Molecular Insights into Pleural Mesothelioma: Unveiling Pathogenic Mechanisms and Therapeutic Opportunities
Abstract
1. Introduction
2. Epidemiology
3. Risk Factors and Pathogenesis
4. Diagnostic Methods
5. MTAP and BAP1—Molecular Biomarkers for Diagnosting and Prognosting PM
5.1. MTAP’s Function as a Tumor Suppressor Gene
5.2. BAP1 Roles in Pathogenesis
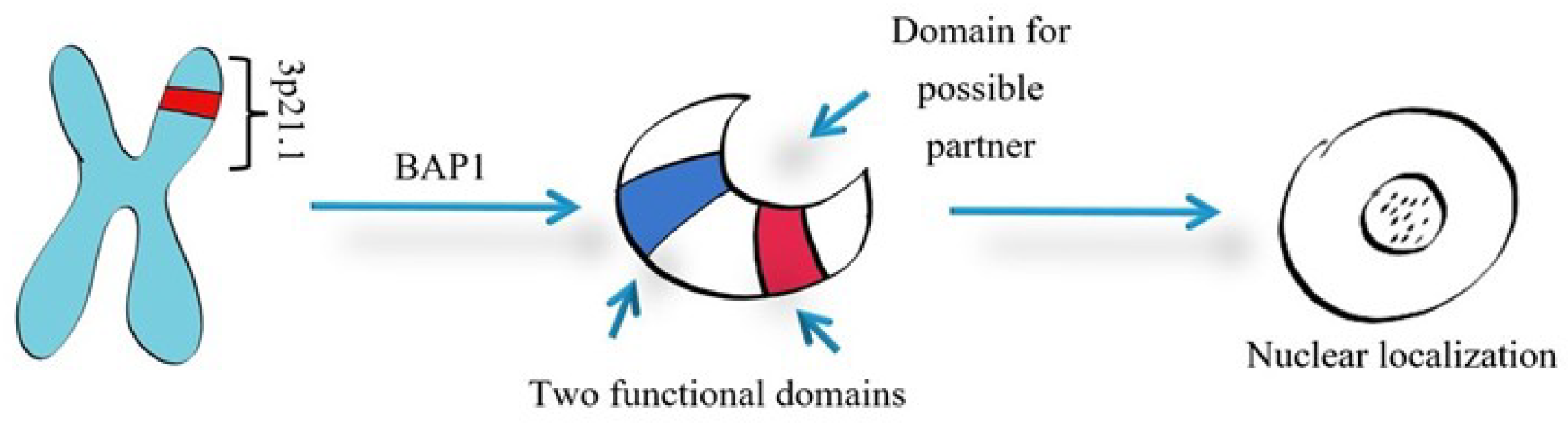
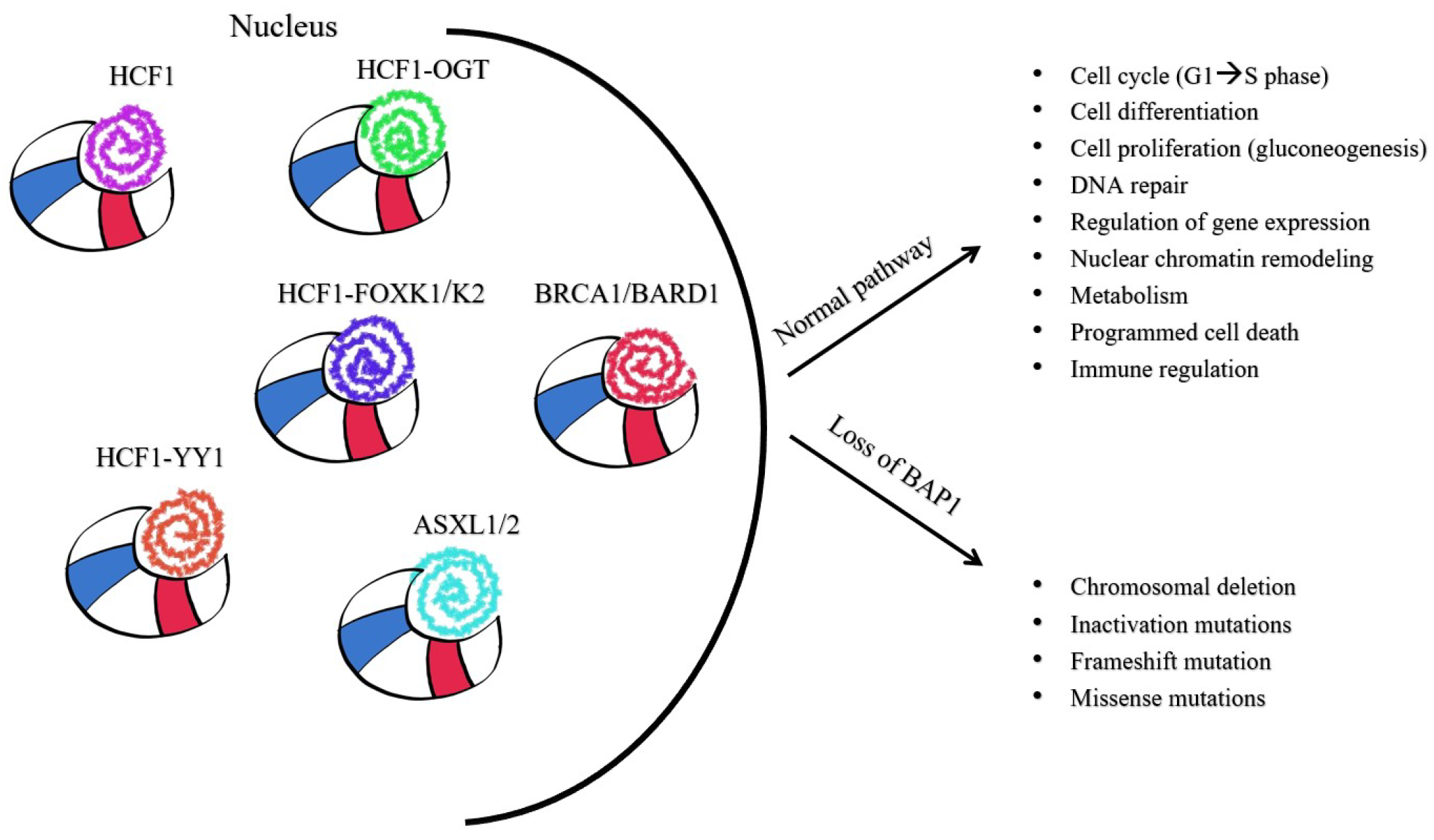
5.2.1. BAP1’s Cellular Roles in Cancer
5.2.2. BAP1 as a Component of Multiprotein Complexes Involved in Cell-Cycle Control
5.2.3. BAP1’s Role in Deubiquitination
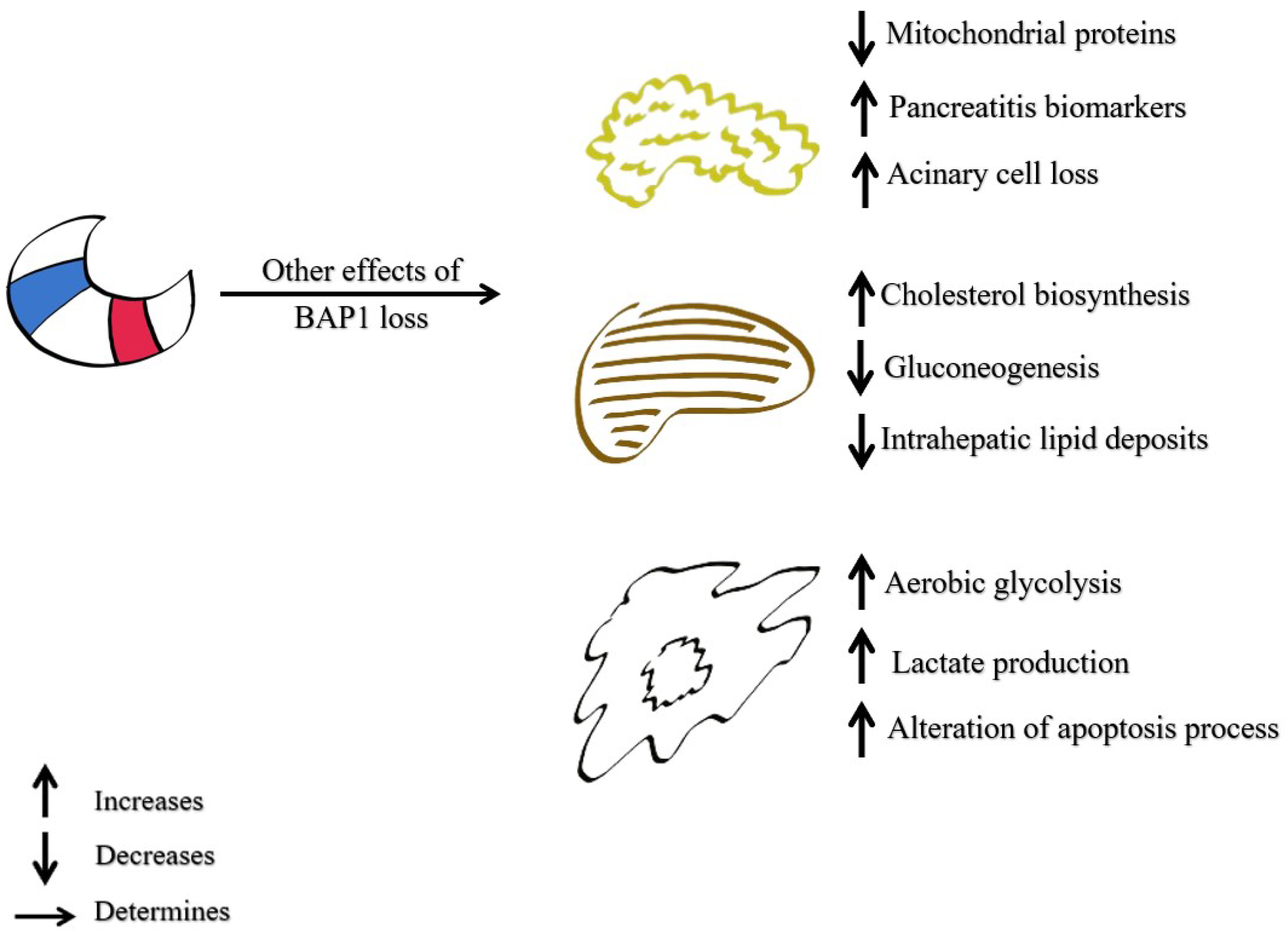
5.2.4. The Role of BAP1 in Malignant Cell Metabolism
5.2.5. The Role of BAP1 in Programmed Cell Death (Regulation of Cell Death)
6. Tumor Inflammatory Microenvironment in PM—Role in Tumor Progression
7. Prognosis of Pleural Mesothelioma
8. Pleural Mesothelioma Treatments in Development
8.1. BAP 1 Target Therapies
8.2. MTAP-Deleted Tumours—New Target Therapies
| Article | Treatment | Mechanism | Dose | Median of Cycles Per Patient | Number of Patients/Samples | Line of Treatment | Median OS | Median PFS | ORR | Side Effects | Observation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadzada 2020 [157] | Pembrolizumab | PD-1 antibody | 200 mg or 2 mg/kg every 3 weeks | 6 | 98 | 4 vs. 63 vs. 31 | 9.5 months | 4.8 months | 18% | Pneumonitis, nefritis, hepatitis, etc. | |
| Hassan 2019 [136] | Platinum-based | Disrupting DNA replication (chemotherapy) | 286 | 1st | OS was higher for the patients with BAP1 mutations | ||||||
| Lam 2020 [158] | AZD4547 | FGF inhibitor | 80 mg × 2/day over 3 weeks | 4 | 24 | 1st/2nd | 7.3 months | 3 months | Hyperphosphatemia, xerostomia, mucositis, retinopathy, etc. | There is no improvement in patient status as a second-line therapy, following treatment with platinum-based chemotherapy | |
| Zauderer 2021 [159] | LY3023414 | Dual PI3K/mTOR inhibitor | 200 mg × 2/day | 42 | 2nd/3rd | 2.83 months | Fatigue, nausea, decreased appetite, vomiting, diarrhea, etc. | The study took into trial patients with advanced mesothelioma (pleural and peritoneal) | |||
| Passiglia 2024 [160] | Niraparib and Dostarlimab | PARP-inhibitor and PD-1 antibody | 17 | 4.2 months | 3.1 months | 6% | Lymphopenia, anemia, hyponatremia, hypokalemia, etc. | The study took into trial patients with pleural mesothelioma or NSCLC | |||
| Hearon 2020 [161] | Pembrolizumab | PD-1 antibody | 200 mg every 3 weeks | 3 | 1 | Fatigue, hypothyroidism, lymphopenia, diabetes type I, etc. | Case study where the effect of pembrolizumab was durable after the drug was stopped | ||||
| Ghafoor 2021 [162] | Olaparib | PARP-inhibitor | 300 mg × 2/day for 3 weeks | 4 | 23 | 2nd/3rd | 8.7 months | 3.6 months | 4% | Nausea, renal toxicity, fatigue, etc. | The study involved patients with mesothelioma (pleural and peritoneal) |
| Forde 2021 [150] | Durvalumab plus platinum–pemetrexed | PD-1 antibody and chemotherapy | 1.120 mg Durvalumab i.v. every 3 weeks | 55 | 1st | 20.4 months | 6.7 months | 56.40% | Fatigue, nausea, anemia, etc. | PFS and OS were statistically better than the PFS and OS of platinum-based monotherapy | |
| Adusumilli 2021 [163] | CAR T cell therapy and pembrolizumab | CAR T cell infusion and PD-1 antibody | 0.3–60 M CAR T cells/kg intrapleural | 23 | 2nd/3rd | 23.9 months | |||||
| Watanabe 2021 [164] | Amrubicine | Inhibition of DNA topoisomerase II | 35 mg/m2 2 days/week for 3 weeks | 3 | 5 | 2nd/3rd | 9.1 months | 2.4 months | 0% | Neutropenia, anemia, decreased appetite, constipation, etc. | There were no responders to Amrubicine, but an SD (stable disease) was observed in three out of five patients |
| Xie 2022 [165] | Crizotinib | Protein kinase inhibitor | 1 | 2nd | 7.6 YEARS | 6 YEARS | The patient has MPM positive for CD74-ROS1 fusion | ||||
| Kindler 2023 [147] | Anetumab Ravtansine vs. Vinorelbine | Antibody anti mesothelin and inhibitor of mitosis | AR: 6.5 mg/kg once over 3 weeks V:30 mg/m2 once every week | 248 | 2nd | 9.5 months vs. 11.6 months | 4.3 months vs. 4.5 months | Neutropenia, pneumonia, dyspnoea, etc. | There was no statistically significant difference between the treatments | ||
| Fennell 2021 [152] | Nivolumab vs. Placebo | PD-1 antibody | 240 mg every 2 weeks | 332 | 2nd | 10.2 months vs. 6.9 months | 3 months vs. 1.8 months | 11% vs. 1% | Dyspnoea, pneumonia, lower inspiratory tract infection, etc. | 95% of the patients had pleural mesothelioma, the rest had peritoneal | |
| Mark 2022 [155] | Lurbinectine | Blocking the cell cycle in the S-phase and activation of the DNA damage response | 42 | 2nd/3rd | 11.5 months | 4.1 months | Viral pneumonitis, dyspnoea, haert failure, etc. | The study classified the group into categories by survival and tried to find a connection between OS and their genes | |||
| Zhang 2022 [154] | Tislelizumab and Anlotinib | PD-1 antibody and tyrosine kinase inhibitor | 200 mg Tislelizumab/day and 10 mg Anlotinib daily for 2 weeks and one week off. | 1 | 2nd | 10 months (until the article was published) | |||||
| Canova 2022 [151] | Durvalumab | PD-1 antibody | 1500 mg Durvalumab every 4 weeks | 3 | 69 | 2nd | 7.3 months | 1.9 months | 10% | Atrial fibrillation, hyper/hypothyroidism, ischemic colitis, diarrhea, etc. | |
| CheckMate 743 [148] | Nivolumab and Ipilimumab | PD-1 antibody and antibody anti CTLA-4 vs. chemotherapy | N: 3 mg/kg i.v. once every two weeks and I:1 mg/kg i.v. once every six weeks | 12 and 4 vs. 6 | 300 vs. 303 | 1st | 18.1 months vs. 14.1 months | 6.8 months vs. 7.2 months | Diarrhoea, pruritus, fatigue, hypothyroidism, nausea, etc. | OS did not differ between histological types of M while using N and I, but differed dramatically while using chemotherapy; 8.8 months for non-epithelioid vs. 16.5 months for epithelioid. | |
| Pinto 2021 [149] | Gemcitabine +/− Ramucirumab | Chemotherapy and antibody anti VEGF/VEGFR | R: 10 mg/kg once every 3 weeks G: 1000 mg/m2 | 7.5 vs. 3.5 | 161 | 2nd | 13.8 months vs. 7.5 months | 6.4 months vs. 3.3 months | Neutropenia, hypertension, thrombembolism, etc. | OS was longer in the gemcitabine plus ramucirumab group than into gemcitabine plus placebo group | |
| Yap 2021 [166] | Pembrolizumab | PD-1 antibody | 200 mg i.v. once every 3 weeks | 6 | 118 | 2nd | 10 months | 2.1 months | Colitis, hyponatraemia, pneumonitis, etc. | Pembrolizumab has a good antitumor activity, regardless of PD-1 status | |
| Costa 2022 [153] | Nivolumab | PD-1antibody | 3 mg/kg once every 2 weeks | 1 | 2nd | Arthralgia | |||||
| Szlosarek 2023 [167] | Pegargiminase and Chematherapy | Arginine deprivation therapy | 36.8 mg/m2 i.m. once per week | 249 | 1st | 9.3 months | 6.2 months | The study was conducted with non-epithelioid pleural mesothelioma patients. |
| Article | Treatment | Mechanism | Dose | Number of Samples | Observation |
|---|---|---|---|---|---|
| Anobile 2021 [168] | Lurbinectedin | Blocking the cell cycle in S-phase and activation of the DNA damage response | 0.07–4.5 nM | 12 | Efficacy independent of the BAP1 status |
| Borchert 2019 [142] | Olaparib | PARP-inhibitor | 1–10 μm | 90 | DDB2 and RAD50 are associated with long survival if given Olaparib |
| Guazzelli 2019 [169] | Gemcitabine | Disrupting DNA replication (chemotherapy) | 0.1–50 μm | Inactivation of BAP1 determines resistance to gemcitabine | |
| Kumar 2019 [170] | Vinorelbine or Mitomycin, vinblastine, or cisplatin | Inhibition of mitosis because of the interaction of tubulin (chemotherapy) | 60 | OS was no different between the treatment arms | |
| Salaroglio 2022 [171] | MLN4924 +/− cisplatin or placebo | Selective NEDD8 inhibitor and chemotherapy | 5 mg/kg cisplatin i.p. once a week; 25 mg/kg MLN4924 s.c. 5 days/week | 40 mice | These two drugs have a synergic anti-tumor effect, independent from the MPM histotype |
| Rossini 2021 [156] | Metformin | Stimulates the apoptotic process, associated with decreased Notch1 activation | 1–50 mM | Metformin succeeded to inhibit cell viability of PM; dose and time dependent |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM | pleural mesothelioma |
| MM | malignant mesothelioma |
| WHO | World Health Organization |
| BAP1 | BRCA associated mesothelial tumors |
| MTAP | metylthioadenosine phosphorylase |
| IHC | immunohistochemistry |
| FISH | fluorescence in situ hybridization |
| TNF α | tumor necrosis factor alpha |
| FGF | fibroblast growth factor |
| ABs | asbestos bodies |
| CT | computer tomography |
| VAT | video-assisted thoracoscopic |
| MRI | magnetic resonance imaging |
| FDG-PET | 18F-fluorodeoxyglucose-positron emission tomography |
| US | ultrasound |
| EMA | epithelial membrane antigen |
| pCEA | polyclonal carcinoembryonic antigen |
| MTR-1-P | methylthioribose-1-phosphate |
| ODC | ornithine deacrboxylase |
| SAM | S-adenosylmethionine |
| DUB | deubiquitinating enzyme |
| YY1-Ying | Yang 1 transcriptional repressor |
| FOXK1/2 | forkhead transcription factor |
| HCF-1 | host cell factor 1 |
| BARD1 | BRCA1-associated RING domain protein 1 |
| PRMT5 | protein arginine methyltransferase |
| MAT2A | metabolic enzyme methionine adenosyltransferase II alpha |
| SDMA | symmetric demethylation of arginine |
| ASXL1/2 | additional sex comb like 1 or 2 |
| OGT | O-linked N-acetylglucosamine transferase |
| BRCA1 | breast cancer gene 1 |
| PR-DUB | polycomb group repressive deubiquitinase complex |
| H2Aub | ubiquitinated H2A |
| HDAC | histone deacetylase inhibitors |
| ER | endoplasmic reticulum |
| IP3R3 | type 3 inositol-1, 4, 5 -triphosphate receptor |
| ROS | reactive oxygen speacies |
| TME | tumor microenvironment |
| ECM | extracellular matrix |
| EMT | epithelial to mesenchymal transition |
| TGF-β | transforming growth factor-beta |
| SMA | smooth muscle actin |
| EGF | epidermal growth factor |
| PFS | progression-free survival |
| pDCs | plasmacytoid dendritic cells |
| PD-L1 | programmed death ligand 1 |
| OS | overall survival |
| CSS | cancer-specific survival |
| LDH | lactate dehydrogenase |
| MPE | malignant pleural effusion |
| SO2 | sulfur dioxide |
| NO2 | nitrogen dioxide |
| MMT | multimodal therapy |
| EZH2 | enhancer of zeste 2 polycomb repressive complex 2 |
| 6TG | 6′-tioguanine |
| 2FA | 2′-fluoroadenine |
References
- Chapel, D.B.; Schulte, J.J.; Husain, A.N.; Krausz, T. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl. Lung Cancer Res. 2020, 9 (Suppl. S1), S3–S27. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, M.; Cesari, D.; Bottaro, M.; Luzzi, L.; Namagerdi, A.; Bertolino, F.M.; Bellan, C.; Proietti, F.; Somma, P.; Micheli, M.; et al. PRMT5 silencing selectively affects MTAP-deleted mesothelioma: In vitro evidence of a novel promising approach. J. Cell Mol. Med. 2020, 24, 5565–5577. [Google Scholar] [CrossRef] [PubMed]
- Dipper, A.; Maskell, N.; Bibby, A. Ancillary diagnostic investigations in malignant pleural mesothelioma. Cancers 2021, 13, 3291. [Google Scholar] [CrossRef]
- Cavone, D.; Caputi, A.; De Maria, L.; Cannone, E.S.S.; Mansi, F.; Birtolo, F.; Delfino, M.C.; Vimercati, L. Epidemiology of Mesothelioma. Environments 2019, 6, 76. [Google Scholar] [CrossRef]
- Astoul, P. Rethought histologic classification of pleural mesothelioma to better treat: Go forward from looking back. Transl. Lung Cancer Res. 2020, 9, 1613–1616. [Google Scholar] [CrossRef]
- McCambridge, A.J.; Napolitano, A.; Mansfield, A.S.; Fennell, D.A.; Sekido, Y.; Nowak, A.K.; Reungwetwattana, T.; Mao, W.; Pass, H.I.; Carbone, M.; et al. Progress in the management of malignant pleural mesothelioma in 2017. J. Thorac. Oncol. 2018, 13, 606–623. [Google Scholar] [CrossRef]
- Brims, F. Epidemiology and clinical aspects of malignant pleural mesothelioma. Cancers 2021, 13, 4194. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Chen, T.; Hemminki, A. Incidence, mortality and survival in malignant pleural mesothelioma before and after asbestos in Denmark, Finland, Norway and Sweden. BMC Cancer 2021, 21, 1189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brcic, L.; Klikovits, T.; Megyesfalvi, Z.; Mosleh, B.; Sinn, K.; Hritcu, R.; Laszlo, V.; Cufer, T.; Rozman, A.; Kern, I.; et al. Prognostic impact of PD-1 and PD-L1 expression in malignant pleural mesothelioma: An international multicenter study. Transl. Lung Cancer Res. 2021, 10, 1594–1607. [Google Scholar] [CrossRef]
- Lettieri, S.; Bortolotto, C.; Agustoni, F.; Lococo, F.; Lancia, A.; Comoli, P.; Corsico, A.G.; Stella, G.M. The evolving landscape of the molecular epidemiology of malignant pleural mesothelioma. J. Clin. Med. 2021, 10, 1034. [Google Scholar] [CrossRef]
- Murali, R.; Park, K.; Leslie, K.O. The pleura in health and disease. Semin. Respir. Crit. Care Med. 2010, 31, 649–673. [Google Scholar] [CrossRef]
- Cotran, R.S.; Kumar, V.; Collins, T. Robbins Pathologic Basis of Disease, 6th ed.; W B Saunders: London, UK, 1998. [Google Scholar]
- Ferrante, D.; Mirabelli, D.; Silvestri, S.; Azzolina, D.; Giovannini, A.; Tribaudino, P.; Magnani, C. Mortality and mesothelioma incidence among chrysotile asbestos miners in Balangero, Italy: A cohort study. Am. J. Ind. Med. 2020, 63, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, T.; Chen, H.; Yang, X. Global magnitude and temporal trend of mesothelioma burden along with the contribution of occupational asbestos exposure in 204 countries and territories from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Crit. Rev. Oncol. Hematol. 2022, 179, 103821. [Google Scholar] [CrossRef] [PubMed]
- Chapel, D.B.; Dubuc, A.M.; Hornick, J.L.; Sholl, L.M. Correlation of methylthioadenosine phosphorylase (MTAP) protein expression with MTAP and CDKN2A copy number in malignant pleural mesothelioma. Histopathology 2021, 78, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- De Rienzo, A.; Chirieac, L.R.; Hung, Y.P.; Severson, D.T.; Freyaldenhoven, S.; Gustafson, C.E.; Dao, N.T.; Meyerovitz, C.V.; Oster, M.E.; Jensen, R.V.; et al. Large-scale analysis of BAP1 expression reveals novel associations with clinical and molecular features of malignant pleural mesothelioma. J. Pathol. 2021, 253, 68–79. [Google Scholar] [CrossRef]
- Boulanger, G.; Andujar, P.; Pairon, J.C.; Billon-Galland, M.A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of short and long asbestos fibers to assess asbestos exposure: A review of fiber size toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wadowski, B.; De Rienzo, A.; Bueno, R. The Molecular Basis of Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020, 30, 383–393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuroda, A. Recent progress and perspectives on the mechanisms underlying Asbestos toxicity. Genes Environ. 2021, 43, 46. [Google Scholar] [CrossRef]
- Benedetti, S.; Nuvoli, B.; Catalani, S.; Galati, R. Reactive oxygen species a double-edged sword for mesothelioma. Oncotarget 2015, 6, 16848–16865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asciak, R.; George, V.; Rahman, N.M. Update on biology and management of mesothelioma. Eur. Respir. Rev. 2021, 30, 200226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghio, A.J.; Stewart, M.; Sangani, R.G.; Pavlisko, E.N.; Roggli, V.L. Asbestos and Iron. Int. J. Mol. Sci. 2023, 24, 12390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korchevskiy, A.; Rasmuson JORasmuson, E.J. Empirical model of mesothelioma potency factors for different mineral fibers based on their chemical composition and dimensionality. Inhal. Toxicol. 2019, 31, 180–191. [Google Scholar] [CrossRef]
- Pascolo, L.; Gianoncelli, A.; Schneider, G.; Salomé, M.; Schneider, M.; Calligaro, C.; Kiskinova, M.; Melato, M.; Rizzardi, C. The interaction of asbestos and iron in lung tissue revealed by synchrotron-based scanning X-ray microscopy. Sci. Rep. 2013, 3, 1123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohar, J.A.; Cheung, M.; Talarchek, J.; Howard, S.E.; Howard, T.D.; Hesdorffer, M.; Peng, H.; Rauscher, F.J.; Testa, R.J. Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res. 2016, 76, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Cavallari, I.; Sharova, E.; Ciccarese, F.; Pasello, G.; Ciminale, V. Metabolic rewiring and redox alterations in malignant pleural mesothelioma. Br. J. Cancer 2020, 122, 52–61. [Google Scholar] [CrossRef]
- Walter, M.; Schenkeveld, W.D.C.; Tomatis, M.; Schelch, K.; Peter-Vörösmarty, B.; Geroldinger, G.; Gille, L.; Bruzzoniti, M.C.; Turci, F.; Kraemer, S.M.; et al. The Potential Contribution of Hexavalent Chromium to the Carcinogenicity of Chrysotile Asbestos. Chem. Res. Toxicol. 2022, 35, 2335–2347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gualtieri, A.F.; Gandolfi, N.B.; Pollastri, S.; Pollok, K.; Langenhorst, F. Where is iron in erionite? A multidisciplinary study on fibrous erionite-Na from Jersey (Nevada, USA). Sci. Rep. 2016, 6, 37981. [Google Scholar] [CrossRef]
- Ugolini, D.; Neri, M.; Ceppi, M.; Cesario, A.; Dianzani, I.; Filiberti, R.; Gemignani, F.; Landi, S.; Magnani, C.; Mutti, L.; et al. Genetic susceptibility to malignant mesothelioma and exposure to asbestos: The influence of the familial factor. Mutat. Res. 2008, 658, 162–171. [Google Scholar] [CrossRef]
- Maltoni, C.; Minardi, F.; Morisi, L. Pleural mesotheliomas in Sprague-Dawley rats by erionite: First experimental evidence. Environ. Res. 1982, 29, 238–244. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kohyama, N. Malignant mesothelioma induced by asbestos and zeolite in the mouse peritoneal cavity. Environ. Res. 1984, 35, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Ozesmi, M.; Patiroglu, T.E.; Hillerdal, G.; Ozesmi, C. Peritoneal mesothelioma and malignant lymphoma in mice caused by fibrous zeolite. Br. J. Ind. Med. 1985, 42, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Attanoos, R.L.; Churg, A.; Galateau-Salle, F.; Gibbs, A.R.; Roggli, V.L. Malignant mesothelioma and its non-asbestos causes. Arch. Pathol. Lab. Med. 2018, 142, 753–760. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakae, D.; Fukumori, N.; Tayama, K.; Maekawa, A.; Imai, K.; Hirose, A.; Nishimura, T.; Ohashi, N.; Ogata, A. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male fischer 344 rats. J. Toxicol. Sci. 2009, 34, 65–76. [Google Scholar] [CrossRef]
- Takagi, A.; Hirose, A.; Nishimura, T.; Fukomori, N.; Ogata, A.; Ohashi, N.; Kitajima, S.; Kanno, J. Induction of mesothelioma in p53 +/− mouse by intraperitoneal application of multi-wall carbon nanotube. J. Toxicol. Sci. 2008, 33, 105–116. [Google Scholar] [CrossRef]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef] [PubMed]
- Mizuhashi, K.; Okamoto, K.; Kishimoto, T. A patient with epithelioid pleural mesothelioma (Myxoid variant) who survived for a long period without treatment. Respir. Med. Case Rep. 2021, 33, 101381. [Google Scholar] [CrossRef]
- Yalcin, N.G.; Choong, C.K.C.; Eizenberg, N. Anatomy and pathophysiology of the pleura and pleural space. Thorac. Surg. Clin. 2013, 23, 1–10. [Google Scholar] [CrossRef]
- Ceruti, P.; Lonni, S.; Baglivo, F.; Marchetti, G. Endoscopic diagnosis and management of pleural effusion in malignant pleural mesothelioma. J. Thorac. Dis. 2018, 10 (Suppl. S2), S269–S275. [Google Scholar] [CrossRef]
- Abbott, D.M.; Bortolotto, C.; Benvenuti, S.; Lancia, A.; Filippi, A.R.; Stella, G.M. Malignant Pleural Mesothelioma: Genetic and Microenviromental Heterogeneity as an Unexpected Reading Frame and Therapeutic Challenge. Cancers 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinha, S.; Swift, A.J.; Kamil, M.A.; Matthews, S.; Bull, M.J.; Fisher, P.; De Fonseka, D.; Saha, S.; Edwards, J.G.; Johns, C.S. The role of imaging in malignant pleural mesothelioma: An update after the 2018 BTS guidelines. Clin. Radiol. 2020, 75, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.L.; Vaughan Dickson, V.; Cacchione, P.Z. A pilot mixed-methods study of malignant pleural mesothelioma symptoms. Oncol. Nurs. Forum 2022, 49, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Feng, W.; Shi, Z.; Shi, H.; Zhang, Y. Prediction modeling using routine clinical parameters to stratify survival in malignant pleural mesothelioma patients complicated with malignant pleural effusion. Thorac. Cancer 2021, 12, 3304–3309. [Google Scholar] [CrossRef]
- Mann, S.; Khawar, S.; Moran, C.; Kalhor, N. Revisiting localized malignant mesothelioma. Ann. Diagn. Pathol. 2019, 39, 74–77. [Google Scholar] [CrossRef]
- Canbeldek, L.; Legesse, T.; Burke, A. Localized mesothelioma of the pleura: Report of 2 cases, from benign to malignant. AJSP Rev. Rep. 2021, 26, 200–202. [Google Scholar] [CrossRef]
- Hirano, H.; Takeda, S.; Sawabata, Y.; Okumura, Y.; Maeda, H.; Hanibuchi, M.; Ito, M.; Nakagawa, M.; Uematsu, K. Localized pleural malignant mesothelioma. Pathol. Int. 2003, 53, 616–621. [Google Scholar] [CrossRef]
- Sauter, J.L.; Dacic, S.; Galateau-Salle, F.; Attanoos, R.L.; Butnor, K.J.; Churg, A.; Husai, N.A.; Kadota, K.; Khoor, A.; Nicholson, G.A.; et al. The 2021 WHO classification of tumors of the Pleura: Advances since the 2015 classification. J. Thorac. Oncol. 2022, 17, 608–622. [Google Scholar] [CrossRef]
- Dacic, S. Pleural mesothelioma classification-update and challenges. Mod. Pathol. 2022, 35 (Suppl. S1), 51–56. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.B.; Galateau-Salle, F.; Dacic, S. Pleural mesothelioma classification update. Virchows Archiv. Int. J. Pathol. 2021, 478, 59–72. [Google Scholar] [CrossRef]
- Plathow, C.; Klopp, M.; Schoebinger, M.; Thieke, C.; Fink, C.; Puderbach, M.; Ley, S.; Weber, M.A.; Sandner, A.; Claussen, C.D.; et al. Monitoring of lung motion in patients with malignant pleural mesothelioma using two-dimensional and threedimensional dynamic magnetic resonance imaging: Comparison with spirometry. Investig. Radiol. 2006, 41, 443–448. [Google Scholar] [CrossRef]
- Schulte, J.J.; Husain, A.N. Morphology and immunohistochemical and molecular markers for diagnosis and guiding therapy in mesothelioma: A narrative review. Shanghai Chest 2023, 7, 31. [Google Scholar] [CrossRef]
- Alì, G.; Bruno, R.; Fontanini, G. The pathological and molecular diagnosis of malignant pleural mesothelioma: A literature review. J. Thorac. Dis. 2018, 10 (Suppl. S2), S276–S284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, A.P.; Hardy, N.; Fanaroff, R.; Legesse, T. Sarcomatoid mesothelioma with bland histologic features: A potential pitfall in diagnosis. AJSP Rev. Rep. 2022, 27, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zuccatosta, L.; Bizzarro, T.; Rossi, G.; Gallo, G.; Gasparini, S.; Ambrosini-Spaltro, A. Immunohistochemistry for Claudin-4 and BAP1 in the Differential Diagnosis between Sarcomatoid Carcinoma and Sarcomatoid Mesothelioma. Diagnostics 2023, 13, 249. [Google Scholar] [CrossRef]
- Kushitani, K.; Takeshima, Y.; Amatya, V.J.; Furonaka, O.; Sakatani, A.; Inai, K. Differential diagnosis of sarcomatoid mesothelioma from true sarcoma and sarcomatoid carcinoma using immunohistochemistry: Sarcomatoid mesothelioma immunostaining. Pathol. Int. 2008, 58, 75–83. [Google Scholar] [CrossRef]
- Piao, Z.H.; Zhou, X.C.; Chen, J.Y. GATA3 is a useful immunohistochemical marker for distinguishing sarcomatoid malignant mesothelioma from lung sarcomatoid carcinoma and organizing pleuritis. Virchows Arch. 2021, 479, 257–263, Erratum in: Virchows Arch. 2021, 479, 437. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.B.; Dacic, S.; Miller, C.; Cheung, S.; Churg, A. Utility of methylthioadenosine phosphorylase compared with BAP1 immunohistochemistry, and CDKN2A and NF2 fluorescence in situ hybridization in separating reactive mesothelial proliferations from epithelioid malignant mesotheliomas. Arch. Pathol. Lab. Med. 2018, 142, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Hida, T.; Hamasaki, M.; Matsumoto, S.; Sato, A.; Tsujimura, T.; Kawahara, K.; Hiroshima, K.; Oda, Y.; Nabeshima, K. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma: MTAP and BAP1 for Mesothelioma Diagnosis. Cancer Cytopathol. 2018, 126, 54–63. [Google Scholar] [CrossRef]
- Lynggård, L.A.; Panou, V.; Szejniuk, W.; Røe, O.D.; Meristoudis, C. Diagnostic capacity of BAP1 and MTAP in cytology from effusions and biopsy in mesothelioma. J. Am. Soc. Cytopathol. 2022, 11, 385–393. [Google Scholar] [CrossRef]
- Schmid, M.; Sen, M.; Rosenbach, M.D.; Carrera, C.J.; Friedman, H.; Carson, D.A. A methylthioadenosine phosphorylase (MTAP) fusion transcript identifies a new gene on chromosome 9p21 that is frequently deleted in cancer. Oncogene 2000, 19, 5747–5754. [Google Scholar] [CrossRef]
- Ladanyi, M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer 2005, 49 (Suppl. S1), S95–S98. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.; Myers, J. Update on Diagnosing and Reporting Malignant Pleural Mesothelioma. Acta Med. Acad. 2021, 50, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Kinoshita, Y.; Yoshimura, M.; Matsumoto, S.; Kamei, T.; Hiroshima, K.; Sato, A.; Tsujimura, T.; Kawahara, K.; Nabeshima, K. Cytoplasmic MTAP expression loss detected by immunohistochemistry correlates with 9p21 homozygous deletion detected by FISH in pleural effusion cytology of mesothelioma. Histopathology 2019, 75, 153–155. [Google Scholar] [CrossRef]
- Hida, T.; Hamasaki, M.; Matsumoto, S.; Sato, A.; Tsujimura, T.; Kawahara, K.; Iwasaki, A.; Okamoto, T.; Oda, Y.; Honda, H.; et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: Comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 2017, 104, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Yuen, M.L.; Rath, E.M.; Johnson, B.; Zhuang, L.; Yu, T.-K.; Aleksova, V.; Linton, A.; Kao, S.; Clarke, C.J.; et al. CDKN2A and MTAP Are Useful Biomarkers Detectable by Droplet Digital PCR in Malignant Pleural Mesothelioma: A Potential Alternative Method in Diagnosis Compared to Fluorescence In Situ Hybridisation. Front. Oncol. 2020, 10, 579327. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Mikhael, P.G.; Ramesh, V.; Dai, Z.; Locasale, J.W. Nutrient availability shapes methionine metabolism in p16/MTAP-deleted cells. Sci Adv. 2019, 5, eaav7769. [Google Scholar] [CrossRef]
- Kadariya, Y.; Tang, B.; Wang, L.; Al-Saleem, T.; Hayakawa, K.; Slifker, M.J.; Kruger, W.D. Germline mutations in mtap cooperate with myc to accelerate tumorigenesis in mice. PLoS ONE 2013, 8, e67635. [Google Scholar] [CrossRef]
- Capella, G.; Caldas, C. MTAP homozygous deletion: An Achilles heel of human cancers ready for clinical use? Cancer Biol. Ther. 2005, 4, 347. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.J.; Pegg, A.E. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 55–91. [Google Scholar] [CrossRef]
- Christopher, S.A.; Diegelman, P.; Porter, C.W.; Kruger, W.D. Methylthioadenosine phosphorylase, a gene frequently codeleted with p16(cdkN2a/ARF), acts as a tumor suppressor in a breast cancer cell line. Cancer Res. 2002, 62, 6639–6644. [Google Scholar]
- Busacca, S.; Zhang, Q.; Sharkey, A.; Dawson, A.G.; Moore, D.A.; Waller, D.A.; Nakas, A.; Jones, C.; Cain, K.; Luo, J.-L.; et al. Transcriptional perturbation of protein arginine methyltransferase-5 exhibits MTAP-selective oncosuppression. Sci. Rep. 2021, 11, 7434. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Wilson, F.H.; Ruth, J.R.; Paulk, J.; Tsherniak, A.; Marlow, S.E.; Vazquez, F.; Weir, A.B.; Fitzgerald, E.M.; Tanaka, M.; et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 2016, 351, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Mavrakis, K.J.; McDonald, E.R., 3rd; Schlabach, M.R.; Billy, E.; Hoffman, G.R.; deWeck, A.; Ruddy, A.D.; Venkatesan, K.; Yu, J.; McALiister, G.; et al. Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 2016, 351, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Marjon, K.; Cameron, M.J.; Quang, P.; Clasquin, M.F.; Mandley, E.; Kunii, K.; McVay, M.; Choe, S.; Kemytsky, A.; Gross, S.; et al. MTAP deletions in cancer create vulnerability to targeting of the MAT2A/PRMT5/RIOK1 axis. Cell Rep. 2016, 15, 574–587. [Google Scholar] [CrossRef]
- Ronai, Z.A.H. PRMT5 function and targeting in cancer. Cell Stress 2020, 4, 199–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKinney, D.C.; McMillan, B.J.; Ranaghan, M.J.; Moroco, J.A.; Brousseau, M.; Mullin-Bernstein, Z.; O’keefe, M.; McCarren, P.; Mesleh, F.M.; Mulvaney, M.K.; et al. Discovery of a first-in-class inhibitor of the PRMT5-substrate adaptor interaction. J. Med. Chem. 2021, 64, 11148–11168. [Google Scholar] [CrossRef]
- Kalev, P.; Hyer, M.L.; Gross, S.; Konteatis, Z.; Chen, C.-C.; Fletcher, M.; Lein, M.; Aguado-Fraile, E.; Frank, V.; Barnett, A.; et al. MAT2A inhibition blocks the growth of MTAP-deleted cancer cells by reducing PRMT5-dependent mRNA splicing and inducing DNA damage. Cancer Cell 2021, 39, 209–224.e11. [Google Scholar] [CrossRef]
- Tang, B.; Lee, H.-O.; An, S.S.; Cai, K.Q.; Kruger, W.D. Specific targeting of MTAP-deleted tumors with a combination of 2′-fluoroadenine and 5′-methylthioadenosine. Cancer Res. 2018, 78, 4386–4395. [Google Scholar] [CrossRef]
- Schunselaar, L.M.; Zwart, W.; Baas, P. Targeting BAP1: A new paradigm for mesothelioma. Lung Cancer 2017, 109, 145–146. [Google Scholar] [CrossRef]
- Jensen, D.E.; Proctor, M.; Marquis, S.T.; Gardner, H.P.; Ha, S.I.; Chodosh, L.A.; Ishov, A.M.; Tommerup, N.; Vissing, H.; Sekido, Y.; et al. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Chekaluk, Y.; Bacares, R.; Ladanyi, M.; Zhang, L. BAP1 missense mutation c.2054 A>T (p.E685V) completely disrupts normal splicing through creation of a novel 5′ splice site in a human mesothelioma cell line. PLoS ONE 2015, 10, e0119224. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.; Zito Marino, F.; Morgillo, F.; Della Corte, C.; Santini, M.; Vicidomini, G.; Guggino, G.; De Dominicis, G.; Campione, S.; Accardo, M.; et al. Inherited predisposition to malignant mesothelioma: Germline BAP1 mutations and beyond. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4236–4246. [Google Scholar] [CrossRef]
- Di Nunno, V.; Frega, G.; Santoni, M.; Gatto, L.; Fiorentino, M.; Montironi, R.; Batteli, N.; Brandi, G.; Massari, F. BAP1 in solid tumors. Future Oncol. 2019, 15, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, A.P.; Wang, L. Emerging multifaceted roles of BAP1 complexes in biological processes. Cell Death Discov. 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Purwin, T.J.; Aplin, A.E. Roles of the BAP1 tumor suppressor in cell metabolism. Cancer Res. 2021, 81, 2807–2814. [Google Scholar] [CrossRef]
- Louie, B.H.; Kurzrock, R. BAP1: Not just a BRCA1-associated protein. Cancer Treat. Rev. 2020, 90, 102091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carbone, M.; Pass, H.I.; Ak, G.; Alexander, H.R., Jr.; Baas, P.; Baumann, F.; Blakely, A.M.; Bueno, R.; Bzura, A.; Cardillo, G.; et al. Medical and surgical care of patients with mesothelioma and their relatives carrying germline BAP1 mutations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 873–889. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Laurin Council, M.; Matatall, A.K.; Helms, C.; Bowcock, M.A.; et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Carbone, M.; Ferris, L.K.; Baumann, F.; Napolitano, A.; Lum, C.A.; Flores, E.G.; Gaudino, G.; Powers, A.; Bryant-Greenwood, P.; Krausz, T.; et al. BAP1 cancer syndrome: Malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J. Transl. Med. 2012, 10, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Napolitano, A.; Pellegrini, L.; Dey, A.; Larson, D.; Tanji, M.; Flores, E.G.; Kendrick, B.; Lapid, D.; Powers, A.; Pastorino, S.; et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016, 35, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kadariya, Y.; Cheung, M.; Pei, J.; Talarchek, J.; Sementino, E.; Tan, Y.; Menges, W.C.; Cai, Q.K.; Litwin, S.; et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014, 74, 4388–4397. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Aspesi, A.; Ferrante, D.; Sculco, M.; Righi, L.; Mirabelli, D.; Napoli, F.; Rondón-Lagos, M.; Casalone, E.; Vignolo Lutati, F.; et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosom. Cancer 2018, 57, 573–583. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinoshita, Y.; Hamasaki, M.; Yoshimura, M.; Matsumoto, S.; Iwasaki, A.; Nabeshima, K. Hemizygous loss of NF2 detected by fluorescence in situ hybridization is useful for the diagnosis of malignant pleural mesothelioma. Mod. Pathol. 2020, 33, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chapel, D.B.; Hornick, J.L.; Barlow, J.; Bueno, R.; Sholl, L.M. Clinical and molecular validation of BAP1, MTAP, P53, and Merlin immunohistochemistry in diagnosis of pleural mesothelioma. Mod. Pathol. Off. J. United States Can. Acad. Pathol. 2022, 35, 1383–1397. [Google Scholar] [CrossRef]
- Terra, S.; Roden, A.C.; Yi, E.S.; Aubry, M.C.; Boland, J.M. Loss of methylthioadenosine phosphorylase by immunohistochemistry is common in pulmonary sarcomatoid carcinoma and sarcomatoid mesothelioma. Am. J. Clin. Pathol. 2022, 157, 33–39. [Google Scholar] [CrossRef]
- Hiroshima, K.; Wu, D.; Hamakawa, S.; Tsuruoka, S.; Ozaki, D.; Orikasa, H.; Hasegawa, M.; Koh, E.; Sekine, Y.; Yonemori, Y.; et al. HEG1, BAP1, and MTAP are useful in cytologic diagnosis of malignant mesothelioma with effusion. Diagn. Cytopathol. 2021, 49, 622–632. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H.; Pass, H.I.; Krausz, T.; Testa, J.R.; Gaudino, G. BAP1 and cancer. Nat. Rev. Cancer 2013, 13, 153–159. [Google Scholar] [CrossRef]
- Carbone, M.; Harbour, J.W.; Brugarolas, J.; Bononi, A.; Pagano, I.; Dey, A.; Krausz, T.; Pass, H.I.; Yang, H.; Gaudino, G. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov. 2020, 10, 1103–1120. [Google Scholar] [CrossRef]
- Nasu, M.; Emi, M.; Pastorino, S.; Tanji, M.; Powers, A.; Luk, H.; Baumann, F.; Zhang, Y.A.; Gazdar, A.; Kanodia, S.; et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J. Thorac. Oncol. 2015, 10, 565–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Din, R.S.; Bahnasy, A.A.; Sabri, N.A.; Abdel-Rahman, C.A.; El Bastawisy, A. BAP1 gene mutations in Egyptian patients with advanced sporadic malignant Pleural mesothelioma (MPM): Relation with clinical outcomes and survival. Cancer Genet. 2018, 228–229, 83–92. [Google Scholar] [CrossRef]
- Farzin, M.; Toon, C.W.; Clarkson, A.; Sioson, L.; Watson, N.; Andrici, J.; Gill, A.J. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology 2015, 47, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, M.; James, M.; Agata, M.; Jennifer, K.; Ryan, D.; Aliya, N.H.; Wickii, V.; Carrie, F.; Thomas, K. BAP1 Immunohistochemistry Has Limited Prognostic Utility as a Complement of CDKN2A (p16) Fluorescence in situ Hybridization in Malignant Pleural Mesothelioma. Hum. Pathol. 2016, 60, 86–94. [Google Scholar] [CrossRef]
- Forest, F.; Patoir, A.; Dal Col, P.; Sulaiman, A.; Camy, F.; Laville, D.; Bayle-Bleuez, S.; Fournel, P.; Habougit, C. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: Prognostic implications. Pathology 2018, 50, 635–641. [Google Scholar] [CrossRef]
- Chou, A.; Toon, C.W.; Clarkson, A.; Sheen, A.; Sioson, L.; Gill, A.J. The epithelioid BAP1-negative and p16-positive phenotype predicts prolonged survival in pleural mesothelioma. Histopathology 2018, 72, 509–515. [Google Scholar] [CrossRef]
- Pulford, E.; Huilgol, K.; Moffat, D.; Henderson, D.W.; Klebe, S. Malignant mesothelioma, BAP1 immunohistochemistry, and VEGFA: Does BAP1 have potential for early diagnosis and assessment of prognosis? Dis. Markers 2017, 2017, 1310478. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.; Brevet, M.; Taylor, B.S.; Shimizu, S.; Ito, T.; Wang, L.; Creaney, J.; Lake, R.A.; Zakowski, M.F.; Reva, B.; et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 2011, 43, 668–672. [Google Scholar] [CrossRef]
- Zauderer, M.G.; Bott, M.; McMillan, R.; Sima, C.S.; Rusch, V.; Krug, L.M.; Ladanyi, M. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, 1430–1433. [Google Scholar] [CrossRef]
- Carbone, M.; Flores, E.G.; Emi, M.; Johnson, T.A.; Tsunoda, T.; Behner, D.; Hoffman, H.; Hesdorffer, M.; Nasu, M.; Napolitano, A.; et al. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015, 11, e1005633. [Google Scholar] [CrossRef]
- Hathaway, F.; Martins, R.; Sorscher, S.; Bzura, A.; Dudbridge, F.; Fennell, D.A. Family matters: Germline testing in thoracic cancers. American Society of Clinical Oncology Educational Book. Am. Soc. Clin. Oncol. Meet. 2023, 43, e389956. [Google Scholar] [CrossRef]
- Masclef, L.; Ahmed, O.; Estavoyer, B.; Larrivée, B.; Labrecque, N.; Nijnik, A.; Affar, E.B. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 2021, 28, 606–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.J.; Pham, T.; Chang, M.T.; Barnes, D.; Cai, A.G.; Noubade, R.; Totpal, K.; Chen, X.; Tran, C.; Hagenbeek, T.; et al. The Tumor Suppressor BAP1 Regulates the Hippo Pathway in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020, 80, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, G.; Sidhu, G.S.; Williamson, E.A.; Jaiswal, A.S.; Najmunnisa, N.; Wilcoxen, K.; Jones, D.; George, J.T., Jr.; Hromas, R. Synthetic lethality in malignant pleural mesothelioma with PARP1 inhibition. Cancer Chemother. Pharmacol. 2017, 80, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Csizmadia, T.; Lőw, P. The Role of Deubiquitinating Enzymes in the Various Forms of Autophagy. Int. J. Mol. Sci. 2020, 21, 4196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheuermann, J.C.; de Ayala Alonso, A.G.; Oktaba, K.; Ly-Hartig, N.; McGinty, R.K.; Fraterman, S.; Wilm, M.; Muir, T.W.; Müller, J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 2010, 465, 243–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bononi, A.; Yang, H.; Giorgi, C.; Patergnani, S.; Pellegrini, L.; Su, M.; Xie, G.; Signorato, V.; Pastorino, S.; Morris, P.; et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017, 24, 1694. [Google Scholar] [CrossRef]
- Carbone 2019 Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [CrossRef]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef]
- Affar, E.B.; Carbone, M. BAP1 regulates different mechanisms of cell death. Cell Death Dis. 2018, 9, 1151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.-S.; Jang, H.-J.; Choi, J.M.; Zhang, J.; de Rosen, V.L.; Wheeler, T.M.; Lee, J.-S.; Tu, T.; Jindra, P.T.; Kerman, R.H.; et al. Comprehensive immunoproteogenomic analyses of malignant pleural mesothelioma. JCI Insight 2018, 3, e98575. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, S.; Lasota, J.; Czapiewski, P.; Langfort, R.; Rys, J.; Szpor, J.; Waloszczyk, P.; Okoń, K.; Biernat, W.; Schrump, D.S.; et al. CD70 expression correlates with a worse prognosis in malignant pleural mesothelioma patients via immune evasion and enhanced invasiveness. J. Pathol. 2020, 250, 205–216. [Google Scholar] [CrossRef]
- Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to Mesenchymal Transition in human mesothelial cells exposed to asbestos fibers: Role of TGF-β as mediator of malignant mesothelioma development or metastasis via EMT event. Int. J. Mol. Sci. 2019, 20, 150. [Google Scholar] [CrossRef]
- Horio, D.; Minami, T.; Kitai, H.; Ishigaki, H.; Higashiguchi, Y.; Kondo, N.; Hirota, S.; Kitajima, K.; Nakajima, Y.; Koda, Y.; et al. Tumor-associated macrophage-derived inflammatory cytokine enhances malignant potential of malignant pleural mesothelioma. Cancer Sci. 2020, 111, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Barbarino, M.; Lonardi, S.; Vermi, W.; Giordano, A.; Bellan, C.; Giurisato, E. Mesothelioma malignancy and the microenvironment: Molecular mechanisms. Cancers 2021, 13, 5664. [Google Scholar] [CrossRef]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): Preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Sule Kutlar Dursun, F.; Alabalik, U. Investigation of pd-l1 (cd274), pd-l2 (pdcd1lg2), and ctla-4 expressions in malignant pleural mesothelioma by immunohistochemistry and real-time polymerase chain reaction methods. Pol. J. Pathol. Off. J. Pol. Soc. Pathol. 2022, 73, 111–119. [Google Scholar] [CrossRef]
- Chen, S.; Yu, W.; Shao, S.; Xiao, J.; Bai, H.; Pu, Y.; Li, M. Establishment of predictive nomogram and web-based survival risk calculator for malignant pleural mesothelioma: A SEER database analysis. Front. Oncol. 2022, 12, 1027149. [Google Scholar] [CrossRef]
- Dynamic Nomogram. Shinyapps.Io. Available online: https://mpmsurvival.shinyapps.io/MPMforOS/ (accessed on 11 May 2025).
- Huh, D.-A.; Choi, Y.-H.; Kim, L.; Park, K.; Lee, J.; Hwang, S.H.; Moon, K.W.; Kang, M.-S.; Lee, Y.-J. Air pollution and survival in patients with malignant mesothelioma and asbestos-related lung cancer: A follow-up study of 1591 patients in South Korea. Environ. Health A Glob. Access Sci. Source 2024, 23, 56. [Google Scholar] [CrossRef]
- Sayan, M.; Bas, A.; Turk, M.S.; Ozkan, D.; Celik, A.; Kurul, İ.C.; Tastepe, A.I. Survival effect of complete multimodal therapy in malignant pleural mesothelioma. J. Chest Surg. 2022, 55, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Hassan, R.; Morrow, B.; Thomas, A.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Gadiraju, M.; Panou, V.; Gao, S.; Mian, I.; et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 9008–9013. [Google Scholar] [CrossRef]
- Krug, L.M.; Kindler, H.L.; Calvert, H.; Manegold, C.; Tsao, S.A.; Fennell, D.; Ohman, R.; Plummer, R.; Eberhardt, E.E.W.; Fukuoka, K.; et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015, 16, 447–456. [Google Scholar] [CrossRef]
- LaFave, L.M.; Beguelin, W.; Koche, R.; Teater, M.; Spitzer, B.; Chramiec, A.; Papalexi, E.; Keller, D.M.; Hricik, T.; Konstantinoff, K.; et al. Loss of BAP1 function leads to EZH2- dependent transformation. Nat. Med. 2015, 21, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Zauderer, M.G.; Szlosarek, P.; Le Moulec, S.; Popat, S.; Taylor, P.; Planchard, D.; Scherpereel, A.; Koczywas, M.; Forster, M.; Cameron, B.R.; et al. Phase 2, multicenter study of the EZH2 inhibitor tazemetostat as monotherapy in adults with relapsed or refractory (R/R) malignant mesothelioma (MM) with BAP1 inactivation. J. Clin. Oncol. 2018, 36, 15. [Google Scholar] [CrossRef]
- George, T.J.; DeRemer, D.L.; Parekh, H.D.; Lee, J.-H.; Markham, M.J.; Daily, K.C.; Chatzkel, A.J.; Ramnaraign, H.N.; Close, J.L.; Ezenwajiaku, N.; et al. Phase II trial of the PARP inhibitor, niraparib, in BAP1 and other DNA damage response (DDR) pathway deficient neoplasms including cholangiocarcinoma. J. Clin. Oncol. 2020, 38, TPS591. [Google Scholar] [CrossRef]
- Hassan, R.; Mian, I.; Wagner, C.; Mallory, Y.; Agra, M.; Morrow, B.; Wei, S.J.; Khan, J.; Thomas, A.; Sengupta, M.; et al. Phase II study of olaparib in malignant mesothelioma (MM) to correlate efficacy with germline and somatic mutations in DNA repair genes. J. Clin. Oncol. 2020, 38, 15. [Google Scholar] [CrossRef]
- Borchert, S.; Wessolly, M.; Schmeller, J.; Mairinger, E.; Kollmeier, J.; Hager, T.; Mairinger, T.; Herold, T.; Christoph, D.C.; Walter, R.F.H.; et al. Gene expression profiling of homologous recombination repair pathway indicates susceptibility for olaparib treatment in malignant pleural mesothelioma in vitro. BMC Cancer 2019, 19, 108. [Google Scholar] [CrossRef]
- Rathkey, D.; Khanal, M.; Murai, J.; Zhang, J.; Sengupta, M.; Jiang, Q.; Morrow, B.; Evans, C.N.; Chari, R.; Fetsch, P.; et al. Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but is enhanced by temozolomide in cells with high-Schlafen 11and low-MGMT expression. J. Thorac. Oncol. 2020, 15, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Peikert, T.; Vasmatzis, G. Chromosomal rearrangements and their neoantigenic potential in mesothelioma. Transl. Lung Cancer Res. 2020, 9, S92. [Google Scholar] [CrossRef]
- American Association for Cancer Research. Dual Checkpoint Blockade Takes Aim at Relapsed B.H. Louie and R. Kurzrock Cancer Treatment Reviews 90 (2020) 102091 8 Mesothelioma. Cancer Discov. 2017, 7, OF7. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Aranda, R.; Bobinski, T.P.; Briere, D.M.; Burns, A.C.; Christensen, J.G.; Clarine, J.; Engstrom, L.D.; Gunn, R.J.; Ivetac, A.; et al. Fragment-Based Discovery of MRTX1719, a Synthetic Lethal Inhibitor of the PRMT5•MTA Complex for the Treatment of MTAP-Deleted Cancers. J. Med. Chem. 2022, 65, 1749–1766. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Novello, S.; Bearz, A.; Ceresoli, G.L.; Aerts, J.G.J.V.; Spicer, J.; Taylor, P.; Nackaerts, K.; Greystoke, A.; Jennens, R.; et al. Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): A randomised, open-label phase 2 trial. Lancet Oncol. 2022, 23, 540–552. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Pinto, C.; Zucali, P.A.; Pagano, M.; Grosso, F.; Pasello, G.; Garassino, M.C.; Tiseo, M.; Soto Parra, H.; Grossi, F.; Cappuzzo, F.; et al. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021, 22, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Anagnostou, V.; Sun, Z.; Dahlberg, S.E.; Kindler, H.L.; Niknafs, N.; Purcell, T.; Santana-Davila, R.; Dudek, A.Z.; Borghaei, H.; et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: Survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat. Med. 2021, 27, 1910–1920. [Google Scholar] [CrossRef]
- Canova, S.; Ceresoli, G.L.; Grosso, F.; Zucali, P.A.; Gelsomino, F.; Pasello, G.; Mencoboni, M.; Rulli, E.; Galli, F.; De Simone, I.; et al. Final results of DIADEM, a phase II study to investigate the efficacy and safety of durvalumab in advanced pretreated malignant pleural mesothelioma. ESMO Open 2022, 7, 100644. [Google Scholar] [CrossRef]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- Costa-Martins, S.; Vicente, I.; Valente, S. Relapsed malignant pleural mesothelioma: An impressive response to Nivolumab monotherapy. Pulmonology 2022, 28, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liang, J.; Lv, Y.; Huang, X.; Guo, W. Tislelizumab combined with anlotinib in the second-line treatment of malignant pleural mesothelioma. Medicine 2022, 101, 52. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Rusakiewicz, S.; Früh, M.; Hayoz, S.; Grosso, F.; Pless, M.; Zucali, P.; Ceresoli, G.L.; Maconi, A.; Schneider, M.; et al. Long-term benefit of lurbinectedin as palliative chemotherapy in progressive malignant pleural mesothelioma (MPM): Final efficacy and translational data of the SAKK 17/16 study. ESMO Open 2022, 7, 100446. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Martini, F.; Torreggiani, E.; Fortini, F.; Aquila, G.; Vieceli Dalla Sega, F.; Patergnani, S.; Pinton, P.; Maniscalco, P.; Cavallesco, G.; et al. Metformin Induces Apoptosis and Inhibits Notch1 in Malignant Pleural Mesothelioma Cells. Front. Cell Dev. Biol. 2021, 8, 534499. [Google Scholar] [CrossRef]
- Ahmadzada, T.; Cooper, A.W.; Holmes, M.; Mahar, A.; Westman, H.; Gill, J.A.; Nordman, I.; Yip, Y.P.; Pal, A.; Zielinski, R.; et al. Retrospective Evaluation of the Use of Pembrolizumab in Malignant Mesothelioma in a Real-World Australian Population. JTO Clin. Res. Rep. 2020, 1, 100075. [Google Scholar] [CrossRef]
- Lam, W.-S.; Creaney, J.; Chen, F.K.; Chin, W.L.; Murugan, S.; Arunachalam, S.; Attia, M.S.; Read, C.; Murray, K.; Millward, M.; et al. A phase II trial of single oral FGF inhibitor, AZD4547, as second or third line therapy in malignant pleural mesothelioma. Lung Cancer 2020, 140, 87–92. [Google Scholar] [CrossRef]
- Zauderer, M.G.; Alley, E.W.; Bendell, J.; Capelletto, E.; Bauer, T.M.; Callies, S.; Szpurka, A.M.; Kang, S.; Willard, M.D.; Wacheck, V.; et al. Phase 1 cohort expansion study of LY3023414, a dual PI3K/mTOR inhibitor, in patients with advanced mesothelioma. Investig. New Drugs 2021, 39, 1081–1088. [Google Scholar] [CrossRef]
- Passiglia, F.; Righi, L.; Bironzo, P.; Listì, A.; Farinea, G.; Capelletto, E.; Novello, S.; Merlini, A.; Scagliotti, G.V. Niraparib plus dostarlimab in pleural mesothelioma or non-small cell lung cancer harboring HRR mutations: Interim results of the UNITO-001 phase II prospective trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 959–964. [Google Scholar] [CrossRef]
- Hearon, B.F.; Redelman, K.N.; Elhomsy, G.C.; Moore, D.F., Jr. Exceptional regression of malignant pleural mesothelioma with pembrolizumab monotherapy. Case Rep. Oncol. 2020, 13, 1483–1489. [Google Scholar] [CrossRef]
- Ghafoor, A.; Mian, I.; Wagner, C.; Mallory, Y.; Agra, G.M.; Morrow, B.; Wei, S.J.; Khan, J.; Thomas, A.; Sengupta, M.; et al. Phase 2 study of olaparib in malignant mesothelioma and correlation of efficacy with germline or somatic mutations in BAP1 gene. JTO Clin. Res. Rep. 2021, 2, 100231. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Okuma, Y.; Kawai, S.; Nagamata, M.; Hosomi, Y. Premature phase II study of amrubicin as palliative chemotherapy for previously treated malignant pleural mesothelioma. Thorac. Cancer 2021, 12, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; You, M.; Meng, E.; Wang, S.; Niu, B.; Huang, W. Complete and durable response to crizotinib in a patient with malignant pleural mesothelioma harboring CD74-ROS1 fusion. J. Cancer Res. Clin. Oncol. 2022, 148, 2561–2566. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Nakagawa, K.; Fujimoto, N.; Kuribayashi, K.; Guren, T.K.; Calabrò, L.; Shapira-Frommer, R.; Gao, B.; Kao, S.; Matos, I.; et al. Efficacy and safety of pembrolizumab in patients with advanced mesothelioma in the open-label, single-arm, phase 2 KEYNOTE-158 study. Lancet Respir. Med. 2021, 9, 613–621. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Creelan, B.C.; Sarkodie, T.; Nolan, L.; Taylor, P.; Olevsky, O.; Grosso, F.; Cortinovis, D.; Chitnis, M.; Roy, A.; et al. ATOMIC-Meso Study Group. Pegargiminase Plus First-Line Chemotherapy in Patients With Nonepithelioid Pleural Mesothelioma: The ATOMIC-Meso Randomized Clinical Trial. JAMA Oncol. 2024, 10, 475–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anobile, D.P.; Bironzo, P.; Picca, F.; Lingua, M.F.; Morena, D.; Righi, L.; Napoli, F.; Papotti, M.G.; Pittaro, A.; Di Nicolantonio, F.; et al. Evaluation of the Preclinical Efficacy of Lurbinectedin in Malignant Pleural Mesothelioma. Cancers 2021, 13, 2332. [Google Scholar] [CrossRef]
- Guazzelli, A.; Meysami, P.; Bakker, E.; Demonacos, C.; Giordano, A.; Krstic-Demonacos, M.; Mutti, L. BAP1 status determines the sensitivity of malignant mesothelioma cells to gemcitabine treatment. Int. J. Mol. Sci. 2019, 20, 429. [Google Scholar] [CrossRef]
- Kumar, N.; Alrifai, D.; Kolluri, K.K.; Sage, E.K.; Ishii, Y.; Guppy, N.; Borg, E.; Falzon, M.; Nankivell, M.; Nicholson, A.G.; et al. Retrospective response analysis of BAP1 expression to predict the clinical activity of systemic cytotoxic chemotherapy in mesothelioma. Lung Cancer 2019, 127, 164–166. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Belisario, D.C.; Bironzo, P.; Ananthanarayanan, P.; Ricci, L.; Digiovanni, S.; Fontana, S.; Napoli, F.S.; Ri, A.; Facolmatà, C.; et al. SKP2 drives the sensitivity to neddylation inhibitors and cisplatin in malignant pleural mesothelioma. J. Exp. Clin. Cancer Res. CR 2022, 41, 75. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahiu, T.; Mihu, C.M.; Bosca, B.A.; Mărginean, M.; Mocan, L.P.; Ștefan, R.-A.; Suflețel, R.T.; Mihu, C.; Melincovici, C.S. Molecular Insights into Pleural Mesothelioma: Unveiling Pathogenic Mechanisms and Therapeutic Opportunities. Diagnostics 2025, 15, 1323. https://doi.org/10.3390/diagnostics15111323
Zahiu T, Mihu CM, Bosca BA, Mărginean M, Mocan LP, Ștefan R-A, Suflețel RT, Mihu C, Melincovici CS. Molecular Insights into Pleural Mesothelioma: Unveiling Pathogenic Mechanisms and Therapeutic Opportunities. Diagnostics. 2025; 15(11):1323. https://doi.org/10.3390/diagnostics15111323
Chicago/Turabian StyleZahiu, Teodora, Carmen Mihaela Mihu, Bianca A. Bosca, Mariana Mărginean, Lavinia Patricia Mocan, Roxana-Adelina Ștefan, Rada Teodora Suflețel, Carina Mihu, and Carmen Stanca Melincovici. 2025. "Molecular Insights into Pleural Mesothelioma: Unveiling Pathogenic Mechanisms and Therapeutic Opportunities" Diagnostics 15, no. 11: 1323. https://doi.org/10.3390/diagnostics15111323
APA StyleZahiu, T., Mihu, C. M., Bosca, B. A., Mărginean, M., Mocan, L. P., Ștefan, R.-A., Suflețel, R. T., Mihu, C., & Melincovici, C. S. (2025). Molecular Insights into Pleural Mesothelioma: Unveiling Pathogenic Mechanisms and Therapeutic Opportunities. Diagnostics, 15(11), 1323. https://doi.org/10.3390/diagnostics15111323







