso regression. Br. J. Surg. 2018, 105. [Google Scholar]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Huszno, J.; Kołosza, Z.; Mrochem-Kwarciak, J.; Telka, E.; Jochymek, B.; Miszczyk, L. Role of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, lymphocyte-monocyte ratio and platelets in prognosis of patients with prostate cancer. Oncol. Lett. 2022, 24, 305. [Google Scholar] [CrossRef]
- Filella, X.; González, Á.; Augé, J.M.; Barco, A.; Carbonell, R.; Gaspar, M.J.; Martínez-Peinado, A.; Barrios, C.P.; Sánchez-Carbayo, M.; Santotoribio, J.D. A new approach to prostate cancer screening. Adv. Lab. Med. Av. Med. Lab. 2023, 4, 207–211. [Google Scholar] [CrossRef]
- Satır, A.; Üstündağ, Y.; Yeşil, M.R.; Huysal, K. Prediction of prostate cancer from routine laboratory markers with automated machine learning. J. Clin. Lab. Anal. 2025, e25143. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Zhang, Y.; Gao, H.; Wang, Y.; Liu, Y.; Gao, X. RPI-MDLStack: Predicting RNA–protein interactions through deep learning with stacking strategy and LASSO. Appl. Soft Comput. 2022, 120, 108676. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Soeterik, T.F.; Wu, X.; Van den Bergh, R.C.; Kesch, C.; Zattoni, F.; Falagario, U.; Martini, A.; Miszczyk, M.; Fasulo, V.; Maggi, M. Personalised prostate cancer diagnosis: Evaluating biomarker-based approaches to reduce unnecessary magnetic resonance imaging and biopsy procedures. Eur. Urol. Open Sci. 2025, 75, 106–119. [Google Scholar] [CrossRef]
- Angioni, D.; Delrieu, J.; Hansson, O.; Fillit, H.; Aisen, P.; Cummings, J.; Sims, J.; Braunstein, J.; Sabbagh, M.; Bittner, T. Blood biomarkers from research use to clinical practice: What must be done? A report from the EU/US CTAD task force. J. Prev. Alzheimer’s Dis. 2022, 9, 569–579. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).