Morphological and Immunohistochemical Support for the Origin of the Carcinoid Component in Strumal Carcinoids: A Case Report and Literature Review
Abstract
1. Introduction
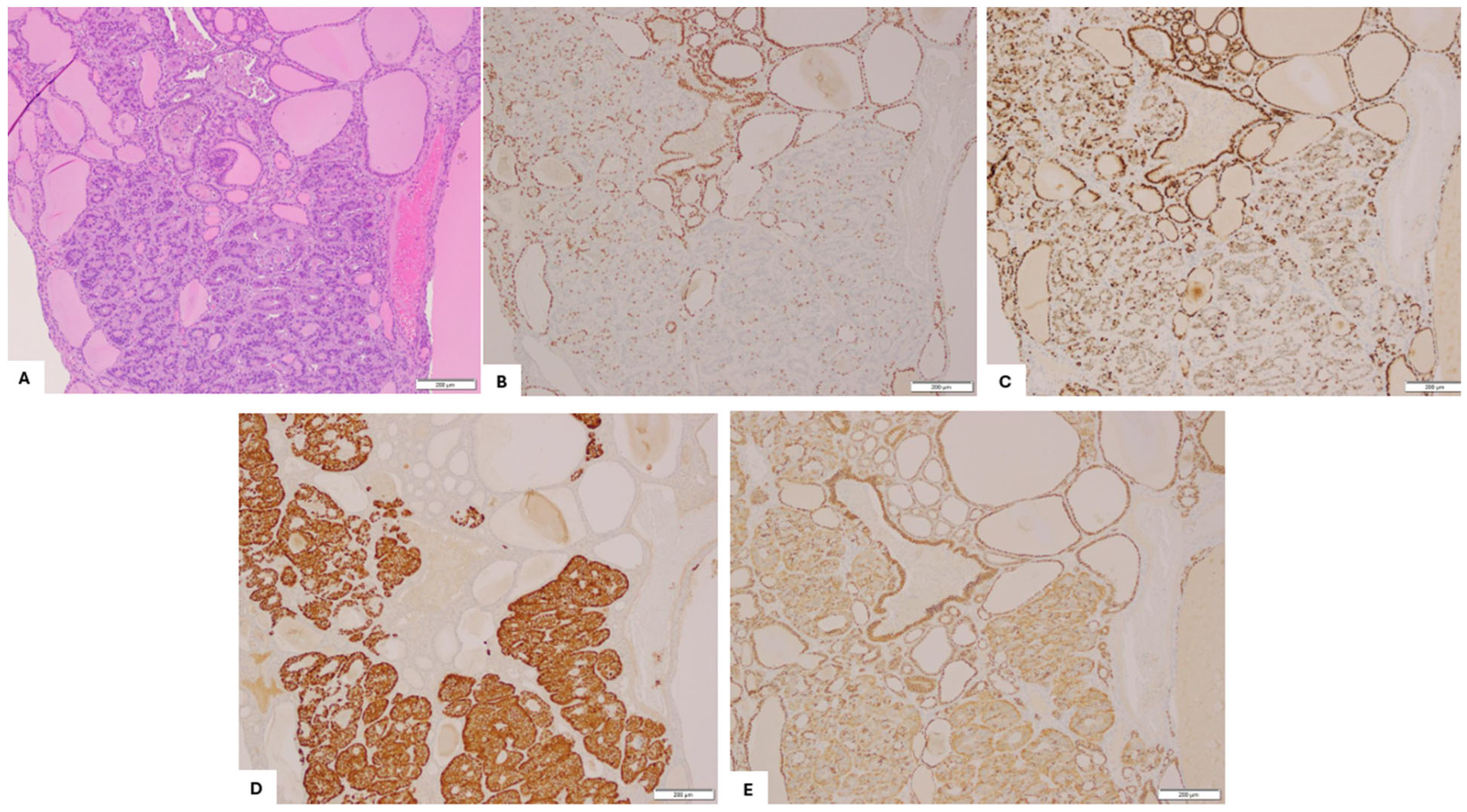
2. Case Presentation
3. Discussion and Literature Review
| Reference | Number of Cases | Immunostaining/Ultrastructural Findings in Strumal Component | Immunostaining/Ultrastructural Findings in Carcinoid Component | Proposed Carcinoid Origin Hypothesis |
|---|---|---|---|---|
| S. Robbo [1] | 50 |
|
| Support unified origin. |
| Takubo, K. et al., 1986 [3] | 1 |
|
| Support unified origin. |
| Tsubura, A., 1986 [4] | 1 |
|
| Support unified origin. Does not support origin from C-cells. |
| Stagno, P. et al., 1987 [5] | 6 |
|
| Support origin from thyroid tissue in strumal ovarii. |
| S. Theurer et al., 2020 [7] | 13 |
|
| Support origin from thyroid tissue in strumal ovarii. Do not support origin from C-cells. |
| L. Greco et al. 1979 [14] | 1 |
|
| Support origin from thyroid tissue in strumal ovarii. |
| M. Senterman et al., 1984 [15] | 1 |
|
| Support origin from thyroid tissue in strumal ovarii. Do not support origin from C-cells. |
| R. Snyder et al., 1986 [2] | 13 |
|
| Support origin from thyroid tissue in strumal ovarii. Do not support origin from C-cells. |
| Kimura, N. et al., 1986 [13] | 1 |
|
| Support origin from hybrid cells having both thyroid and neuroendocrine features. |
| S. Hamazaki et al., 2002 [6] | 2 |
|
| Divergent differentiation from a common progenitor. Do not support origin from C-cells. |
| K. Macháleková, 2018 [18] | 2 |
|
| Do not support origin from C-cells. |
| C. Apostol et al., 2017 [17] | 1 |
|
| Do not support origin from C-cells. |
| Blaustein, A., 1979 [8] | 1 | N/A |
| Support origin from C- cells. |
| Ulbright, T. et al., 1982 [9] | 2 |
|
| Support origin from C-cells. |
| A.Turla et al., 2022 [16] | 117 |
|
| Support origin from C-cells. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robboy, S.J.; Scully, R.E. Strumal carcinoid of the ovary: An analysis of 50 cases of a distinctive tumor composed of thyroid tissue and carcinoid. Cancer 1980, 46, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.R.; Tavassoli, F.A. Ovarian strumal carcinoid: Immunohistochemical, ultrastructural, and clinicopathologic observations. Int. J. Gynecol. Pathol. 1986, 5, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Takubo, K.; Yasui, H.; Toi, K. Strumal carcinoid of the ovary: A case report. Acta Pathol. Jpn. 1986, 36, 765–771. [Google Scholar]
- Tsubura, A.; Sasaki, M. Strumal carcinoid of the ovary: Ultrastructural and immunohistochemical study. Acta Pathol. Jpn. 1986, 36, 1383–1390. [Google Scholar]
- Stagno, P.A.; Petras, R.E.; Hart, W.R. Strumal carcinoids of the ovary: An immunohistologic and ultrastructural study. Arch. Pathol. Lab. Med. 1987, 111, 440–446. [Google Scholar]
- Hamazaki, S.; Okino, T.; Tsukayama, C.; Okada, S. Expression of thyroid transcription factor-1 in strumal carcinoid and struma ovarii: An immunohistochemical study. Pathol. Int. 2002, 52, 458–462. [Google Scholar] [CrossRef]
- Theurer, S.; Ingenwerth, M.; Herold, T.; Herrmann, K.; Schmid, K.W. Immunohistochemical Profile and 47-Gene Next-Generation Sequencing (NGS) Solid Tumor Panel Analysis of a Series of 13 Strumal Carcinoids. Endocr. Pathol. 2020, 31, 101–107. [Google Scholar] [CrossRef]
- Blaustein, A. Calcitonin secreting struma-carcinoid tumor of the ovary. Hum. Pathol. 1979, 10, 222–228. [Google Scholar] [CrossRef]
- Ulbright, T.M.; Roth, L.M.; Ehrlich, C.E. Ovarian strumal carcinoid: An immunocytochemical and ultrastructural study of two cases. Am. J. Clin. Pathol. 1982, 77, 622–631. [Google Scholar] [CrossRef]
- Hart, W.R.; Regezi, J.A. Strumal carcinoid of the ovary: Ultrastructural observations and long-term follow-up study. Am. J. Clin. Pathol. 1978, 69, 356–359. [Google Scholar] [CrossRef]
- Sarcevic, B.; Separovic, V.; Scukanec-Spoljar, M.; Pejsa, V. Strumal carcinoid tumor of the ovary—Histologic and electron microscopy characteristics of the tumor. Lijec. Vjesn. 1990, 112, 381–383. [Google Scholar]
- Scully, R.E. Recent progress in ovarian cancer. Hum. Pathol. 1970, 1, 73–98. [Google Scholar] [CrossRef]
- Kimura, N.; Sasano, N.; Namiki, T. Evidence of hybrid cell of thyroid follicular cell and carcinoid cell in strumal carcinoid. Int. J. Gynecol. Pathol. 1986, 5, 269–277. [Google Scholar] [CrossRef]
- Greco, M.A.; LiVolsi, V.A.; Pertschuk, L.P.; Bigelow, B. Strumal carcinoid of the ovary: An analysis of its components. Cancer 1979, 43, 1380–1388. [Google Scholar] [CrossRef]
- Senterman, M.K.; Cassidy, P.N.; Fenoglio, C.M.; Ferenczy, A. Histology, ultrastructure, and immunohistochemistry of strumal carcinoid: A case report. Int. J. Gynecol. Pathol. 1984, 3, 232–240. [Google Scholar] [CrossRef]
- Turla, A.; Zamparini, M.; Milione, M.; Grisanti, S.; Amoroso, V.; Pedersini, R.; Cosentini, D.; Berruti, A. Ovarian Strumal Carcinoid: Case Report, Systematic Literature Review and Pooled Analysis. Front. Endocrinol. 2022, 13, 871210. [Google Scholar] [CrossRef]
- Ciobanu Apostol, D.G.; BuTureanu, T.A.; Socolov, D.G.; Scripcaru, D.C.; Rosin, O.L.; Lozneanu, L. Ovarian strumal carcinoid—Case report. Rom. J. Morphol. Embryol. 2017, 58, 1035–1040. [Google Scholar]
- Machalekova, K.; Kolnikova, G.; Redecha, M.; Zubor, P.; Kajo, K. Strumal carcinoid of the ovary—Report of two cases and review of literature. Ceska Gynekol. 2018, 83, 452–457. [Google Scholar]
- Shia, J.; Tang, L.H.; Weiser, M.R.; Brenner, B.; Adsay, N.V.; Stelow, E.B.; Saltz, L.B.; Qin, J.; Landmann, R.; Leonard, G.D.; et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am. J. Surg. Pathol. 2008, 32, 719–731. [Google Scholar] [CrossRef]
- Jukic, Z.; Limani, R.; Luci, L.G.; Nikic, V.; Mijic, A.; Tomas, D.; Kruslin, B. hGH and GHR expression in large cell neuroendocrine carcinoma of the colon and rectum. Anticancer Res. 2012, 32, 3377–3381. [Google Scholar]
- Motoyama, T.; Katayama, Y.; Watanabe, H.; Okazaki, E.; Shibuya, H. Functioning ovarian carcinoids induce severe constipation. Cancer 1992, 70, 513–518. [Google Scholar] [CrossRef]
- Matsunami, K.; Takagi, H.; Ichigo, S.; Murase, T.; Ikeda, T.; Imai, A. Peptide YY producing strumal carcinoid tumor of the ovary. Eur. J. Gynaecol. Oncol. 2011, 32, 201–202. [Google Scholar] [PubMed]
- Noh, H.K.; Kwon, B.S.; Kim, Y.H.; Lee, N.K.; Choi, K.U.; Suh, D.S.; Lee, D.H.; Kim, K.H. Peptide YY producing strumal carcinoid tumor of the ovary in a postmenopausal woman: A rare cause of chronic constipation. Obstet. Gynecol. Sci. 2017, 60, 602–607. [Google Scholar] [CrossRef] [PubMed]


| Tumor Marker | Results | Reference Range |
|---|---|---|
| CA125 | 12.3 U/mL | <35 U/mL |
| CEA | 1.3 ng/mL | <5 ng/mL |
| CA19-9 | 7.7 U/mL | <35.1 U/mL |
| Inhibin B | 106 pg/mL | <261 pg/mL |
| AFP | 2.1 ng/mL | <15 ng/mL |
| LDH | 243 U/L | <201 U/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Feroze, A.; Yang, L.; Balakrishnan, R. Morphological and Immunohistochemical Support for the Origin of the Carcinoid Component in Strumal Carcinoids: A Case Report and Literature Review. Diagnostics 2025, 15, 1249. https://doi.org/10.3390/diagnostics15101249
Liu Y, Feroze A, Yang L, Balakrishnan R. Morphological and Immunohistochemical Support for the Origin of the Carcinoid Component in Strumal Carcinoids: A Case Report and Literature Review. Diagnostics. 2025; 15(10):1249. https://doi.org/10.3390/diagnostics15101249
Chicago/Turabian StyleLiu, Yu, Asra Feroze, Liz Yang, and Ridin Balakrishnan. 2025. "Morphological and Immunohistochemical Support for the Origin of the Carcinoid Component in Strumal Carcinoids: A Case Report and Literature Review" Diagnostics 15, no. 10: 1249. https://doi.org/10.3390/diagnostics15101249
APA StyleLiu, Y., Feroze, A., Yang, L., & Balakrishnan, R. (2025). Morphological and Immunohistochemical Support for the Origin of the Carcinoid Component in Strumal Carcinoids: A Case Report and Literature Review. Diagnostics, 15(10), 1249. https://doi.org/10.3390/diagnostics15101249






