Abstract
Background/Objectives: Although Osgood–Schlatter disease (OSD) is often self-limiting following apophyseal closure, it may cause persistent symptoms into adulthood, affecting physical and functional activities. The purpose of this systematic review is to summarize the current evidence on the efficacy of hyperosmolar dextrose injection for patients with OSD unresponsive to conservative treatment. Methods: Multiple databases were searched for studies investigating the efficacy of hyperosmolar dextrose injection in patients with OSD. Two reviewers independently extracted data and evaluated the risk of bias. Meta-analyses were performed to compare hyperosmolar dextrose injection with placebo injections. Results: Four studies including three randomized controlled trials (RCTs) and one case series involving a total of 166 (162 males and 4 females) patients with 184 knees were included in this review. At three months, there was no significant difference in patient-reported improvement from baseline between hyperosmolar dextrose injection and placebo injections (standardized mean difference [SMD] = 1.92, 95% confidence interval [CI], −0.12 to 3.96; I2 = 96.2%). However, a meta-analysis of two RCTs including athletic pediatric patients found a pooled risk ratio of 2.11 (95% CI: 1.12 to 3.98, I2 = 30.73%) for pain-free return to sports at three months. In addition, at one year, a meta-analysis of two RCTs showed greater patient-reported improvement from baseline with hyperosmolar dextrose injection compared to placebo (SMD = 1.09, 95% CI, 0.62 to 1.56; I2= 0%). Conclusions: Based on the limited number of RCTs, although no improvement in patient-reported outcomes is seen at three months, hyperosmolar dextrose injection may safely facilitate a pain-free return to sports at three months and lead to patient-reported improvement at one year. However, further high-quality RCTs are needed to substantiate these findings.
1. Introduction
Osgood–Schlatter disease (OSD) is a common cause of anterior knee pain in adolescents, with an estimated incidence of 3.8 per 1000 person-years [1]. It predominantly affects active males more than females [2]. OSD is an apophysitis of the tibial tubercle caused by repetitive traction of the patellar tendon on the developing tibial tuberosity. This occurs particularly during periods of rapid femur growth, placing physically active adolescents undergoing rapid growth spurts at higher risk [3]. While OSD is typically self-limiting, a subset of patients may develop persistent pain, functional limitations, and swelling of the tibial tubercle [4].
Conventional management of OSD includes activity modification, physical therapy with a focus on strengthening and stretching the quadriceps, hip flexors, hamstrings, and pelvic stabilizers, patellar tendon straps, protective knee pads, oral and/or topical nonsteroidal anti-inflammatory drugs (NSAIDs), and cold therapy [4]. However, some patients do not respond to these conventional therapies. In one retrospective study, 26 out of 43 patients reported OSD-related knee pain during a median follow-up of 3.75 years, despite attempting strength exercises, stretches, activity modification, and oral medications [4]. Therefore, additional treatments, such as hyperosmolar dextrose injection, may be warranted for these patients.
Hyperosmolar dextrose injection, the most commonly used agent in prolotherapy, has been utilized for several decades to treat various musculoskeletal conditions [5]. It is hypothesized to work by creating an osmotic gradient that dehydrates and lyses local cells, leading to localized inflammation and initiation of the healing cascade. This inflammatory response stimulates cellular proliferation, extracellular matrix deposition, and collagen synthesis, promoting tissue repair and remodeling [6].
Although several randomized controlled trials (RCTs) [7,8,9] have evaluated the efficacy of hyperosmolar dextrose injections for OSD, the evidence is conflicting whether dextrose is superior to placebo. This systematic review with meta-analysis aims to summarize the current evidence on hyperosmolar dextrose injections for patients with OSD who did not respond to conservative management. We hypothesized that hyperosmolar dextrose injections may improve pain and facilitate a return to sports compared to placebo injections.
2. Materials and Methods
2.1. Study Design
PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) 2020 and PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance 2022 were used to conduct and report this review [10,11]. Our protocol was registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202520020). Our Population, Intervention, Comparison, and Outcome framework is as follows:
- -
- Participants: Patients diagnosed with Osgood–Schlatter disease (tibial tuberosity apophysitis).
- -
- Intervention: Hyperosmolar dextrose injection (prolotherapy) near/to the tibial tuberosity.
- -
- Comparison: Placebo injection (saline or lidocaine), other injectable treatments, physical therapy, sham procedures, or no intervention.
- -
- Outcome: Clinically relevant measures, such as Nirschl Pain Phase Scale (NPPS) and Victorian Institute of Sport Assessment (VISA), return to activity/sport, and adverse events.
2.2. Search Strategy
The literature was searched by a medical librarian for the concepts of OSD and dextrose or prolotherapy. Search strategies were created using a combination of keywords and standardized index terms. Searches were run on 6 February 2025 in ClinicalTrials.gov (2000+), Ovid Cochrane Central Register of Controlled Trials (1991+), Google Scholar through Harzing’s Publish or Perish for Windows, Ovid Embase (1974+), Ovid Medline (1946+ including epub ahead of print, in-process, and other non-indexed citations), Scopus (1788+), Web of Science Core Collection (Science Citation Index Expanded 1975+ and Emerging Sources Citation Index 2015+), and the World Health Organization’s ICTRP clinical trial registry (2005+). Full search strategies are provided in Supplemental Tables S1–S8.
2.3. Study Screening
All references were uploaded to Covidence and independently screened by two authors. Discrepancies between the two authors were resolved through discussion with a third author.
2.4. Study Selection
The systematic review aimed to include RCTs, prospective and retrospective comparative studies, and case series evaluating the efficacy of hyperosmolar dextrose injection for OSD. Reviews, case reports, studies conducted on animals, cadavers, or in vitro settings, letters to the editor, and technical descriptions were excluded. Studies lacking details on the intervention procedure, patient diagnosis, follow-up, clinical examination, or statistical analysis were excluded.
2.5. Data Extraction
Two authors independently reviewed each study identified in the initial search and conducted data extraction. Extracted variables included study design, inclusion and exclusion criteria, patient demographics, intervention details (dextrose concentration, volume, number of injections), rehabilitation protocols, follow-up durations, outcome measures, and adverse events. Any statistical information such as mean and standard deviation relevant for meta-analysis was also extracted.
2.6. Risk-of-Bias Assessment
All RCTs included in this review were assessed for the risk of bias using a revised Cochrane risk-of-bias tool for randomized trials. This tool assesses the risk of bias from five different domains, including the process of participant randomization, anomalies from interventions planned, absences of the outcome data, outcome measurements, and selective reporting of the result [12]. If all five domains were assessed to have a low risk of bias, then the overall risk of bias in the study was considered low. If up to two domains were assessed to have some concerns in risk of bias, then the overall risk of bias of the study was considered to have some concerns. Finally, if three or more domains were assessed to have some concerns in risk of bias or at least one domain was evaluated to have a high risk of bias, then the overall risk of bias of the study was determined to be high. Two authors assessed the risk of bias, and any discrepancies between the two authors were resolved through discussion with a third author.
2.7. Statistical Analysis
The standardized mean difference (SMD) was used to calculate the effect size because Topol et al. reported NPPS scores [7], while Nakase et al. and Wu et al. reported VISA scores [8,9], which represent two different patient-reported outcome measures. To provide a consistent interpretation of the effect size, the direction of NPPS scores was reversed (by multiplying by −1) [13], as lower NPPS scores indicate better outcomes, while higher VISA scores indicate better outcomes.
Wu et al. reported mean and standard errors for follow-up VISA scores instead of standard deviations (SDs), and therefore the SDs were calculated using the following formula [13]:
SD = SE × √n.
Meta-analysis was performed for outcomes at three months and at one year, as all studies reported three-month outcomes, and two studies reported one-year outcomes. A random effects meta-analysis was used to compare patient-reported improvement (measured by VISA or NPPS) from baseline to follow-up (three months or one year) in order to account for expected heterogeneity, including variability in patient populations and interventions. Because the included studies did not report within-group SDs for changes in scores, the following formula with an assumed correlation coefficient (r) of 0.5 was used [13,14]:
Subgroup analysis was performed with two studies reporting VISA scores at three months, using a weighted mean difference (WMD). Furthermore, since the mean age of patients was significantly higher in the RCT by Wu et al. [8], we conducted a meta-analysis including only pediatric patients from the two RCTs [7,9]. From the same two RCTs, a meta-analysis of risk ratios (RRs) was conducted using the fixed-effects model in order to analyze the proportion of pain-free patients during sports at three months.
Statistical heterogeneity was assessed using Cochran’s Q test and I2 statistics, with I2 values categorized as low (≤25%), moderate (26–50%), or high (>50%) heterogeneity [15]. Publication bias was not assessed because less than 10 studies were included in the analysis. All analyses were conducted using STATA Version 16 (StataCorp, LLC, College Station, TX, USA).
3. Results
3.1. Study Selection
A total of 278 citations were retrieved. Deduplication was performed automatically in Covidence, leaving 225 citations for screening. After screening studies with titles and abstracts, 213 studies were removed, resulting in a total of 4 remaining studies, which all met the eligibility criteria (Figure 1). The four studies included in this review, including three RCTs [7,8,9] and one case series [16], had a total of 166 (162 males and 4 females) patients with 184 knees.
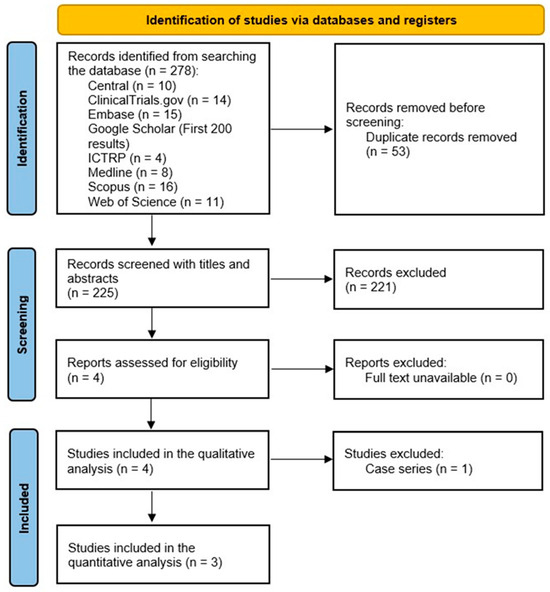
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses demonstrating the study selection process.
3.2. Study Characteristics
Population: Two RCTs [7,9] included exclusively pediatric patients (mean age 12–13 years) who participated in sports and were members of sports teams or clubs. One RCT [8] included male military officers and soldiers (mean age: 21 years). One cases series [16] included five pediatric patients (age range: 11–16) and one adult patient. The inclusion and exclusion criteria, along with demographic information, are summarized in Table 1 and Table 2.

Table 1.
Demographic characteristics of the included studies.

Table 2.
Inclusion and exclusion criteria characteristics and main findings of the included studies.
Intervention: For hyperosmolar dextrose injection, the dextrose concentration ranged from 12.5% to 20%, with sterile water or lidocaine added to the injection mixture. Two studies [8,9] used ultrasound guidance, while the other studies [7,16] performed landmark-guided injections. Three RCTs [7,8,9] administered three injections monthly at 0, 1, and 2 months. The needle size ranged from 27 to 30 gauge. Details of the injectates and injection methods are summarized in Table 3.

Table 3.
Treatment intervention characteristics of the included studies.
Two RCTs [8,9] did not impose any activity restrictions, whereas one RCT [7] specified no running or kicking motions for one week following the first injection and for three days following the second and third injections.
Comparator: In two RCTs [8,9], lidocaine with saline injection was used as a placebo injection, while one RCT [7] included a placebo injection arm with lidocaine only and a usual care group undergoing supervised physical therapy.
Outcome: Two RCTs [8,9] evaluated VISA as their primary outcome measure, and one RCT [7] used the NPPS. Additionally, pain-free return to activities was also reported in two RCTs [7,9]. Follow-up durations varied from 1 month to 12 months. Two studies [7,16] did not report adverse events, whereas the other two studies explicitly stated that no adverse events occurred [8,9].
3.3. Risk-of-Bias Assessment
The RCTs included in this study were assessed to have some concerns about the risk of bias for their reported outcomes, including VISA, NPPS, and VISA-P (Figure 2) [7,8,9]. The primary reason for this assessment was due to the absence of clinical trial protocols important in the evaluation of selective reporting of the result domain. Therefore, all of the included studies were judged to have some concerns for risk of bias in this domain, resulting in their overall risk of bias being of at least some concern.
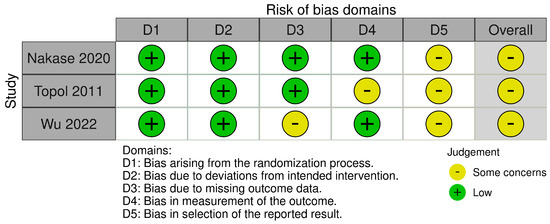
Figure 2.
Risk-of-bias assessment for the included randomized controlled trials [7,8,9].
3.4. Meta-Analysis Results
A meta-analysis of three RCTs [7,8,9] demonstrated that at three months, there was no significant difference in patient-reported improvement from baseline between hyperosmolar dextrose injection and placebo injections (SMD = 1.92, 95% confidence interval [CI], −0.12 to 3.96; I2 =96.2%). However, at one year, a meta-analysis of two RCTs [7,8] showed greater patient-reported improvement from baseline with hyperosmolar dextrose injection compared to placebo (SMD = 1.09, 95% CI, 0.62 to 1.56; I2= 0%).
At three months, there was no significant difference in improvement from baseline between hyperosmolar dextrose injection and placebo injections in terms of VISA scores (WMD = 17.24, 95% CI −0.82 to 35.3; I2 = 90.1%) [8,9], or in pediatric patients (SMD = 0.819, 95% CI: −0.039 to1.676; I2 = 73.1%) [7,9].
Finally, a meta-analysis of two RCTs [7,9] including athletic pediatric patients found a pooled risk ratio of 2.11 (95% CI: 1.12 to 3.98, I2 = 30.73%) for pain-free return to sports at 3 months, indicating that patients receiving hyperosmolar injection therapy were 2.11 times more likely to become pain-free during sports at 3 months compared to the control group.
4. Discussion
Contrary to our hypothesis, there was no significant difference in patient-reported outcome improvement at three months between hyperosmolar dextrose and placebo injections. However, based on two RCTs, hyperosmolar dextrose injections may increase the likelihood of adolescent athletes with OSD returning to sports pain-free at three months and improving patient-reported outcomes at one year.
OSD is often described as a self-limiting condition with symptoms resolving in most patients upon apophyseal closure. Seldomly, symptoms can persist through adulthood, affecting physical or functional activities. Previous studies have reported that between 10% and 60% of patients may experience persistent symptoms into adulthood despite conventional management [17,18], although these figures should be interpreted with caution due to the small sample sizes in these studies. Nonetheless, athletes with a history of OSD have been found to exhibit higher levels of disability, as measured by the Knee Outcome Survey Activities of Daily Living Scale and the Sports Activity Scale [19]. These studies highlight the importance of adequate and early treatment of OSD at the time of initial diagnosis and the need for alternative interventions if conservative measures—such as activity modification, physical therapy (strengthening and stretching), and oral/topical medications—fail. Corticosteroid injections are commonly used for musculoskeletal conditions in adults [20], but they are not recommended in pediatric populations due to increased risks of potential growth plate injury [21] and tendon weakening or rupture [22,23]. Furthermore, there is a paucity of literature on treatments beyond conservative management, posing unique challenges in treating OSD patients with persistent symptoms.
The results of our study suggest that hyperosmolar dextrose injection may be a potential treatment option for young athletes who do not respond to initial conservative treatment but wish to return to sports, as well as for patients with persistent symptoms despite apophyseal closure. Hyperosmolar dextrose injection is a regenerative medicine therapy thought to stimulate local inflammatory responses and promote tissue healing through several proposed mechanisms. These include the increased production of platelet-derived growth factor (PDGF) [24], insulin growth factor-1 (IGF-1) [25], and transforming growth factor (TGF)-beta [26], which may lead to fibroblast proliferation and extracellular matrix deposition. While the exact mechanism of action is not fully understood, several systematic reviews have demonstrated potential benefits in various musculoskeletal conditions, including Achilles tendinopathy [27], plantar fasciitis [28], common extensor tendinopathy [29], rotator cuff tendinopathy [30], and osteoarthritis [31]. While our study is based on a limited number of trials, it contributes to the existing body of evidence supporting hyperosmolar injection therapy as a potential treatment option for patients with OSD.
4.1. Clinical Application
Given the limited number of studies included in the meta-analysis, the evidence is not robust enough to recommend hyperosmolar dextrose injections for all patients with OSD. Notably, analysis of our main outcome, patient-reported outcomes at three months, did not show any statistically significant difference between the hyperosmolar dextrose injections and placebo injections. Furthermore, our results should be interpreted with caution, as the potential benefits of hyperosmolar dextrose injections do not justify their routine use in these patients. However, based on our findings, it may be reasonable to consider this treatment for young athletes with persistent symptoms despite initial conservative measures, particularly those seeking additional options to facilitate their return to sports. Additionally, one of the included RCTs [8], which evaluated a slightly older population with apophyseal closure, highlights that symptoms of OSD may persist into adulthood and suggests that hyperosmolar dextrose injection therapy may be considered in this subset of the population.
Although only two studies in our review [8,9] utilized ultrasound guidance, ultrasound-guided injections are highly recommended to ensure precise delivery of the injectate, especially when it is delivered at multiple sites. In one RCT [9], injections were administered into the infrapatellar fat pad, deep infrapatellar bursa, and superficial infrapatellar bursa. In the other RCT [8], injections were placed in the superficial and deep layers of the patellar tendons. These anatomic targets may therefore be considered in clinical practice.
The included studies utilized 27–30-gauge needles, a factor that may be particularly relevant when treating pediatric patients, especially those who are averse to needles. Additionally, a potential advantage of hyperosmolar dextrose injections based on the reviewed studies is the lack of prolonged activity restrictions following injections. Two RCTs imposed no post-injection activity restrictions [8,9], while one RCT advised avoiding running and kicking for one week after the first injection and for two or three days after the second and third injections [7]. Most importantly, no studies reported any complications associated with the injections.
4.2. Future Research Direction
All three RCTs included in our review administered three injections at 0, 1, and 2 months. In his case series, Kidd reported that all but one patient responded well to just one or two injections [16], raising the possibility that fewer injections may still provide clinical benefit. Future studies should consider comparisons of different injection frequencies (e.g., single vs. multiple injections) and intervals to determine the most cost-effective and clinically beneficial dosing regimen. This is particularly important given that hyperosmolar dextrose injection in the United States is typically an out-of-pocket expense, which may be a barrier to access to this treatment.
Furthermore, while OSD is more prevalent in males, all three RCTs primarily included male patients. Future trials should consider more balanced sex representation or perform subgroup analyses to assess potential sex-specific differences in treatment response.
Lastly, standardized reporting of outcome measures, ultrasound guidance techniques, and injection formulations would enhance comparability across studies and facilitate evidence synthesis.
4.3. Limitations
The primary limitation of this meta-analysis is the small number of studies included. Therefore, caution is needed when interpreting the results of this review. A limited number of studies reduces statistical power and increases the likelihood that findings may be influenced by study-level variability, rather than true treatment effects. We also noted differences in patient populations, which may limit the generalizability of our findings to specific age groups. Wu et al. included adult military personnel [8], while the other RCTs focused on pediatric athletes [7,9]. However, we performed subgroup analyses to account for these demographic differences.
Variations in hyperosmolar dextrose injection formulations were also observed, with concentrations ranging from 12.5% to 20%, and some studies incorporated lidocaine or sterile water. Additionally, injection locations varied slightly across the studies, with two studies using landmark-guided injections. These methodological differences could contribute to clinical heterogeneity and may impact treatment efficacy. Given that patients may present with differing symptomatology and ultrasound findings, judicious clinical assessment remains essential to tailor the injection location, formulation, and volume to individual needs.
Lastly, all included RCTs were assessed as having some concerns regarding the risk of bias, mainly due to the lack of protocol registration or availability. The absence of a priori protocol registration may raise concerns about selective reporting, deviations from intended interventions, and incomplete reporting. To address these issues, future trials should register their protocols in advance, implement blinding when feasible, ensure adequate sample sizes, and report outcomes based on standardized guidelines.
These methodological limitations reduce the certainty of the evidence synthesized in this review, and therefore clinical applicability should be interpreted with caution until larger, well-designed, and transparently reported RCTs confirm these results.
5. Conclusions
Based on the limited number of RCTs, no significant difference in patient-reported outcome improvement was observed at three months between hyperosmolar dextrose and placebo injections. However, hyperosmolar dextrose injection may safely facilitate a pain-free return to sports at three months and lead to patient-reported improvement at one year. Further high-quality RCTs are needed to substantiate these findings.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics15101282/s1: Table S1: ClinicalTrials.gov; Table S2: Cochrane Central Register of Controlled Trials; Table S3: Embase; Table S4: Google Scholar; Table S5: International Clinical Trials Registry Platform; Table S6: MEDLINE; Table S7: Scopus; Table S8: Web of Science
Author Contributions
Conceptualization, H.C.R., L.B.B., and C.H.; methodology, H.C.R.; software, H.C.R.; formal analysis, H.C.R.; investigation, H.C.R., L.B.B., J.S., J.P., and C.H.; resources, L.B.B., K.-M.J., and C.H.; data curation, H.C.R. and J.S.; writing—original draft preparation, H.C.R., L.B.B., J.S., J.P., and K.C.M.; writing—review and editing, S.E.D., K.-M.J., and C.H.; visualization, H.C.R. and J.S.; supervision, K.-M.J. and C.H.; funding acquisition, K.-M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Korea University grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from H.C.R. upon reasonable request.
Acknowledgments
The authors would like to thank the Mayo Clinic librarian (Dana Gerberi) for helping us with building search terms and identifying relevant studies for this review.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CI | Confidence interval |
| NPPS | Nirschl Pain Phase Scale |
| NSAID | Nonsteroidal anti-inflammatory drug |
| OSD | Osgood–Schlatter disease |
| SD | Standard deviation |
| SMD | Standardized mean difference |
| RCT | Randomized controlled trial |
| VISA | Victorian Institute of Sport Assessment |
| WMD | Weighted mean difference |
References
- van Leeuwen, G.J.; de Schepper, E.I.; Rathleff, M.S.; Bindels, P.J.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Incidence and management of Osgood-Schlatter disease in general practice: Retrospective cohort study. Br. J. Gen. Pract. 2022, 72, e301–e306. [Google Scholar] [CrossRef]
- Gaulrapp, H.; Nührenbörger, C. The Osgood-Schlatter disease: A large clinical series with evaluation of risk factors, natural course, and outcomes. Int. Orthop. 2022, 46, 197–204. [Google Scholar] [CrossRef]
- Ladenhauf, H.N.; Seitlinger, G.; Green, D.W. Osgood-Schlatter disease: A 2020 update of a common knee condition in children. Curr. Opin. Pediatr. 2020, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Guldhammer, C.; Rathleff, M.S.; Jensen, H.P.; Holden, S. Long-term Prognosis and Impact of Osgood-Schlatter Disease 4 Years After Diagnosis: A Retrospective Study. Orthop. J. Sports Med. 2019, 7, 2325967119878136. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Lackner, J.B.; Steilen-Matias, D.; Harris, D.K. A Systematic Review of Dextrose Prolotherapy for Chronic Musculoskeletal Pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Steilen, D.; Hauser, R.; Woldin, B.; Sawyer, S. Chronic neck pain: Making the connection between capsular ligament laxity and cervical instability. Open Orthop. J. 2014, 8, 326–345. [Google Scholar] [CrossRef]
- Topol, G.A.; Podesta, L.A.; Reeves, K.D.; Raya, M.F.; Fullerton, B.D.; Yeh, H.W. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics 2011, 128, e1121–e1128. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, X.; Tu, Z. Hyperosmolar dextrose injection for Osgood-Schlatter disease: A double-blind, randomized controlled trial. Arch. Orthop. Trauma Surg. 2022, 142, 2279–2285. [Google Scholar] [CrossRef]
- Nakase, J.; Oshima, T.; Takata, Y.; Shimozaki, K.; Asai, K.; Tsuchiya, H. No superiority of dextrose injections over placebo injections for Osgood-Schlatter disease: A prospective randomized double-blind study. Arch. Orthop. Trauma Surg. 2020, 140, 197–202. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sports Med. 2022, 56, 175–195. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 143–176. [Google Scholar]
- Wiebe, N.; Vandermeer, B.; Platt, R.W.; Klassen, T.P.; Moher, D.; Barrowman, N.J. A systematic review identifies a lack of standardization in methods for handling missing variance data. J. Clin. Epidemiol. 2006, 59, 342–353. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Kidd, R.F. Treatment of Osgood-Schlatter disease by prolotherapy: A case report. J. Orthop. Med. 1993, 15, 62–63. [Google Scholar] [CrossRef]
- Kujala, U.M.; Kvist, M.; Heinonen, O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am. J. Sports Med. 1985, 13, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.L.; Williams, J.P.; Catterall, A. Natural history of Osgood-Schlatter disease. J. Pediatr. Orthop. 1990, 10, 65–68. [Google Scholar] [CrossRef]
- Ross, M.D.; Villard, D. Disability levels of college-aged men with a history of Osgood-Schlatter disease. J. Strength Cond. Res. 2003, 17, 659–663. [Google Scholar] [CrossRef]
- Rhim, H.C.; Ruiz, J.; Taseh, A.; Afunugo, W.; Crockett, Z.; Schon, J.; Pan, X.; Shin, J.; Schowalter, S.; Jang, K.M.; et al. Nonsteroidal Anti-Inflammatory Drug Injections versus Steroid Injections in the Management of Upper and Lower Extremity Orthopedic Conditions: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 1132. [Google Scholar] [CrossRef]
- Sanchez, C.P.; He, Y.Z. Alterations in the growth plate cartilage of rats with renal failure receiving corticosteroid therapy. Bone 2002, 30, 692–698. [Google Scholar] [CrossRef]
- Vallone, G.; Vittorio, T. Complete Achilles tendon rupture after local infiltration of corticosteroids in the treatment of deep retrocalcaneal bursitis. J. Ultrasound 2014, 17, 165–167. [Google Scholar] [CrossRef]
- Lu, H.; Yang, H.; Shen, H.; Ye, G.; Lin, X.J. The clinical effect of tendon repair for tendon spontaneous rupture after corticosteroid injection in hands: A retrospective observational study. Medicine 2016, 95, e5145. [Google Scholar] [CrossRef]
- Okuda, Y.; Adrogue, H.J.; Nakajima, T.; Mizutani, M.; Asano, M.; Tachi, Y.; Suzuki, S.; Yamashita, K. Increased production of PDGF by angiotensin and high glucose in human vascular endothelium. Life Sci. 1996, 59, 1455–1461. [Google Scholar] [CrossRef]
- Lam, S.; van der Geest, R.N.; Verhagen, N.A.; van Nieuwenhoven, F.A.; Blom, I.E.; Aten, J.; Goldschmeding, R.; Daha, M.R.; van Kooten, C. Connective tissue growth factor and igf-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose. Diabetes 2003, 52, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Lee, M.Y.; Yun, S.P.; Han, H.J. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J. Cell. Physiol. 2010, 224, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Morath, O.; Kubosch, E.J.; Taeymans, J.; Zwingmann, J.; Konstantinidis, L.; Südkamp, N.P.; Hirschmüller, A. The effect of sclerotherapy and prolotherapy on chronic painful Achilles tendinopathy-a systematic review including meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 4–15. [Google Scholar] [CrossRef]
- Chutumstid, T.; Susantitaphong, P.; Koonalinthip, N. Effectiveness of dextrose prolotherapy for the treatment of chronic plantar fasciitis: A systematic review and meta-analysis of randomized controlled trials. PMR 2023, 15, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Rabago, D.; Chung, V.C.; Reeves, K.D.; Wong, S.Y.; Sit, R.W. Effects of Hypertonic Dextrose Injection (Prolotherapy) in Lateral Elbow Tendinosis: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 2209–2218. [Google Scholar] [CrossRef]
- Catapano, M.; Zhang, K.; Mittal, N.; Sangha, H.; Onishi, K.; de Sa, D. Effectiveness of Dextrose Prolotherapy for Rotator Cuff Tendinopathy: A Systematic Review. PMR 2020, 12, 288–300. [Google Scholar] [CrossRef]
- Waluyo, Y.; Artika, S.R.; Insani Nanda, W.; Gunawan, A.; Zainal, A.T.F. Efficacy of Prolotherapy for Osteoarthritis: A Systematic Review. J. Rehabil. Med. 2023, 55, 2572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).