Diagnostic Stratification of Prostate Cancer Through Blood-Based Biochemical and Inflammatory Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
Diagnostic Workflow and Histological Confirmation
- Initial clinical evaluation, including digital rectal examination (DRE);
- Serum prostate-specific antigen (PSA) measurement (performed in all patients);
- Multiparametric magnetic resonance imaging (mpMRI) in 378 out of 514 patients (73.5%), evaluated according to PI-RADS v2.0 criteria. A PI-RADS score ≥ 3 prompted further evaluation;
- Transrectal ultrasound (TRUS)-guided prostate biopsy, performed in all patients with a minimum of 12 cores sampled;
- Histopathological evaluation of biopsy specimens, based on Gleason scoring and assignment to International Society of Urological Pathology (ISUP) Grade Groups;
- In selected cases, radical prostatectomy was performed to confirm ISUP grading and assess pathological staging (pT, pN);
- Radiological staging (e.g., CT, bone scintigraphy, or PSMA-PET) was performed in patients with PSA > 20 ng/mL or ISUP grade ≥ 3 to evaluate potential metastatic disease.
2.2. Clinical and Laboratory Parameters
2.3. Statistical Analysis
3. Results
3.1. Univariate Analysis
3.2. Distribution of ISUP Grades in Confirmed PCa Patients
3.3. Post-Hoc Analysis
3.4. Multivariate Analysis
3.5. Biplot Analysis
3.6. Joint Analysis
4. Discussion
4.1. Biological Interpretation of Significant Biomarkers
4.2. Strengths of the Study
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Adaptive Box–Cox transformation |
| AIOM | Italian Association of Medical Oncology |
| AISI | Acute inflammation systemic index |
| AUC | Area under the curve |
| BRCA1/BRCA2 | Breast cancer gene 1/breast cancer gene 2 |
| BPH | Benign prostatic hyperplasia |
| DRE | Digital rectal examination |
| FDR | False discovery rate |
| HGB | Hemoglobin |
| HDW | Hemoglobin distribution width |
| IIEF | International Index of Erectile Function |
| KW | Kruskal–Wallis |
| LASSO | Least absolute shrinkage and selection operator |
| MLR | Monocyte-to-lymphocyte ratio |
| mpMRI | Multiparametric magnetic resonance imaging |
| NLR | Neutrophil-to-lymphocyte ratio |
| PCa | Prostate cancer |
| PL | Precancerous lesions |
| PLR | Platelet-to-lymphocyte ratio |
| PPCA | Probabilistic principal component analysis |
| PSA | Prostate-specific antigen |
| RBC | Red blood cells |
| RDW | Red cell distribution width |
| ROC | Receiver operating characteristic |
| SIU | Italian Society of Urology |
| SIRI | Systemic inflammatory response index |
| SNV | Standard normal variate |
| TRUSS | Transrectal ultrasound scan |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Tesfai, A.; Norori, N.; Harding, T.A.; Wong, Y.H.; Hobbs, M.D. Variation in harms and benefits of prostate-specific antigen screening for prostate cancer by socio-clinical risk factors: A rapid review. BJUI Compass 2024, 5, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Solinas, T.; Azara, E.; Culeddu, N.; Cruciani, S.; Zinellu, A.; Medici, S.; Maioli, M.; Madonia, M.; Carru, C. Plasma polyamine biomarker panels: Agmatine in support of prostate cancer diagnosis. Biomolecules 2022, 12, 514. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K. Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K. Advanced prostate cancer: AUA/ASTRO/SUO guideline part II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Desai, M.M.; Cacciamani, G.E.; Gill, K.; Zhang, J.; Liu, L.; Abreu, A.; Gill, I.S. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw. Open 2022, 5, e222246. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A. Underlying features of prostate cancer—Statistics, risk factors, and emerging methods for its diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Ziglioli, F.; Patera, A.; Isgrò, G.; Campobasso, D.; Guarino, G.; Maestroni, U. Impact of modifiable lifestyle risk factors for prostate cancer prevention: A review of the literature. Front. Oncol. 2023, 13, 1203791. [Google Scholar] [CrossRef]
- Coradduzza, D.; Medici, S.; Chessa, C.; Zinellu, A.; Madonia, M.; Angius, A.; Carru, C.; De Miglio, M.R. Assessing the predictive power of the hemoglobin/red cell distribution width ratio in cancer: A systematic review and future directions. Medicina 2023, 59, 2124. [Google Scholar] [CrossRef]
- Matsukawa, A.; Yanagisawa, T.; Bekku, K.; Parizi, M.K.; Laukhtina, E.; Klemm, J.; Chiujdea, S.; Mori, K.; Kimura, S.; Fazekas, T. Comparing the performance of digital rectal examination and prostate-specific antigen as a screening test for prostate cancer: A systematic review and meta-analysis. Eur. Urol. Oncol. 2024, 7, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Albaladejo, M.D.; Allué, J.A.; Castaño, M.A.; Morell-Garcia, D.; Ruiz, M.À.; Santamaría, M.; Torrejón, M.J.; Giménez, N. Prostate cancer screening: Guidelines review and laboratory issues. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Gondoputro, W.; Thompson, J.E.; Reesink, D.J.; van Riel, L.A.; Zhang, D.; Blazevski, A.; Doan, P.; Agrawal, S.; Matthews, J. Diagnostic accuracy of multiparametric magnetic resonance imaging to detect residual prostate cancer following irreversible electroporation—A multicenter validation study. Eur. Urol. Focus 2022, 8, 1591–1598. [Google Scholar] [CrossRef]
- Hoffman, R.M. Screening for prostate cancer. N. Engl. J. Med. 2011, 365, 2013–2019. [Google Scholar] [CrossRef]
- Ho, M.D.; Ross, A.E.; Eggener, S.E. Risk stratification of low-risk prostate cancer: Individualizing care in the era of active surveillance. J. Urol. 2023, 210, 38–45. [Google Scholar] [CrossRef]
- Coradduzza, D.; Solinas, T.; Balzano, F.; Culeddu, N.; Rossi, N.; Cruciani, S.; Azara, E.; Maioli, M.; Zinellu, A.; De Miglio, M.R. miRNAs as molecular biomarkers for prostate cancer. J. Mol. Diagn. 2022, 24, 1171–1180. [Google Scholar] [CrossRef]
- Sharma, U.; Sahu, A.; Shekhar, H.; Sharma, B.; Haque, S.; Kaur, D.; Tuli, H.S.; Mishra, A.; Ahmad, F. The heat of the battle: Inflammation’s role in prostate cancer development and inflammation-targeted therapies. Discov. Oncol. 2025, 16, 108. [Google Scholar] [CrossRef]
- Wang, M.; Dong, H.; He, Z.-Y. A novel prognosis and drug-susceptibility predictor based inflammatory-related genes signature in prostate cancer. J. Men’s Health 2024, 20, 26–38. [Google Scholar]
- Liu, X.; Yin, L.; Shen, S.; Hou, Y. Inflammation and cancer: Paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2023, 10, 151–164. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef] [PubMed]
- Melichar, B. Biomarkers in the management of lung cancer: Changing the practice of thoracic oncology. Clin. Chem. Lab. Med. (CCLM) 2023, 61, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, X.; Shen, L.; Xu, K.; Wen, Z.; Gao, N.; Fan, T.; Xun, S.; Zhu, Q.; Qu, X. Amplification-free analysis of bladder cancer micrornas on wrinkled silica nanoparticles with DNA-functionalized quantum dots. Anal. Chem. 2024, 96, 4860–4867. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wu, J.; Kong, Y.; Xu, F.; Zhou, Y.; Sun, Q.; Gao, Q.; Cai, Z.; Yang, C.; Huang, Y. Associations of systemic immune-inflammation index with high risk for prostate cancer in middle-aged and older US males: A population-based study. Immun. Inflamm. Dis. 2024, 12, e1327. [Google Scholar] [CrossRef] [PubMed]
- Orabi, H.; Howard, L.; Amling, C.L.; Aronson, W.J.; Cooperberg, M.R.; Kane, C.J.; Terris, M.K.; Klaassen, Z.; Janes, J.L.; Freedland, S.J. Red blood cell distribution width is associated with all-cause mortality but not adverse cancer-specific outcomes in men with clinically localized prostate cancer treated with radical prostatectomy: Findings based on a multicenter shared equal access regional cancer hospital registry. Eur. Urol. Open Sci. 2022, 37, 106–112. [Google Scholar]
- Schumacher, O.; Galvao, D.A.; Taaffe, D.R.; Chee, R.; Spry, N.; Newton, R.U. Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Carlisi, M.; Presti, R.L.; Plano, F.; Mancuso, S.; Siragusa, S.; Caimi, G. Changes in RDW according to prognostic predictors in newly diagnosed multiple myeloma. Sci. Rep. 2024, 14, 2832. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Munitz, A. T cell-eosinophil crosstalk—A new road for effective immune checkpoint blockade in breast cancer? Cancer Cell 2023, 41, 9–11. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Grass, D.G.; Torres-Roca, J.; Eschrich, S.A.; Gordon, Y.; Dolitzky, A.; Hazut, I.; Avlas, S.; Jacobsen, E.A. Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations. Oncoimmunology 2021, 10, 1859732. [Google Scholar] [CrossRef]
- Jacobse, J.; Aziz, Z.; Sun, L.; Chaparro, J.; Pilat, J.M.; Kwag, A.; Buendia, M.; Wimbiscus, M.; Nasu, M.; Saito, T. Eosinophils exert antitumorigenic effects in the development of esophageal squamous cell carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 961–983. [Google Scholar] [CrossRef]
- Yu, H.; Sang, P.; Huan, T. Adaptive box–cox transformation: A highly flexible feature-specific data transformation to improve metabolomic data normality for better statistical analysis. Anal. Chem. 2022, 94, 8267–8276. [Google Scholar] [CrossRef]
- Atkinson, A.C.; Riani, M.; Corbellini, A. The box–cox transformation: Review and extensions. Statist. Sci. 2021, 36, 239–255. [Google Scholar] [CrossRef]
- Fanari, F.; Carboni, G.; Desogus, F.; Grosso, M.; Wilhelm, M. A chemometric approach to assess the rheological properties of durum wheat dough by indirect FTIR measurements. Food Bioprocess Technol. 2022, 15, 1040–1054. [Google Scholar] [CrossRef]
- Sibono, L.; Grosso, M.; Tejedor-Calvo, E.; Casula, M.; Marco-Montori, P.; Garcia-Barreda, S.; Manis, C.; Caboni, P. A critical analysis of adaptive box-cox transformation for skewed distributed data management: Metabolomics of spanish and argentinian truffles as a case study. Anal. Chim. Acta 2025, 1345, 343704. [Google Scholar] [CrossRef]
- Sibono, L.; Grosso, M.; Tronci, S.; Errico, M.; Addis, M.; Vacca, M.; Manis, C.; Caboni, P. Investigation of seasonal variation in fatty acid and mineral concentrations of pecorino romano pdo cheese: Imputation of missing values for enhanced classification and metabolic profile reconstruction. Metabolites 2023, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Jonas, R.; Cook, J. Lasso regression. Br. J. Surg. 2018, 105. [Google Scholar]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Huszno, J.; Kołosza, Z.; Mrochem-Kwarciak, J.; Telka, E.; Jochymek, B.; Miszczyk, L. Role of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, lymphocyte-monocyte ratio and platelets in prognosis of patients with prostate cancer. Oncol. Lett. 2022, 24, 305. [Google Scholar] [CrossRef]
- Filella, X.; González, Á.; Augé, J.M.; Barco, A.; Carbonell, R.; Gaspar, M.J.; Martínez-Peinado, A.; Barrios, C.P.; Sánchez-Carbayo, M.; Santotoribio, J.D. A new approach to prostate cancer screening. Adv. Lab. Med. Av. Med. Lab. 2023, 4, 207–211. [Google Scholar] [CrossRef]
- Satır, A.; Üstündağ, Y.; Yeşil, M.R.; Huysal, K. Prediction of prostate cancer from routine laboratory markers with automated machine learning. J. Clin. Lab. Anal. 2025, e25143. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Zhang, Y.; Gao, H.; Wang, Y.; Liu, Y.; Gao, X. RPI-MDLStack: Predicting RNA–protein interactions through deep learning with stacking strategy and LASSO. Appl. Soft Comput. 2022, 120, 108676. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Soeterik, T.F.; Wu, X.; Van den Bergh, R.C.; Kesch, C.; Zattoni, F.; Falagario, U.; Martini, A.; Miszczyk, M.; Fasulo, V.; Maggi, M. Personalised prostate cancer diagnosis: Evaluating biomarker-based approaches to reduce unnecessary magnetic resonance imaging and biopsy procedures. Eur. Urol. Open Sci. 2025, 75, 106–119. [Google Scholar] [CrossRef]
- Angioni, D.; Delrieu, J.; Hansson, O.; Fillit, H.; Aisen, P.; Cummings, J.; Sims, J.; Braunstein, J.; Sabbagh, M.; Bittner, T. Blood biomarkers from research use to clinical practice: What must be done? A report from the EU/US CTAD task force. J. Prev. Alzheimer’s Dis. 2022, 9, 569–579. [Google Scholar] [CrossRef] [PubMed]
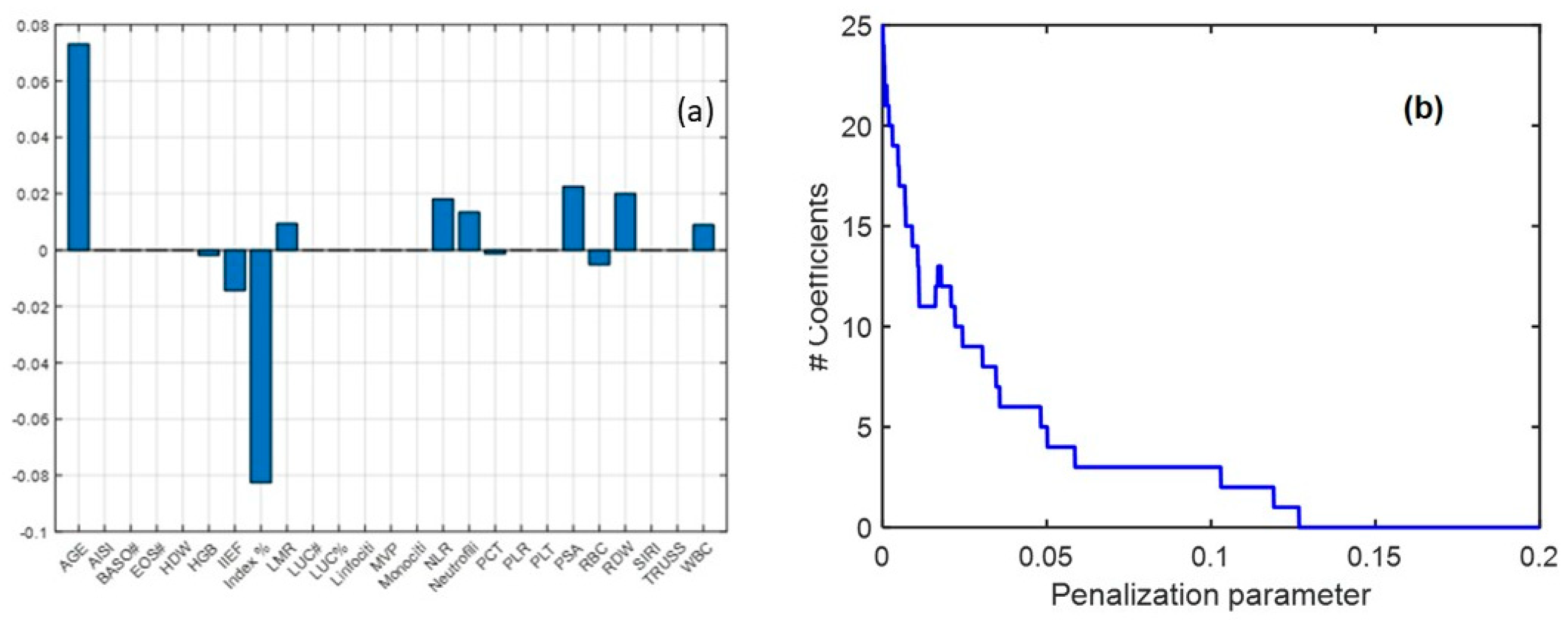

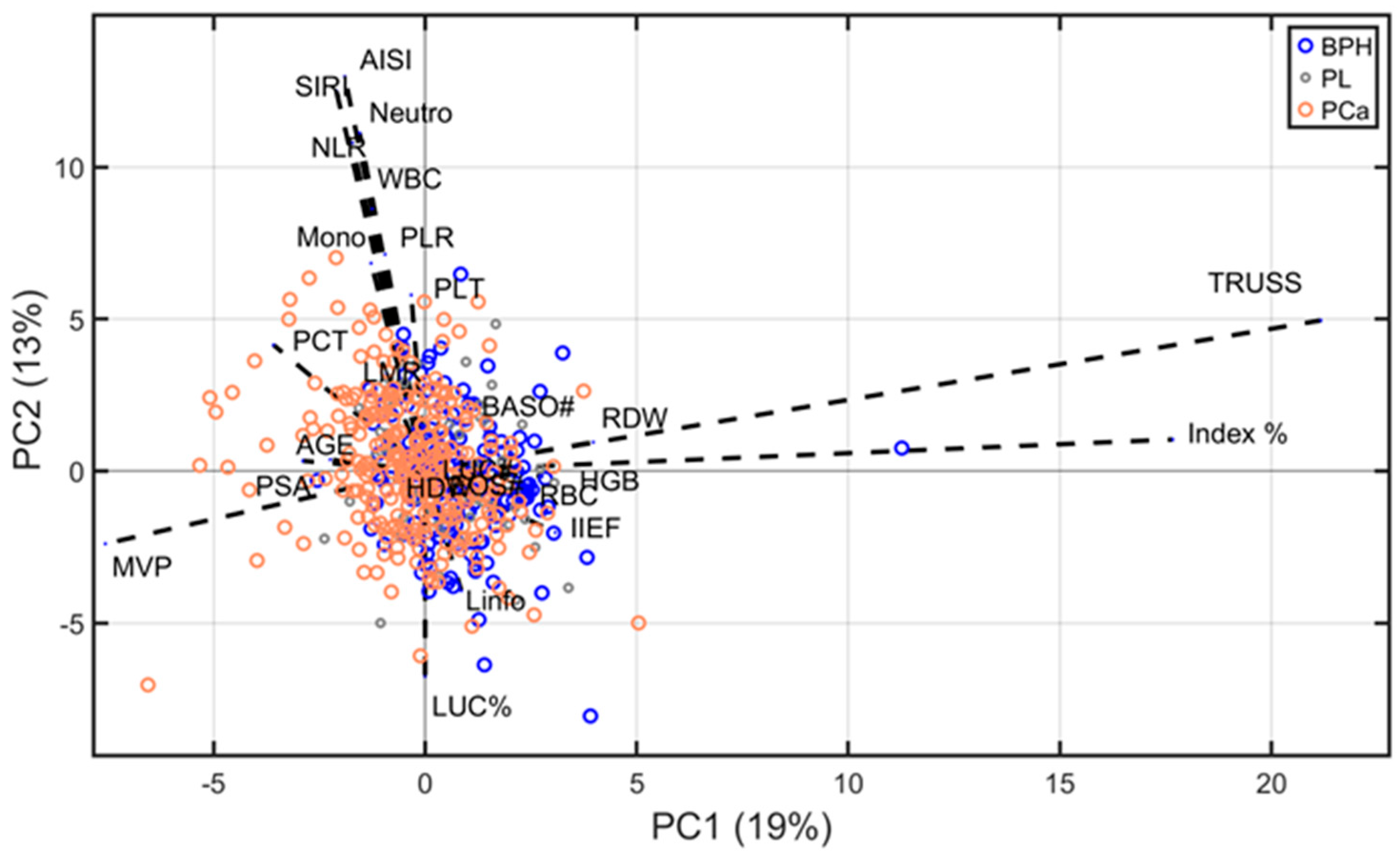
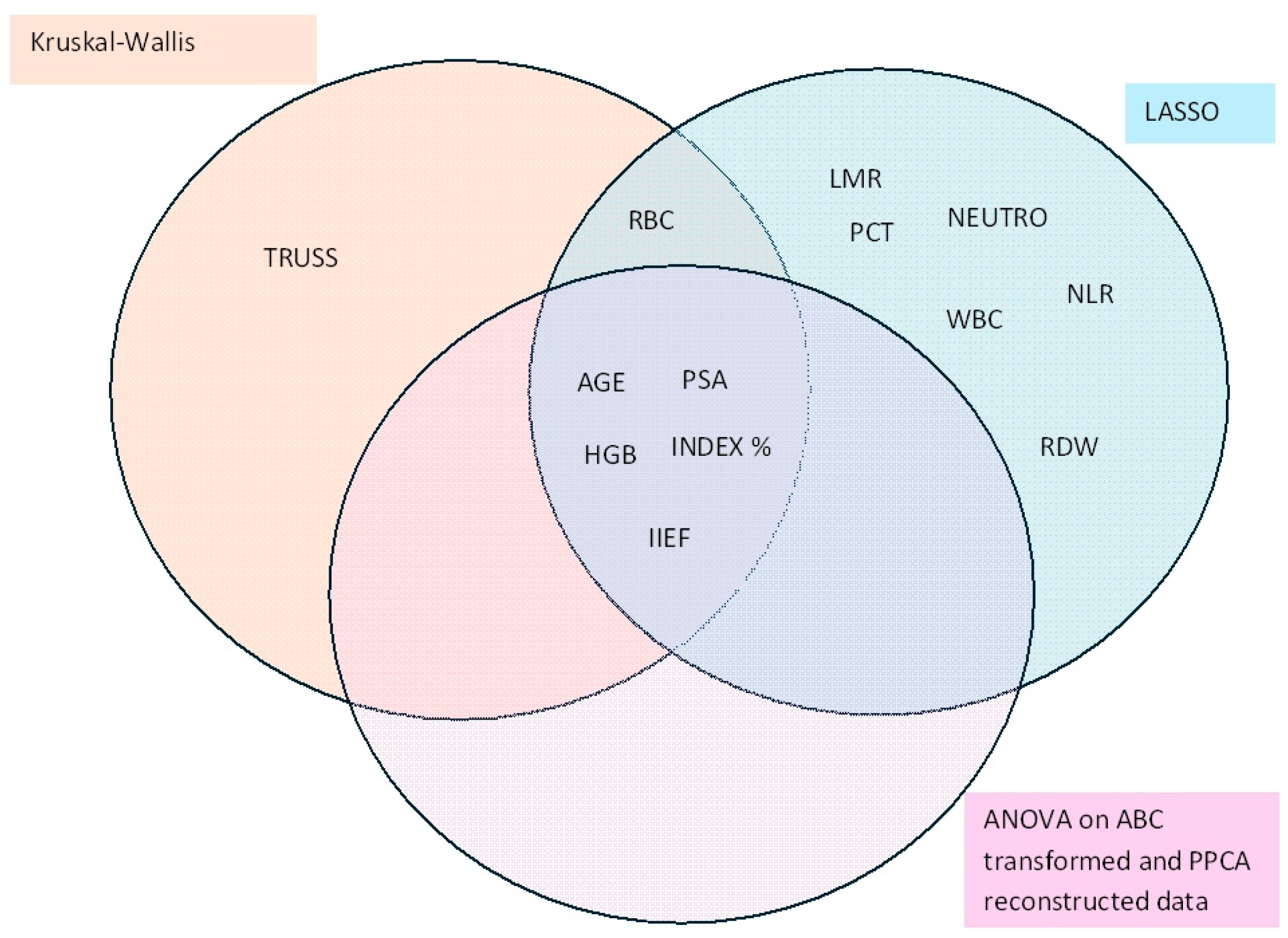
| Kruskall–Wallis | ANOVA on ABC Transformed Data | ||||
|---|---|---|---|---|---|
| Variable | p-Value | Benjamini–Hochberg Corrected p-Value | p-Value | Benjamini–Hochberg Corrected p-Value | λ |
| AGE | <0.01 | <0.01 | <0.01 | <0.01 | 2.87 |
| PSA | <0.01 | <0.01 | <0.01 | <0.01 | 0.56 |
| Index % | <0.01 | <0.01 | <0.01 | <0.01 | 0.47 |
| WBC | 0.12 | 0.21 | 0.07 | 0.15 | 0.25 |
| RBC | 0.01 | 0.04 | 0.04 | 0.09 | −0.10 |
| HGB | <0.01 | 0.003 | <0.01 | 0.004 | 3.0 |
| RDW | 0.08 | 0.16 | 0.16 | 0.27 | 0.10 |
| HDW | 0.05 | 0.11 | 0.03 | 0.093 | −1.33 |
| MVP | 0.70 | 0.83 | 0.63 | 0.81 | 1.11 |
| PLT | 0.57 | 0.75 | 0.65 | 0.81 | 0.06 |
| PCT | 0.67 | 0.83 | 0.81 | 0.86 | 0.16 |
| NEUTROPHILS | 0.03 | 0.08 | 0.02 | 0.07 | 0.03 |
| LIMPHO | 0.42 | 0.6 | 0.31 | 0.45 | 0.30 |
| MONO | 0.43 | 0.6 | 0.40 | 0.56 | −0.50 |
| EOS# | 0.95 | 0.96 | 0.89 | 0.89 | 0.01 |
| BASO# | 0.85 | 0.93 | 0.83 | 0.86 | 0.01 |
| LUC# | 0.85 | 0.93 | 0.68 | 0.81 | 0.01 |
| LUC% | 0.38 | 0.6 | 0.19 | 0.29 | 0.46 |
| LMR | 0.04 | 0.10 | 0.14 | 0.25 | −3.0 |
| NLR | 0.04 | 0.09 | 0.01 | 0.052 | −0.3 |
| PLR | 0.89 | 0.93 | 0.72 | 0.82 | −0.26 |
| SIRI | 0.02 | 0.07 | 0.01 | 0.05 | −0.24 |
| AISI | 0.10 | 0.17 | 0.12 | 0.23 | −0.32 |
| IIEF | 0.01 | 0.02 | <0.01 | <0.01 | 2.55 |
| TRUSS | <0.01 | <0.01 | 0.05 | 0.12 | 0.11 |
| Variable | BPH (Mean ± SD) | PL (Mean ± SD) | PCa (Mean ± SD) | p-Value (ANOVA) | Regulation |
|---|---|---|---|---|---|
| Age (years) | 65.0 ± 7.6 | 66.8 ± 8.5 | 69.6 ± 6.4 | <0.001 | PCa |
| PSA (ng/mL) | 6.3 ± 3.8 | 8.6 ± 4.5 | 14.8 ± 13.1 | <0.001 | PCa |
| Hemoglobin (HGB) (g/dL) | 14.5 ± 1.1 | 14.2 ± 1.2 | 13.7 ± 1.4 | <0.001 | BPH |
| White blood cells (WBC) (×10⁹/L) | 6.5 ± 1.5 | 6.8 ± 1.7 | 7.0 ± 1.8 | 0.024 | PCa |
| Neutrophils (×10⁹/L) | 3.8 ± 1.2 | 4.1 ± 1.3 | 4.5 ± 1.5 | <0.001 | PCa |
| NLR | 2.1 ± 0.9 | 2.4 ± 1.0 | 3.2 ± 1.4 | <0.001 | PCa |
| SIRI | 1.1 ± 0.6 | 1.3 ± 0.7 | 1.7 ± 0.9 | <0.001 | PCa |
| Hemoglobin distribution width (HDW) (%) | 2.4 ± 0.4 | 2.5 ± 0.5 | 2.7 ± 0.6 | 0.019 | PCa |
| IIEF-5 Score | 17.8 ± 5.3 | 16.5 ± 5.7 | 14.2 ± 6.0 | <0.001 | BPH |
| Variable | Class 0 vs. Class 1 (BPH vs. PL) | Class 0 vs. Class 2 (BPH vs. PCa) | Class 1 vs. Class 2 (PL vs. PCa) |
|---|---|---|---|
| Age | 0.98 | <0.01 | <0.01 |
| PSA | 0.93 | <0.01 | <0.01 |
| Index % | 0.99 | <0.01 | <0.01 |
| WBC | 0.89 | 0.07 | 0.55 |
| RBC | 0.99 | 0.05 | 0.21 |
| HGB | 0.75 | <0.01 | 0.01 |
| RDW | 0.93 | 0.26 | 0.32 |
| HDW | 0.31 | 0.36 | 0.03 |
| MVP | 0.99 | 0.63 | 0.87 |
| PLT | 0.76 | 0.69 | 0.98 |
| PCT | 0.82 | 0.89 | 0.95 |
| NEUTROPHILS | 0.87 | 0.02 | 0.40 |
| LIMPHO | 0.67 | 0.29 | 0.99 |
| MONO | 0.50 | 0.49 | 0.90 |
| EOS# | 0.88 | 0.97 | 0.94 |
| BASO# | 0.83 | 0.90 | 0.95 |
| LUC# | 0.68 | 0.83 | 0.87 |
| LUC% | 0.85 | 0.35 | 0.28 |
| LMR | 0.38 | 0.14 | 0.99 |
| NLR | 0.57 | <0.01 | 0.62 |
| PLR | 0.93 | 0.697 | 0.98 |
| SIRI | 0.29 | 0.0104 | 0.92 |
| AISI | 0.59 | 0.0976 | 0.93 |
| IIEF | 0.99 | <0.01 | 0.04 |
| TRUSS | 0.74 | 0.04 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coradduzza, D.; Sibono, L.; Tedde, A.; Marra, S.; De Miglio, M.R.; Zinellu, A.; Medici, S.; Mangoni, A.A.; Grosso, M.; Madonia, M.; et al. Diagnostic Stratification of Prostate Cancer Through Blood-Based Biochemical and Inflammatory Markers. Diagnostics 2025, 15, 1385. https://doi.org/10.3390/diagnostics15111385
Coradduzza D, Sibono L, Tedde A, Marra S, De Miglio MR, Zinellu A, Medici S, Mangoni AA, Grosso M, Madonia M, et al. Diagnostic Stratification of Prostate Cancer Through Blood-Based Biochemical and Inflammatory Markers. Diagnostics. 2025; 15(11):1385. https://doi.org/10.3390/diagnostics15111385
Chicago/Turabian StyleCoradduzza, Donatella, Leonardo Sibono, Alessandro Tedde, Sonia Marra, Maria Rosaria De Miglio, Angelo Zinellu, Serenella Medici, Arduino A. Mangoni, Massimiliano Grosso, Massimo Madonia, and et al. 2025. "Diagnostic Stratification of Prostate Cancer Through Blood-Based Biochemical and Inflammatory Markers" Diagnostics 15, no. 11: 1385. https://doi.org/10.3390/diagnostics15111385
APA StyleCoradduzza, D., Sibono, L., Tedde, A., Marra, S., De Miglio, M. R., Zinellu, A., Medici, S., Mangoni, A. A., Grosso, M., Madonia, M., & Carru, C. (2025). Diagnostic Stratification of Prostate Cancer Through Blood-Based Biochemical and Inflammatory Markers. Diagnostics, 15(11), 1385. https://doi.org/10.3390/diagnostics15111385









