Three Neglected STARD Criteria Reduce the Uncertainty of the Liver Fibrosis Biomarker FibroTest-T2D in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethics
2.2. Patients
2.3. Methods
2.3.1. First Aim: To Compare Two Scoring Systems, Both with 5-Tiers, eF Being More Sensitive than the Standard CRN
2.3.2. Second Aim: To Assess the Performance of FT-T2D Using wAUROC or the Adjusted Binary AUROC Instead of the Standard Binary AUROC (bAUROC)
2.3.3. Third Aim: To Assess the Impact of Biopsy Sample Length
2.3.4. Fourth Aim: To Assess the Trajectories of Fibrosis, Inflammation, and Steatosis Stratified by Sex, T2D, and Obesity
2.4. Sensitivity Analyses
3. Results
3.1. First Aim: Advantage of a More Sensitive (eF) Histological Comparator
3.2. Second Aim: Performance of FT-T2D vs. FibroTest Using wAUROC or bAUROC
3.3. Third Aim: Impact of the Biopsy Sample Length, a Major Factor of Uncertainty
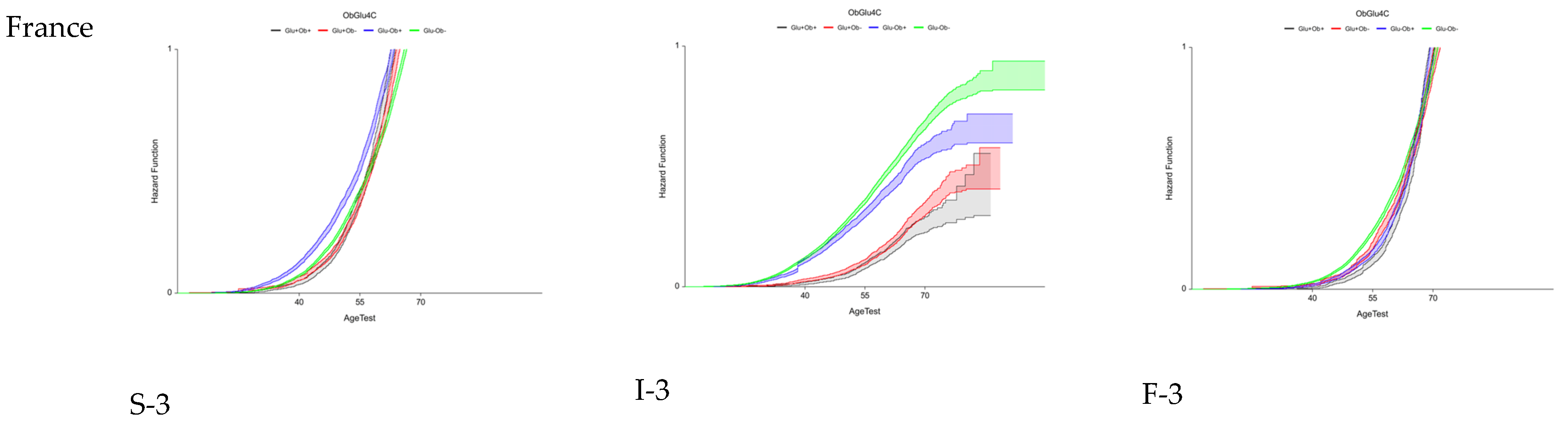
3.4. Fourth Aim: Variability of Early Fibrosis Trajectories in Large Populations
3.5. Sensitivity Analyses (Supplementary File S7)
4. Discussion
4.1. Limitations
4.2. Strengths
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- López Tórrez, S.M.; Ayala, C.O.; Ruggiro, P.B.; Costa, C.A.D.; Wagner, M.B.; Padoin, A.V.; Mattiello, R. Accuracy of prognostic serological biomarkers in predicting liver fibrosis severity in people with metabolic dysfunction-associated steatotic liver disease: A meta-analysis of over 40,000 participants. Front. Nutr. 2024, 11, 1284509. [Google Scholar] [CrossRef]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- Syn, N.L.; Cummings, D.E.; Wang, L.Z.; Lin, D.J.; Zhao, J.J.; Loh, M.; Koh, Z.J.; Chew, C.A.; Loo, Y.E.; Tai, B.C.; et al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: A one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet 2021, 397, 1830–1841. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.-C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e5. [Google Scholar] [CrossRef]
- Pais, R.; Aron-Wisnewsky, J.; Bedossa, P.; Ponnaiah, M.; Oppert, J.; Siksik, J.; Genser, L.; Charlotte, F.; Thabut, D.; Clement, K.; et al. Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery. Hepatology 2022, 76, 456–468. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Castera, L.; Wong, V.W.-S. Noninvasive Assessment of Liver Fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 2026–2039. [Google Scholar] [CrossRef]
- McHugh, L.C.; Snyder, K.; Yager, T.D. The effect of uncertainty in patient classification on diagnostic performance estimations. Sartori G, editor. PLoS ONE 2019, 14, e0217146. [Google Scholar]
- Mehta, S.H.; Lau, B.; Afdhal, N.H.; Thomas, D.L. Exceeding the limits of liver histology markers. J. Hepatol. 2009, 50, 36–41. [Google Scholar] [CrossRef]
- Poynard, T.; Lenaour, G.; Vaillant, J.C.; Capron, F.; Munteanu, M.; Eyraud, D.; Ngo, Y.; M’Kada, H.; Ratziu, V.; Hannoun, L.; et al. Liver Biopsy Analysis Has a Low Level of Performance for Diagnosis of Intermediate Stages of Fibrosis. Clin. Gastroenterol. Hepatol. 2012, 10, 657–663.e7. [Google Scholar] [CrossRef]
- Bedossa, P.; Dargere, D.; Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; LIDO Study Group. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Poynard, T.; Paradis, V.; Mullaert, J.; Deckmyn, O.; Gault, N.; Marcault, E.; Manchon, P.; Mohammed, N.S.; Parfait, B.; Ibberson, M.; et al. Prospective external validation of a new non-invasive test for the diagnosis of non-alcoholic steatohepatitis in patients with type 2 diabetes. Aliment. Pharmacol. Ther. 2021, 54, 952–966. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Shankar, S.S.; Yates, K.P.; Bolognese, J.; Daly, E.; Dehn, C.A.; Neuschwander-Tetri, B.; Kowdley, K.; Vuppalanchi, R.; Behling, C.; et al. Diagnostic performance of circulating biomarkers for non-alcoholic steatohepatitis. Nat. Med. 2023, 29, 2656–2664. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Clin. Chem. 2015, 61, 1446–1452. [Google Scholar] [CrossRef]
- Boursier, J.; De Ledinghen, V.; Poynard, T.; Guéchot, J.; Carrat, F.; Leroy, V.; Wong, G.L.; Friedrich-Rust, M.; Fraquelli, M.; Plebani, M.; et al. An extension of STARD statements for reporting diagnostic accuracy studies on liver fibrosis tests: The Liver-FibroSTARD standards. J. Hepatol. 2015, 62, 807–815. [Google Scholar] [CrossRef]
- Lambert, J.; Halfon, P.; Penaranda, G.; Bedossa, P.; Cacoub, P.; Carrat, F. How to Measure the Diagnostic Accuracy of Noninvasive Liver Fibrosis Indices: The Area Under the ROC Curve Revisited. Clin. Chem. 2008, 54, 1372–1378. [Google Scholar] [CrossRef]
- Poynard, T.; Halfon, P.; Castera, L.; Munteanu, M.; Imbert-Bismut, F.; Ratziu, V.; Benhamou, Y.; Bourlière, M.; de Ledinghen, V.; FibroPaca Group. Standardization of ROC Curve Areas for Diagnostic Evaluation of Liver Fibrosis Markers Based on Prevalences of Fibrosis Stages. Clin. Chem. 2007, 53, 1615–1622. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Carrieri, P.; Carrat, F.; Di Beo, V.; Bourlière, M.; Barré, T.; De Ledinghen, V.; Pageaux, G.-P.; Bureau, M.; Cagnot, C.; Dorival, C.; et al. Severe liver fibrosis in the HCV cure era: Major effects of social vulnerability, diabetes, and unhealthy behaviors. JHEP Rep. 2022, 4, 100481. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Gozar, M.; Da, B.L.; Bernstein, D.; Satapathy, S.K. Pharmacotherapeutic efficacy on noninvasive fibrosis progression in nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 102–111. [Google Scholar] [CrossRef]
- Poynard, T.; Deckmyn, O.; Peta, V.; Paradis, V.; Gautier, J.; Brzustowski, A.; Bedossa, P.; Castera, L.; Pol, S.; Valla, D.; et al. Prospective direct comparison of non-invasive liver tests in outpatients with type 2 diabetes using intention-to-diagnose analysis. Aliment. Pharmacol. Ther. 2023, 58, 888–902. [Google Scholar] [CrossRef]
- Poynard, T.; Deckmyn, O.; Peta, V.; Sakka, M.; Lebray, P.; Moussalli, J.; Pais, R.; Housset, C.; Ratziu, V.; Pasmant, E.; et al. Clinical and genetic definition of serum bilirubin levels for the diagnosis of Gilbert syndrome and hypobilirubinemia. Hepatol. Commun. 2023, 7, e0245. [Google Scholar] [CrossRef]
- Poynard, T.; Deckmyn, O.; Munteanu, M.; Ngo, Y.; Drane, F.; Castille, J.M.; Housset, C.; Ratziu, V. Awareness of the severity of liver disease re-examined using software-combined biomarkers of liver fibrosis and necroinflammatory activity. BMJ Open 2015, 5, e010017. [Google Scholar] [CrossRef]
- Deckmyn, O.; Poynard, T.; Bedossa, P.; Paradis, V.; Peta, V.; Pais, R.; Ratziu, V.; Thabut, D.; Brzustowski, A.; Gautier, J.F.; et al. Clinical Interest of Serum Alpha-2 Macroglobulin, Apolipoprotein A1, and Haptoglobin in Patients with Non-Alcoholic Fatty Liver Disease, with and without Type 2 Diabetes, before or during COVID-19. Biomedicines 2022, 10, 699. [Google Scholar] [CrossRef]
- Poynard, T.; Munteanu, M.; Deckmyn, O.; Ngo, Y.; Drane, F.; Messous, D.; Castille, J.M.; Housset, C.; Ratziu, V.; Imbert-Bismut, F. Applicability and precautions of use of liver injury biomarker FibroTest. A reappraisal at 7 years of age. BMC Gastroenterol. 2011, 11, 39. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Vali, Y.; Lee, J.; Boursier, J.; Petta, S.; Wonders, K.; Tiniakos, D.; Bedossa, P.; Geier, A.; Francque, S.; Allison, M.; et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): A comparative diagnostic accuracy study. Lancet Gastroenterol. Hepatol. 2023, 8, 714–725. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Jha, P.; Kleiner, D.E. Digital pathology for nonalcoholic steatohepatitis assessment. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 57–69. [Google Scholar] [CrossRef]
- Poynard, T.; Peta, V.; Deckmyn, O.; Pais, R.; Ngo, Y.; Charlotte, F.; Ngo, A.; Munteanu, M.; Imbert-Bismut, F.; Monneret, D.; et al. Performance of liver biomarkers, in patients at risk of nonalcoholic steato-hepatitis, according to presence of type-2 diabetes. Eur. J. Gastroenterol. Hepatol. 2020, 32, 998–1007. [Google Scholar] [CrossRef]
- Poynard, T.; Munteanu, M.; Deckmyn, O.; Ngo, Y.; Drane, F.; Castille, J.M.; Housset, C.; Ratziu, V.; Imbert-Bismut, F. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: Proof of concept and first application in a large population. J. Hepatol. 2012, 57, 541–548. [Google Scholar] [CrossRef]
- Ratziu, V.; Massard, J.; Charlotte, F.; Messous, D.; Imbert-Bismut, F.; Bonyhay, L.; Tahiri, M.; Munteanu, M.; Thabut, D.; Cadranel, J.F.; et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006, 6, 6. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Hollebecque, A.; Buob, D.; Leteurtre, E.; Arnalsteen, L.; Louvet, A.; Pigeyre, M.; Raverdy, V.; Verkindt, H.; et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur. J. Gastroenterol. Hepatol. 2011, 23, 499–506. [Google Scholar] [CrossRef]
- Munteanu, M.; Tiniakos, D.; Anstee, Q.; Charlotte, F.; Marchesini, G.; Bugianesi, E.; Trauner, M.; Romero Gomez, M.; Oliveira, C.; Day, C.; et al. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment. Pharmacol. Ther. 2016, 44, 877–889. [Google Scholar] [CrossRef]
- Arai, T.; Takahashi, H.; Seko, Y.; Toyoda, H.; Hayashi, H.; Yamaguchi, K.; Iwaki, M.; Yoneda, M.; Shima, T.; Fujii, H.; et al. Accuracy of the Enhanced Liver Fibrosis Test in Patients With Type 2 Diabetes Mellitus and Its Clinical Implications. Clin. Gastroenterol. Hepatol. 2024, 22, 789–797.e8. [Google Scholar] [CrossRef]
- Bertot, L.C.; Jeffrey, G.P.; De Boer, B.; Wang, Z.; Huang, Y.; Garas, G.; MacQuillan, G.; Wallace, M.; Smith, B.W.; Adams, L.A. Comparative Accuracy of Clinical Fibrosis Markers, Hepascore and Fibroscan® to Detect Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2023, 68, 2757–2767. [Google Scholar] [CrossRef]
- Jin, S.; Li, S.; Fang, P.; Pan, C.; Huang, S. Association of hysterectomy with nonalcoholic fatty liver disease among US women. Lipids Health Dis. 2024, 23, 34. [Google Scholar] [CrossRef]
- Netanel, C.; Goitein, D.; Rubin, M.; Kleinbaum, Y.; Katsherginsky, S.; Hermon, H.; Tsaraf, K.; Tachlytski, I.; Herman, A.; Safran, M.; et al. The impact of bariatric surgery on nonalcoholic fatty liver disease as measured using non-invasive tests. Am. J. Surg. 2021, 222, 214–219. [Google Scholar] [CrossRef]
- Codjia, T.; Rebibo, L.; François, A.; Lagnel, C.; Huet, E.; Bekri, S.; Pattou, F.; Régimbeau, J.-M.; Schwarz, L. Evolution of Non-alcoholic Fatty Liver Disease (NAFLD) Biomarkers in Response to Weight Loss 1 Year After Bariatric Surgery—A Post Hoc Analysis of the FibroTest Prospective Study. Obes. Surg. 2021, 31, 3548–3556. [Google Scholar] [CrossRef]
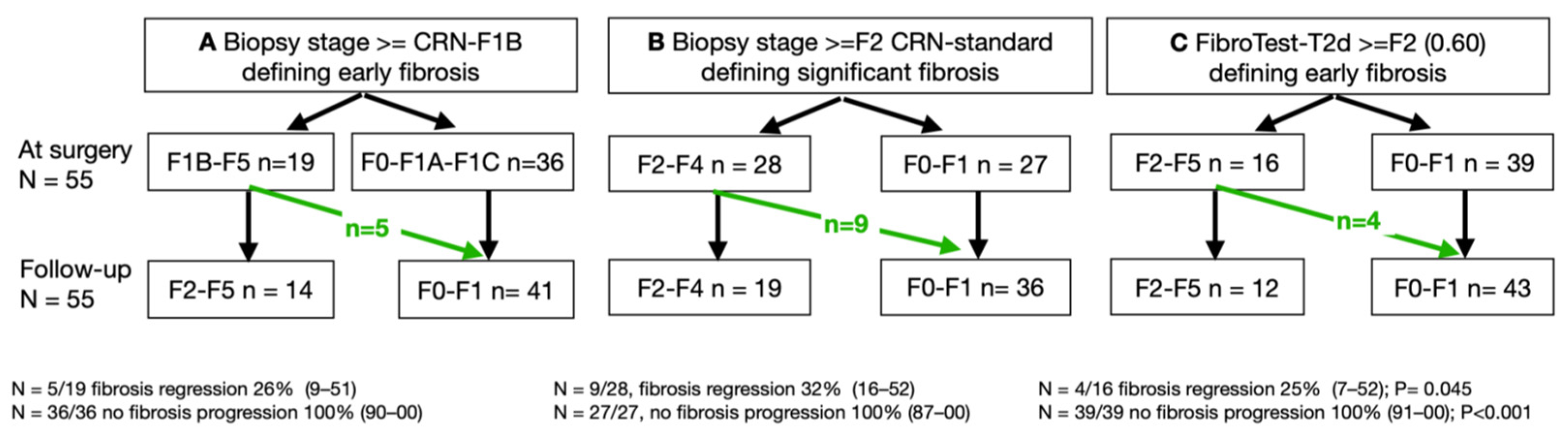
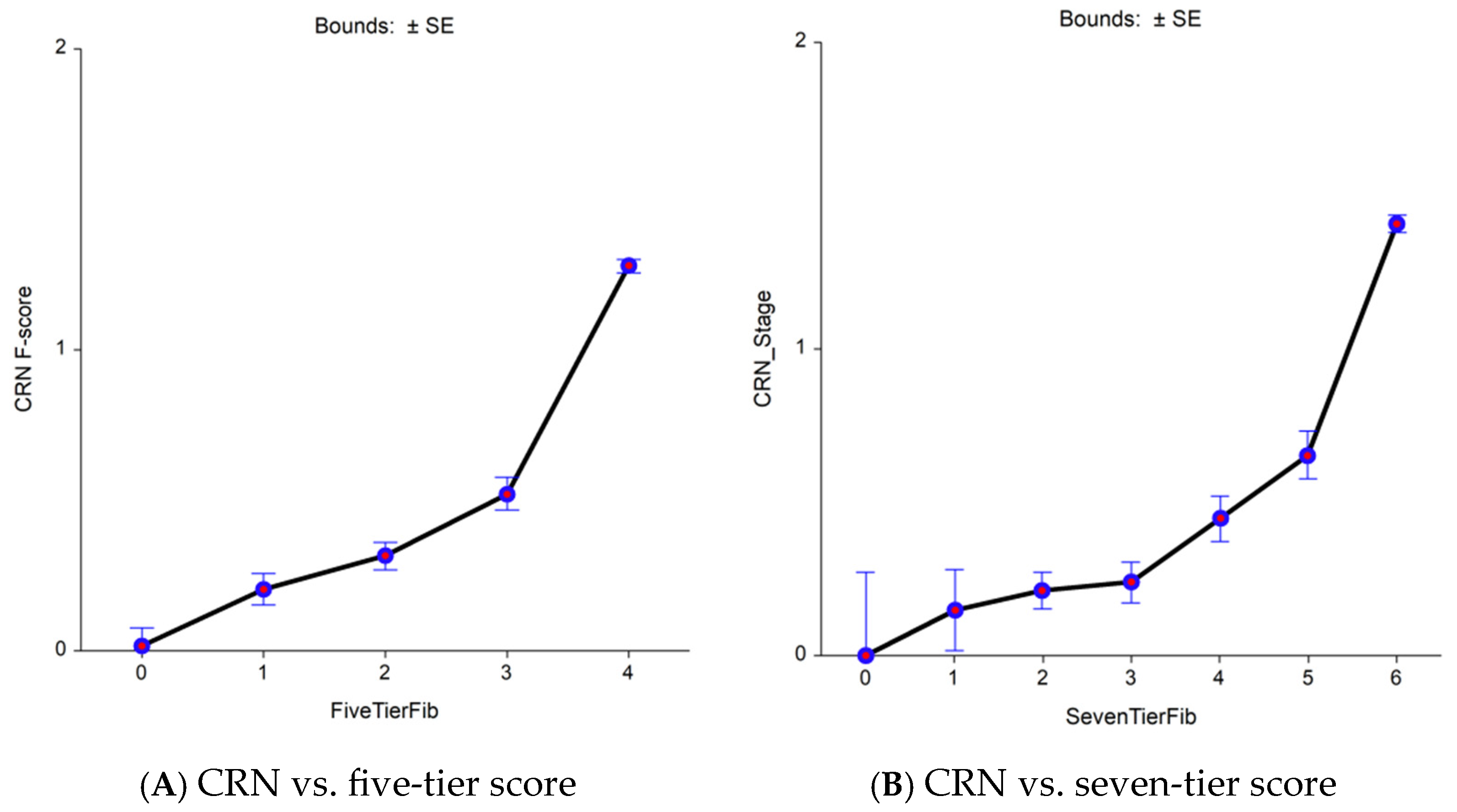
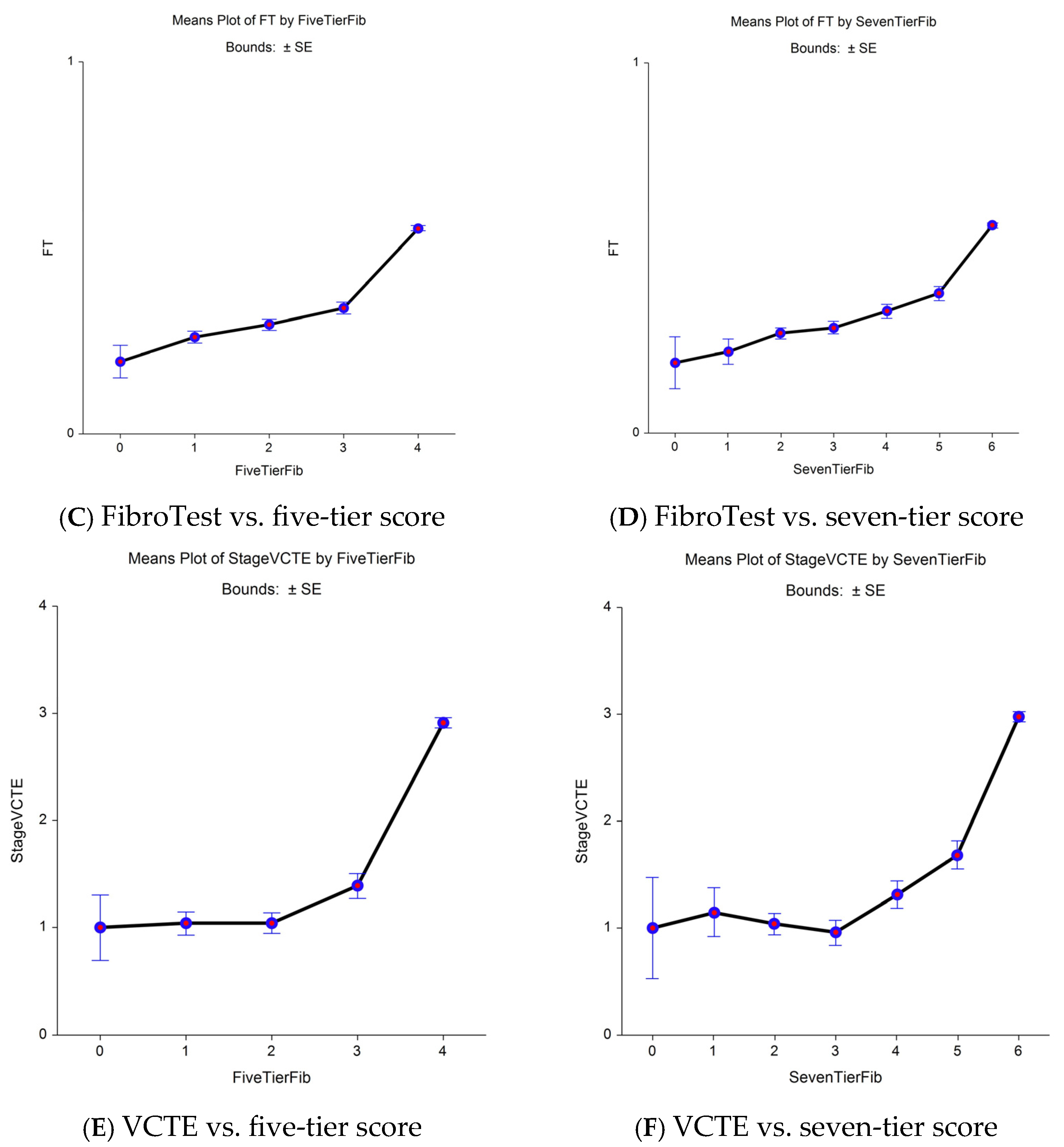
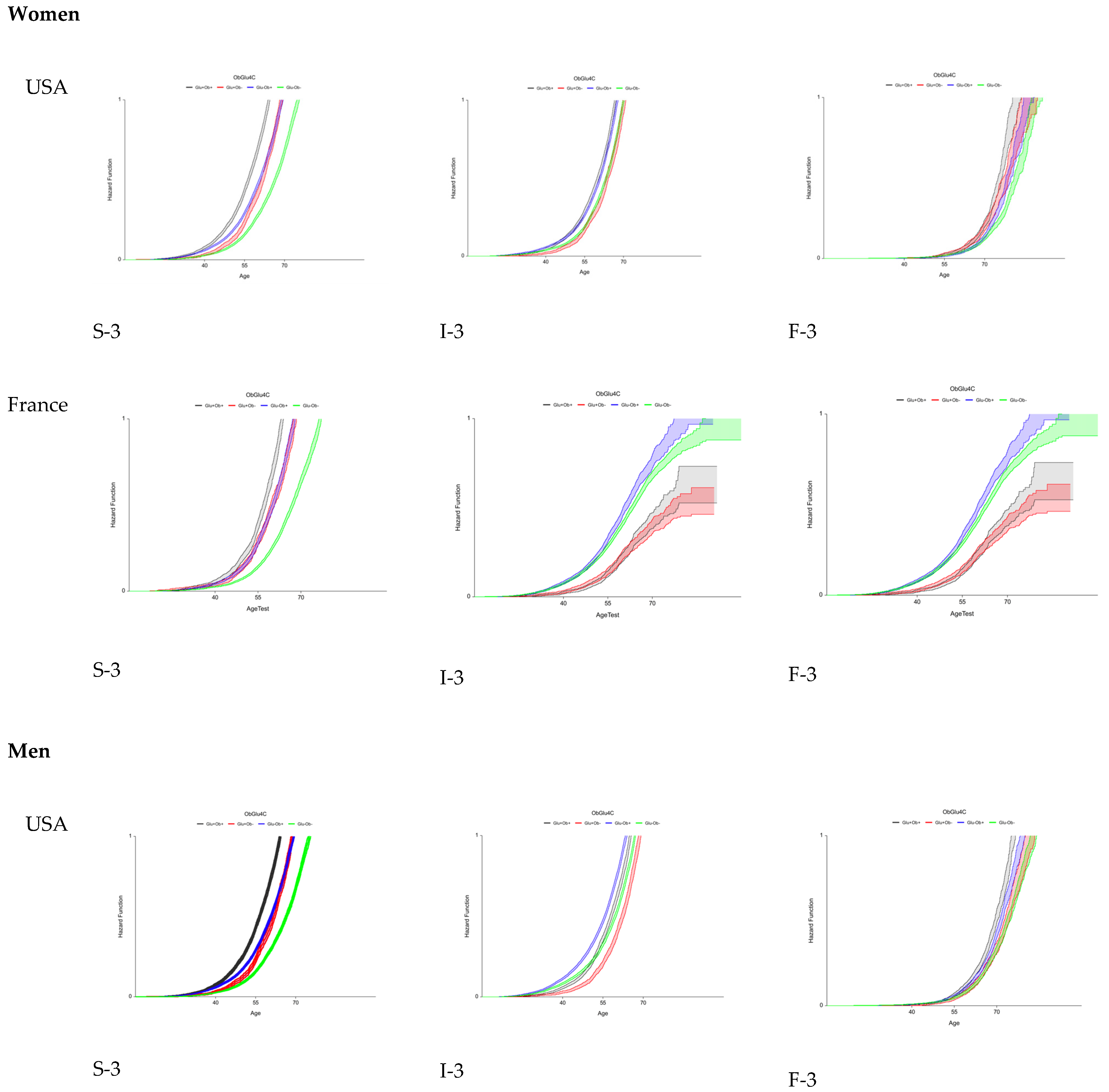
| Characteristics | Three STARD Methods | FT-T2D Proxy3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Aim | Second Aim | Third Aim | Fourth Aim | |||||||
| Seven Subsets Reference | Disease | Number All/Biopsy/Controls | Age, Years Mean (Range or SD) | Female% | BMI, kg/m2 Median (Range) | T2D % | Early Fibrosis | Weighted AUROC | Liver Biopsy Sample Length | Confounding Factors of Fibrosis Progression |
| BARICAN Pais [6] | Bariatric surgery | 55/110/0 | 55 (SD = 8) | 62 | 44 (26–61) | 64 | Yes | Yes | Yes | Yes |
| QuidNash Poynard [14,23] | Type 2 diabetes | 402/402/0 | 58 (SD = 10) | 40 | 34 (19–54) | 100 | Yes | Yes | Yes | Yes |
| LIDO Ratziu [13] | MASLD | 51/102/0 | 55 (31–73) | 39 | 32 (22–45) | 33 | Yes | No | Yes | Yes |
| Fibrosis-TAGS Poynard [11] | MASLD | 909/1293/4 | 46 (SD = 12) | 39 | Not available | Not available | No | Yes | Yes | Yes |
| UK BioBank Poynard [24] | At risk of MASLD | 159,794/0/0 | 57 (SD = 8) | 53 | 31 (12–75) | 6 | No | No | No | Yes |
| France- FibroTest Poynard [25] | At risk of MASLD | 67,278/0/0 | 53 (SD = 14) | 41 | 28 (SD = 6) | 16 | No | No | No | Yes |
| USA- FibroTest Deckmyn [26] | At risk of MASLD | 72,026/0/0 | 56 (SD = 14) | 54 | 31 (10–79) | 22 | No | No | No | Yes |
| Characteristics | eF (Cutoff ≥ F1B Early Fibrosis) | CRN Standard (Cutoff ≥ F2 Bridging Fibrosis) | ||||
|---|---|---|---|---|---|---|
| QUIDNASH COHORT N = 402 | ||||||
| Biopsy Length | ≥Median 17 mm | <Median 17 mm | All | ≥Median 17 mm | <Median 17 mm | All |
| Method = weighted AUROC (SD) | ||||||
| FibroTest * | 0.84 (0.02) | 0.80 (0.02) | 0.82 (0.01) | 0.87 (0.01) | 0.85 (0.02) | 0.86 (0.01) |
| p-value FibroTest-T2D vs. FibroTest | 0.12 | 0.002 | 0.001 | 0.02 | 0.002 | 0.0002 |
| p-value between biopsy lengths | FT-T2D < 0.001 | FT-T2D < 0.001 | ||||
| FT < 0.001 | FT < 0.001 | |||||
| Standard = bAUROC (95% CI) | ||||||
| N (prevalence) | n = 136/211 (64%) | n = 116/191 (61%) | n = 156/402 (63%) | n = 79/211 (37%) | n = 71/211 (37%) | n = 150/402 (37%) |
| FibroTest-T2D | 0.72 (0.64–0.78) | 0.77 (0.69–0.83) | 0.74 (0.69–0.79) | 0.80 (0.73–0.85) | 0.74 (0.66–0.80) | 0.77 (0.72–0.81) |
| FibroTest | 0.69 (0.61–0.76) | 0.67 (0.58–0.74) | 0.68 (0.62–0.74) | 0.74 (0.66–0.80) | 0.67 (0.58–0.74) | 0.70 (0.65–0.75) |
| BARICAN COHORT N = 110 | ||||||
| Biopsy Length | ≥Median 20 mm | <Median 20 mm | All | ≥Median 20 mm | <Median 20 mm | All |
| Method = weighted AUROC (SD) | ||||||
| FibroTest-T2D * | 0.93 (0.03) p < 0.001 | 0.88 (0.02) p < 0.001 | 0.90 (0.02) p < 0.001 | 0.91 (0.03) p < 0.001 | 0.84 (0.03) p < 0.001 | 0.87 (0.02) p < 0.001 |
| FibroTest * | 0.94 (0.03) p < 0.001 | 0.86 (0.03) p < 0.01 | 0.89 (0.02) p < 0.001 | 0.92 (0.03) p < 0.001 | 0.84 (0.03) p < 0.01 | 0.87 (0.02) p < 0.001 |
| p-value FibroTest-T2D vs. FibroTest * | 0.001 | 0.35 | 0.59 | 0.60 | 0.98 | 0.87 |
| p-value between biopsy length groups | FT-T2D < 0.001 | FT-T2D < 0.001 | ||||
| FT < 0.001 | FT < 0.001 | |||||
| Standard = bAUROC (95% CI), | ||||||
| N (prevalence) | n = 8/73 (11%) | n = 12/37 (17%) | n = 18/110 (27%) | n = 11/43 (26%) | n = 16/67 (24%) | n = 27/110 (28%) |
| FibroTest-T2D | 0.84 (0.64–0.93) | 0.72 (0.47–0.90) | 0.78 (0.63–0.87) | 0.84 (0.65–0.93) | 0.83 (0.67–0.92) | 0.83 (0.72–0.90) |
| FibroTest | 0.80 (0.46–0.94) | 0.49 (0.25–0.68) | 0.67 (0.50–0.80) | 0.78 (0.57–0.89) | 0.76 (0.57–0.88 | 0.77(0.63–0.86) |
| 22 Comparisons in 18 Published Studies Author Year | N | eF | CRN F0/F1/F2/F3/F4 | DANA Index | Adjusted AUROC F3F4 vs. F0F1F2 | Weighted AUROC | Binary AUROC Standard F3/F4 vs. F0/F1/F2 | Biopsy Length Median (mm) |
|---|---|---|---|---|---|---|---|---|
| Uniform spectrum model | 100 | 20/20/20/20/20 | 2.50 | 0.800 | 0.800 | >0.800 | >30 | |
| FibroTest | ||||||||
| Ratziu 2006 [33] first | 97 | 0 | 26/40/15/12/4 | 2.39 | 0.910 | 0.878 | 0.810 | 18 |
| Ratziu 2006 [33] validation | 170 | 0 | 76/54/31/9/0 | 2.28 | 0.873 | 0.920 | 0.920 | 20 |
| Lassailly 2011 [34] | 288 | 0 | 170/98/13/2/5 | 3.27 | 0.911 | 0.847 | 0.971 | NA |
| Adams 2011 * | 242 | 0 | 87/58/44/30/23 | 2.38 | 0.784 | NA | 0.802 | 16 |
| Munteanu 2016 [35] | 600 | 0 | 122/184/140/121/33 | 2.17 | 0.744 | 0.878 | 0.749 | 20 |
| Boursier 2016 [17] *,^,w | 452 | 0 | 41/117/120/114/58 | 2.06 | 0.735 | 0.722 | 0.734 | 27 |
| Bril 2020 * | 151 | 0 | 38/63/25/19/6 | 2.34 | 0.722 | NA | 0.720 | NA |
| Poynard 2023 [23] 2,^,l,r,w | 402 | 1 | 117/66/63/85/71 | 2.67 | 0.789 | 0.842 | 0.709 | 17 |
| FT-T2D | ||||||||
| Poynard 2023 [23] 2,^,b,l,r,w | 402 | 1 | 117/66/63/85/71 | 2.67 | 0.789 | 0.879 | 0.774 | 17 |
| ELF | ||||||||
| Miele 2017 * | 82 | 0 | 6/32/29/5/10 | 2.32 | 0.759 | NA | 0.948 | >16 |
| Anstee 2019 * | 3202 | 0 | 246/276/418/979/128 | 2.38 | 0.764 | NA | 0.800 | 22 |
| Guillaume 2019 ^,w,* | 417 | 0 | 38/98/114/135/32 | 1.89 | 0.720 | 0.764 | 0.793 | 29 |
| Arai 2024 [36] r | 1228 | 0 | 214/411/327/237/39 | 2.02 | 0.803 | NA | 0.828 | NA |
| Hepascore | ||||||||
| Adams 2011 *,^ | 242 | 0 | 87/58/44/30/23 | 2.38 | 0.788 | NA | 0.814 | 16 |
| Bertot 2023 [37] r | 271 | 0 | 101/67/20/36/47 | 3.00 | 0.842 | NA | 0.880 | NA |
| Boursier 2016 [17] 2,^,w | 452 | 0 | 41/117/120/114/58 | 2.06 | 0.735 | 0.765 | 0.778 | |
| FibroMeter NAFLD | ||||||||
| Cales 2009 * | 235 | 0 | 102/68/21/19/25 | 2.99 | 0.889 | NA | 0.928 | 30 |
| Aykut 2014 t,* | 88 | 0 | 23/21/17/27/9 | 2.35 | 0.761 | NA | 0.937 | NA |
| Boursier 2016 [17] ^,w, | 452 | 0 | 41/117/120/114/58 | 2.06 | 0.735 | 0.886 | 0.759 | 27 |
| Subasi 2015 t,* | 142 | 0 | 40/50/22/20/10 | 2.49 | 0.774 | NA | 0.761 | 20 |
| FibroMeter V2G | ||||||||
| Boursier 2016 [17] 2,^,w | 452 | 0 | 41/117/120/114/58 | 2.06 | 0.735 | 0.798 | 0.817 | 27 |
| Guillaume 2019 ^,w,* | 417 | 0 | 38/98/114/135/32 | 1.89 | 0.720 | 0.763 | 0.804 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poynard, T.; Deckmyn, O.; Pais, R.; Aron-Wisnewsky, J.; Peta, V.; Bedossa, P.; Charlotte, F.; Ponnaiah, M.; Siksik, J.-M.; Genser, L.; et al. Three Neglected STARD Criteria Reduce the Uncertainty of the Liver Fibrosis Biomarker FibroTest-T2D in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Diagnostics 2025, 15, 1253. https://doi.org/10.3390/diagnostics15101253
Poynard T, Deckmyn O, Pais R, Aron-Wisnewsky J, Peta V, Bedossa P, Charlotte F, Ponnaiah M, Siksik J-M, Genser L, et al. Three Neglected STARD Criteria Reduce the Uncertainty of the Liver Fibrosis Biomarker FibroTest-T2D in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Diagnostics. 2025; 15(10):1253. https://doi.org/10.3390/diagnostics15101253
Chicago/Turabian StylePoynard, Thierry, Olivier Deckmyn, Raluca Pais, Judith Aron-Wisnewsky, Valentina Peta, Pierre Bedossa, Frederic Charlotte, Maharajah Ponnaiah, Jean-Michel Siksik, Laurent Genser, and et al. 2025. "Three Neglected STARD Criteria Reduce the Uncertainty of the Liver Fibrosis Biomarker FibroTest-T2D in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" Diagnostics 15, no. 10: 1253. https://doi.org/10.3390/diagnostics15101253
APA StylePoynard, T., Deckmyn, O., Pais, R., Aron-Wisnewsky, J., Peta, V., Bedossa, P., Charlotte, F., Ponnaiah, M., Siksik, J.-M., Genser, L., Clement, K., Leanour, G., & Valla, D. (2025). Three Neglected STARD Criteria Reduce the Uncertainty of the Liver Fibrosis Biomarker FibroTest-T2D in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Diagnostics, 15(10), 1253. https://doi.org/10.3390/diagnostics15101253








