Detection of Cadherin 12 in Plasma and Peritoneal Fluid Among Women with Endometriosis Using Novel Surface Plasmon Resonance Imaging (SPRi) Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Patients’ Characteristics and Sample Collection
2.3. SPRi Analysis
2.4. Data Analysis
3. Results
3.1. Peritoneal Fluid
3.1.1. Clinical Characteristics of Patients
3.1.2. CDH12 Concentrations in Peritoneal Fluid
3.2. Plasma
3.2.1. Clinical Characteristics of Patients
3.2.2. CDH12 Concentration Levels for Different Clinical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Materials and Reagents
Appendix A.2. Materials and ReagentsSPRi Analysis
Appendix B
References
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Mackenzie, S.C.; Morrison, L.; Schrepf, A.; Zondervan, K.T.; Horne, A.W.; Missmer, S.A. Endometriosis: A Review. JAMA 2025. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar] [PubMed]
- Dull, A.M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation—The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77, e12622. [Google Scholar] [CrossRef]
- Laudanski, P.; Charkiewicz, R.; Tolwinska, A.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. Profiling of Selected MicroRNAs in Proliferative Eutopic Endometrium of Women with Ovarian Endometriosis. BioMed Res. Int. 2015, 2015, 760698. [Google Scholar] [CrossRef]
- Leckband, D.; Prakasam, A. Mechanism and Dynamics of Cadherin Adhesion. Annu. Rev. Biomed. Eng. 2006, 8, 259–287. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Santos, L.; Dimitriadis, E. Characterization of the role for cadherin 6 in the regulation of human endometrial receptivity. Reprod. Biol. Endocrinol. 2020, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Lu, J.; Wang, P.; Feng, H.; Zong, Y.; Ou, B.; Zheng, M.; Lu, A. Cadherin-12 enhances proliferation in colorectal cancer cells and increases progression by promoting EMT. Tumor Biol. 2016, 37, 9077–9088. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Liu, X.; Ding, Y.; Gao, R.; Qiu, Y.; Wang, Y.; He, J. Exposure of mice to benzo(a)pyrene impairs endometrial receptivity and reduces the number of implantation sites during early pregnancy. Food Chem. Toxicol. 2014, 69, 244–251. [Google Scholar] [CrossRef]
- Ishida, M.; Takebayashi, A.; Kimura, F.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hanada, T.; Tsuta, K.; Murakami, T. Induction of the epithelial-mesenchymal transition in the endometrium by chronic endometritis in infertile patients. PLoS ONE 2021, 16, e0249775. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dong, F.; Yue, W.; Ouyang, Y.-C.; Wang, Z.-B.; Hou, Y.; Schatten, H.; Sun, Q.-Y. CENP-T regulates both the G2/M transition and anaphase entry by acting through CDH1 in meiotic oocytes. J. Cell Sci. 2020, 133, jcs238105. [Google Scholar] [CrossRef]
- Goławski, K.; Soczewica, R.; Kacperczyk-Bartnik, J.; Mańka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczyński, R.; Piekarski, P.; Banaszewska, B.; et al. The Role of Cadherin 12 (CDH12) in the Peritoneal Fluid among Patients with Endometriosis and Endometriosis-Related Infertility. Int. J. Environ. Res. Public Health 2022, 19, 11586. [Google Scholar] [CrossRef]
- Oldak, L.; Lukaszewski, Z.; Leśniewska, A.; Goławski, K.; Laudański, P.; Gorodkiewicz, E. Development of an SPRi Test for the Quantitative Detection of Cadherin 12 in Human Plasma and Peritoneal Fluid. Int. J. Mol. Sci. 2023, 24, 16894. [Google Scholar] [CrossRef]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and Label-free Bio-sensing of Molecular Interactions by Surface Plasmon Resonance: A Laboratory Medicine Perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar]
- Scarano, S.; Mascini, M.; Turner, A.P.F.; Minunni, M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron. 2010, 25, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Puiu, M.; Bala, C. SPR and SPR Imaging: Recent Trends in Developing Nanodevices for Detection and Real-Time Monitoring of Biomolecular Events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef]
- Laudański, P.; Rogalska, G.; Warzecha, D.; Lipa, M.; Mańka, G.; Kiecka, M.; Spaczyński, R.; Piekarski, P.; Banaszewska, B.; Jakimiuk, A.; et al. Autoantibody screening of plasma and peritoneal fluid of patients with endometriosis. Hum. Reprod. 2023, 38, 629–643. [Google Scholar] [CrossRef]
- Wojtyla, C.; Tołwiński, I.; Laudański, P. The Use of the Neoglycolipid-Based Oligosaccharide Microarray System in the Diagnosis of Endometriosis—Preliminary Study. J. Inflamm. Res. 2024, 17, 899–908. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Vitonis, A.F.; Vincent, K.; Rahmioglu, N.; Fassbender, A.; Louis, G.M.B.; Hummelshoj, L.; Giudice, L.C.; Stratton, P.; Adamson, G.D.; Becker, C.M.; et al. World Endometriosis Research Foundation Endometriosis Phenome and biobanking harmonization project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil. Steril. 2014, 102, 1223–1232. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Fassbender, A.; Vitonis, A.F.; Tworoger, S.S.; Hummelshoj, L.; D’Hooghe, T.M.; Adamson, G.D.; Giudice, L.C.; Becker, C.M.; Zondervan, K.T.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil. Steril. 2014, 102, 1233–1243. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Kong, S.K.; Kabashin, A.V. Phase-sensitive surface plasmon resonance biosensors: Methodology, instrumentation and applications. Ann. Phys. 2012, 524, 637–662. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef]
- Komarowska, M.; Szymańska, B.; Ołdak, Ł.; Sankiewicz, A.; Matuszczak, E.; Gorodkiewicz, E.; Debek, W.; Milewski, R.; Hermanowicz, A. Plasma level of laminin 5 and collagen IV in cryptorchidism. Adv. Med. Sci. 2020, 65, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kajdos, M.; Szymanski, J.; Jerczynska, H.; Stetkiewicz, T.; Wilczynski, J.R. Microvesicles released from ectopic endometrial foci as a potential biomarker of endometriosis. Ginekol. Pol. 2023, 94, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Soliman, A.M.; Rahal, Y.; Robert, C.; Defoy, I.; Nisbet, P.; Leyland, N. Prevalence, Symptomatic Burden, and Diagnosis of Endometriosis in Canada: Cross-Sectional Survey of 30,000 Women. J. Obstet. Gynaecol. Can. 2020, 42, 829–838. [Google Scholar] [CrossRef]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg MLou Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef]
- Hudelist, G.; Fritzer, N.; Thomas, A.; Niehues, C.; Oppelt, P.; Haas, D.; Tammaa, A.; Salzer, H. Diagnostic delay for endometriosis in Austria and Germany: Causes and possible consequences. Hum. Reprod. 2012, 27, 3412–3416. [Google Scholar] [CrossRef]
- Singh, R.; Ansari, J.A.; Maurya, N.; Mandhani, A.; Agrawal, V.; Garg, M. Epithelial-to-Mesenchymal Transition and Its Correlation with Clinicopathologic Features in Patients with Urothelial Carcinoma of the Bladder. Clin. Genitourin. Cancer 2017, 15, e187–e197. [Google Scholar] [CrossRef]
- Liu, G.L.; Yang, H.J.; Liu, T.; Lin, Y.Z. Expression and significance of E-cadherin, N-cadherin, transforming growth factor-β1 and Twist in prostate cancer. Asian Pac. J. Trop. Med. 2014, 7, 76–82. [Google Scholar] [CrossRef]
- Pascal, L.E.; Dhir, R.; Balasubramani, G.K.; Chen, W.; Hudson, C.N.; Srivastava, P.; Green, A.; DeFranco, D.B.; Yoshimura, N.; Wang, Z.; et al. E-cadherin expression is inversely correlated with aging and inflammation in the prostate. Am. J. Clin. Exp. Urol. 2021, 9, 140–149. [Google Scholar]

| Variable | Endometriosis (n = 41) | Controls (n = 32) | p-Value |
|---|---|---|---|
| Cadherin 12 (CDH12) [ng/mL] PF | 0.35 | ||
| Me ± IQR; QCV; n | 17.11 ± 17.16; 100%; 41 | 17.63 ± 24.88; 141%; 32 | |
| (Min–Max) | (1.08–52.70) | (2.15–48.15) | |
| Age [years] | 0.07 | ||
| M ± SD; CV; n | 32.2 ± 4.4; 13.7; 40 | 29.9 ± 6.1; 20.3; 32 | |
| 95% PU | (30.8–33.6) | (27.7–32.1) | |
| Day of cycle | 0.07 | ||
| Me ± IQR; QCV; n | 12.0 ± 9.5; 79%; 40 | 10.0 ± 8.0; 80%; 32 | |
| (Min–Max) | (5.0–26.0) | (5.0–26.0) | |
| Infertility | 25 (61%) | 16 (50%) | 0.35 |
| Primary infertility | 21 (51%) | 12 (38%) | 0.27 |
| Secondary infertility | 4 (10%) | 4 (13%) | 0.69 |
| First phase of cycle | 25 (63%) | 23 (72%) | 0.42 |
| Variable | Patients with Infertility | Patients Without Infertility | p-Value |
|---|---|---|---|
| Cadherin 12 (CDH12) [ng/mL] PF | 0.05 | ||
| Me ± IQR; QCV; n | 14.772 ± 13.205; 89%; 41 | 22.179 ± 26.322; 119%; 32 | |
| (Min–Max) | (1.081–42.290) | (1.964–52.700) |
| Variable | Endometriosis (n = 52) | Controls (n = 44) | p-Value |
|---|---|---|---|
| Cadherin 12 (CDH12) [ng/mL] PF | 0.3561 | ||
| Me ± IQR; QCV; n | 25.15 ± 15.73; 62%; 52 | 22.05 ± 26.6; 107%; 44 | |
| (Min–Max) | (3.25–55.46) | (2.15–67.85) | |
| Age [years] | 0.6564 | ||
| Me ± IQR; QCV; n | 31.50 ± 6.50; 21%; 52 | 31.0 ± 7.0; 23%; 43 | |
| (Min–Max) | (24.0–43.0) | (19.0–46.0) | |
| Day of cycle | 0.1261 | ||
| Me ± IQR; QCV; n | 11.0 ± 7.5; 68%; 52 | 10.00 ± 6.50; 65%; 44 | |
| (Min–Max) | (4.0–26.0) | (1.0–27.0) | |
| Infertility (n; %) | 32; 61.54% | 26; 59.09% | 0.8068 |
| Primary infertility (n; %) | 26; 50.00% | 19; 43.18% | 0.5046 |
| Secondary infertility (n; %) | 6; 11.54% | 7; 15.91% | 0.5330 |
| First phase of cycle (n; %) | 16; 30.77% | 10; 22.73% | 0.4417 |
| Factor | p-Value |
|---|---|
| Age (N = 96) | 0.046 * |
| Age in research group (n = 52) | 0.044 * |
| Age in control group (n = 44) | 0.573 |
| Infertility (N = 38 + 58) | 0.597 |
| Primary infertility (N = 51 + 45) | 0.186 |
| Secondary infertility (N = 83 + 13) | 0.243 |
| Phase od cycle (N = 70 + 26) | 0.141 |
| Ovarian cysts (N = 68 + 28) | 0.945 |
| Stage o endometriosis (I, II vs. III, IV, N = 27 + 23) | 0.186 |
| Day of cycle (N = 96) | 0.343 * |
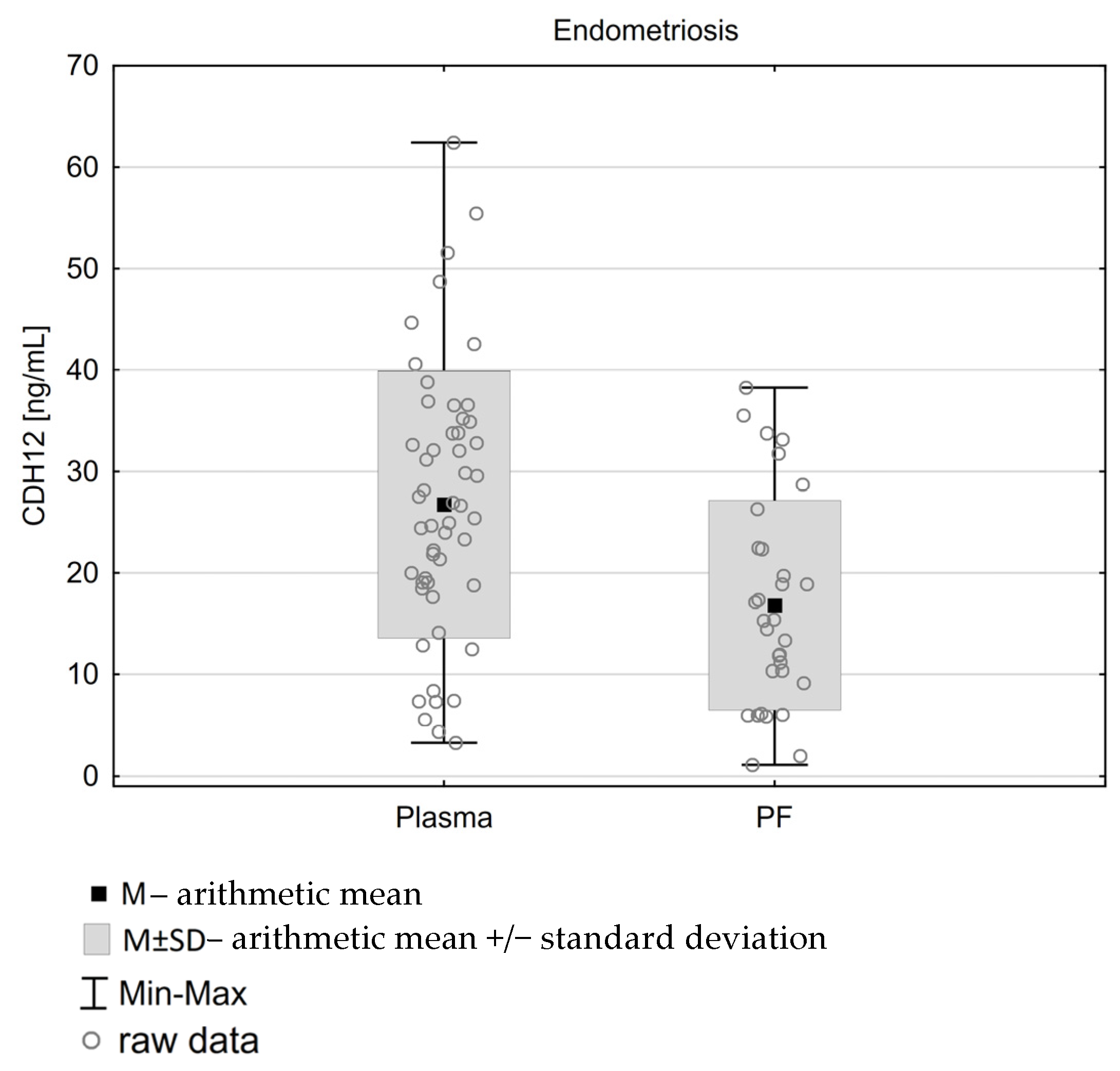
| M | SD | CV | T | p | rS | p | ||
|---|---|---|---|---|---|---|---|---|
| Study group N = 31 | Plasma | 27.2 | 14.485 | 53% | 2.90 | 0.007 | −0.29 | 0.112 |
| Peritoneal fluid | 16.79 | 10.331 | 62% | |||||
| Control N = 26 | Plasma | 24.78 | 17.43 | 70% | 1.22 | 0.234 | 0.18 | 0.234 |
| Peritneal fluid | 19.85 | 12.58 | 63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goławski, K.; Zielińska, Z.; Wojtyła, C.; Ołdak, Ł.; Kuźmicki, M.; Ławicki, S.; Ciebiera, M.; Issat, T.; Gorodkiewicz, E.; Pierzyński, P.; et al. Detection of Cadherin 12 in Plasma and Peritoneal Fluid Among Women with Endometriosis Using Novel Surface Plasmon Resonance Imaging (SPRi) Method. Diagnostics 2025, 15, 1366. https://doi.org/10.3390/diagnostics15111366
Goławski K, Zielińska Z, Wojtyła C, Ołdak Ł, Kuźmicki M, Ławicki S, Ciebiera M, Issat T, Gorodkiewicz E, Pierzyński P, et al. Detection of Cadherin 12 in Plasma and Peritoneal Fluid Among Women with Endometriosis Using Novel Surface Plasmon Resonance Imaging (SPRi) Method. Diagnostics. 2025; 15(11):1366. https://doi.org/10.3390/diagnostics15111366
Chicago/Turabian StyleGoławski, Ksawery, Zuzanna Zielińska, Cezary Wojtyła, Łukasz Ołdak, Mariusz Kuźmicki, Sławomir Ławicki, Michał Ciebiera, Tadeusz Issat, Ewa Gorodkiewicz, Piotr Pierzyński, and et al. 2025. "Detection of Cadherin 12 in Plasma and Peritoneal Fluid Among Women with Endometriosis Using Novel Surface Plasmon Resonance Imaging (SPRi) Method" Diagnostics 15, no. 11: 1366. https://doi.org/10.3390/diagnostics15111366
APA StyleGoławski, K., Zielińska, Z., Wojtyła, C., Ołdak, Ł., Kuźmicki, M., Ławicki, S., Ciebiera, M., Issat, T., Gorodkiewicz, E., Pierzyński, P., & Laudański, P. (2025). Detection of Cadherin 12 in Plasma and Peritoneal Fluid Among Women with Endometriosis Using Novel Surface Plasmon Resonance Imaging (SPRi) Method. Diagnostics, 15(11), 1366. https://doi.org/10.3390/diagnostics15111366







