Ultra-High-Frequency Ultrasound Mapping of the Superficial Circumflex Iliac and Superficial Inferior Epigastric Vessels: An Anatomical Study
Abstract
1. Introduction
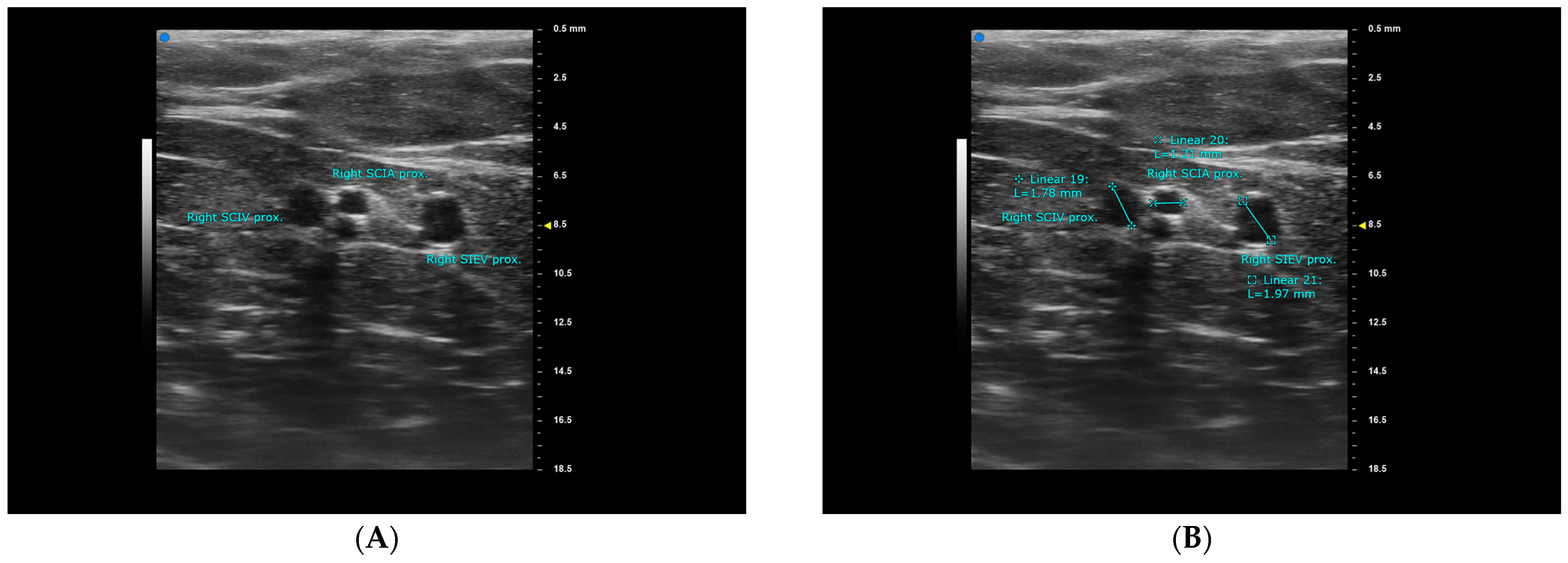
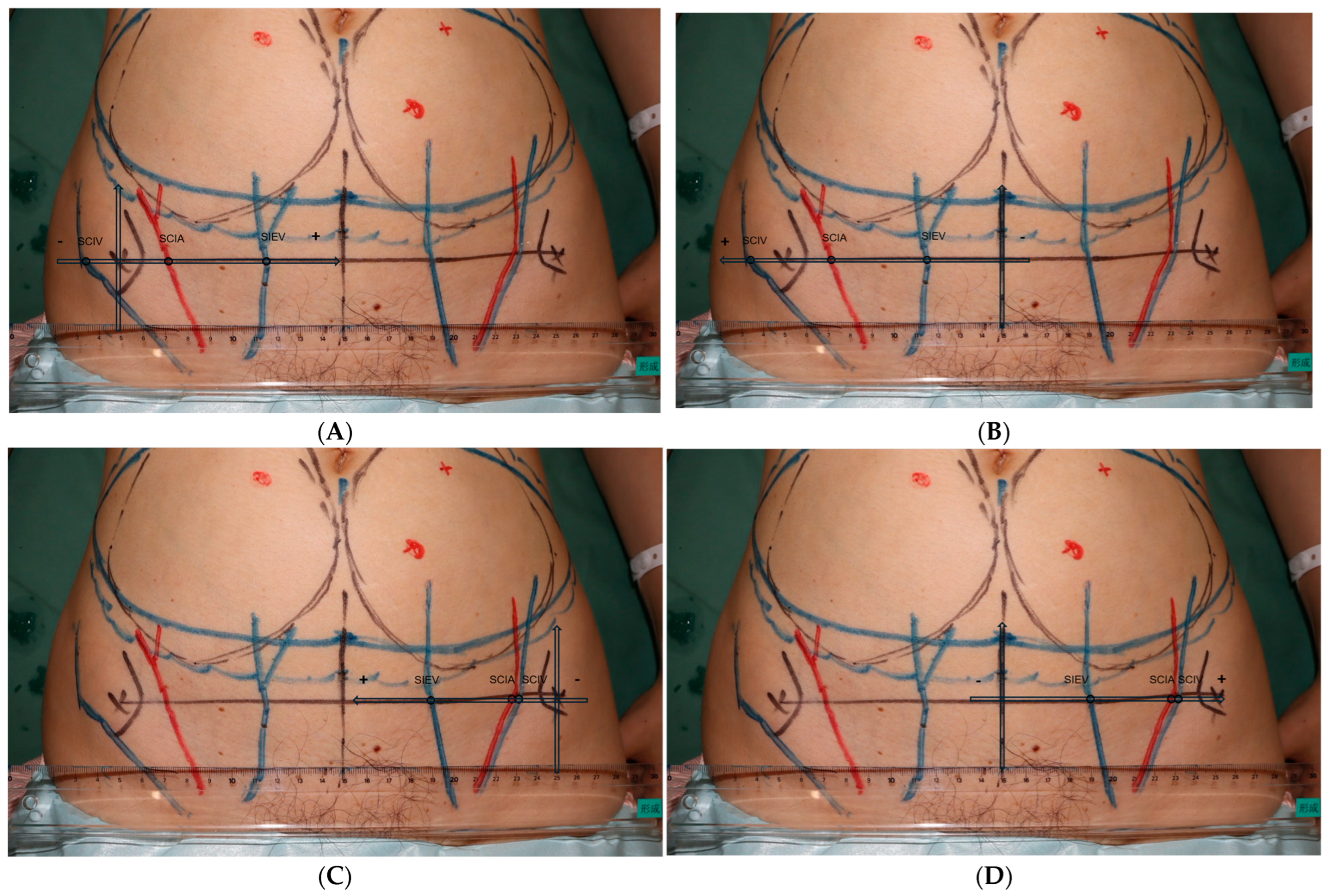
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshimatsu, H.; Yamamoto, T.; Hayashi, N.; Kato, M.; Iida, T.; Koshima, I. Reconstruction of the ankle complex wound with a fabricated superficial circumflex iliac artery chimeric flap including the sartorius muscle: A case report. Microsurgery 2017, 37, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Yoshimatsu, H.; Karakawa, R.; Fuse, Y.; Kuramoto, Y.; Shibata, T.; Suesada, N.; Miyashita, H. Use of a combined SIEA and SCIP based double pedicled abdominal flap for breast reconstruction. Microsurgery 2021, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Saito, T.; Ishiura, R.; Iida, T. Quadruple-component superficial circumflex iliac artery perforator (SCIP) flap: A chimeric SCIP flap for complex ankle reconstruction of an exposed artificial joint after total ankle arthroplasty. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2016, 69, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Daniel, B.W.; Rodriguez, J.R.; Kageyama, T.; Sakai, H.; Fuse, Y.; Tsukuura, R.; Yamamoto, N. Radical reduction and reconstruction for male genital elephantiasis: Superficial circumflex iliac artery perforator (SCIP) lymphatic flap transfer after elephantiasis tissue resection. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2022, 75, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.L.; Li, J.; Zhou, X.; Luo, X.C.; Zou, Y.G. Repair of multiple hand defects with superficial circumflex iliac artery perforator flap. Injury 2023, 54, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, N.J.; Taylor, D.M. Pedicled chimeric superficial circumflex iliac artery perforator (SCIP) flap with external oblique fascia for vesicocutaneous bladder fistula repair: A case report and literature review on the utility of pedicled chimeric SCIP. Microsurgery 2024, 44, e31138. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Sun, S.H.; Ben-Nakhi, M. Modified superficial circumflex iliac artery perforator flap and supermicrosurgery technique for lower extremity reconstruction: A new approach for moderate-sized defects. Ann. Plast. Surg. 2013, 71, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.L.H.; Park, S.W.; Cho, J.Y.; Choi, J.W.; Hong, J.P. The search for the ideal thin skin flap: Superficial circumflex iliac artery perforator flap--a review of 210 cases. Plast. Reconstr. Surg. 2015, 135, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, C.W.; Greyson, M.A.; Iorio, M.L. Superficial Circumflex Iliac Artery Perforator Flap Reconstruction of the Upper Extremity. Hand Clin. 2024, 40, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Satake, T.; Muto, M.; Kou, S.; Yasumura, K.; Ishikawa, T.; Maegawa, J. Contralateral unaffected breast augmentation using zone IV as a SIEA flap during unilateral DIEP flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2019, 72, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Grünherz, L.; Wolter, A.; Andree, C.; Grüter, L.; Staemmler, K.; Munder, B.; Schulz, T.; Stambera, P.; Hagouan, M.; Fleischer, O.; et al. Autologous Breast Reconstruction with SIEA Flaps: An Alternative in Selected Cases. Aesthetic Plast. Surg. 2020, 44, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Min, J.C.; Pak, C.J.; Hong, J.P.J.; Suh, H.P. Maximizing the Versatility of Thin Flap from the Groin Area as a Workhorse Flap: The Selective Use of Superficial Circumflex Iliac Artery Perforator (SCIP) Free Flap and Superficial Inferior Epigastric Artery (SIEA) Free Flap with Precise Preoperative Planning. J. Reconstr. Microsurg. 2023, 39, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Hayashi, N.; Yoshimatsu, H.; Yamamoto, T. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J. Surg. Oncol. 2018, 117, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, G.I.; Watson, N. One-stage repair of compound leg defects with free, revascularized flaps of groin skin and iliac bone. Plast. Reconstr. Surg. 1978, 61, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Steinbacher, J.; Meng, S.; Hamscha, U.M.; Weninger, W.J.; Tinhofer, I.E.; Harima, M.; Fuse, Y.; Yamamoto, T.; Tzou, C.H.J. Superficial Circumflex Iliac Artery Perforator Flap: An Anatomical Study of the Correlation of the Superficial and the Deep Branches of the Artery and Evaluation of Perfusion from the Deep Branch to the Sartorius Muscle and the Iliac Bone. Plast. Reconstr. Surg. 2019, 143, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Koshima, I.; Nanba, Y.; Tsutsui, T.; Takahashi, Y.; Urushibara, K.; Inagawa, K.; Hamasaki, T.; Moriguchi, T. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast. Reconstr. Surg. 2004, 113, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Mihara, M.; Yoshimatsu, H.; Narushima, M.; Koshima, I. Versatility of the superficial circumflex iliac artery perforator flap in head and neck reconstruction. Ann. Plast. Surg. 2014, 72, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Iida, T. Superficial circumflex iliac perforator (SCIP) flap: Variations of the SCIP flap and their clinical applications. J. Reconstr. Microsurg. 2014, 30, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.P.; Jeong, H.H.; Choi, D.H.; Hong, J. Study of the Medial Superficial Perforator of the Superficial Circumflex Iliac Artery Perforator Flap Using Computed Tomographic Angiography and Surgical Anatomy in 142 Patients. Plast. Reconstr. Surg. 2017, 139, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Yamamoto, T.; Hayashi, A.; Iida, T. Proximal-to-Distally Elevated Superficial Circumflex Iliac Artery Perforator Flap Enabling Hybrid Reconstruction. Plast. Reconstr. Surg. 2016, 138, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xi, W.; Zhang, Z.; Tremp, M.; Schaefer, D.J.; Sadigh, P.L.; Zhang, W.; Zhang, Y.X. A reappraisal of the surgical planning of the superficial circumflex iliac artery perforator flap. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2017, 70, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Fukunaga, Y.; Arikawa, M.; Kagaya, Y.; Miyamoto, S. Anatomy of the arterial and venous systems of the superficial inferior epigastric artery flap: A retrospective study based on computed tomographic angiography. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2020, 73, 870–875. [Google Scholar] [CrossRef] [PubMed]


| Patient number | 25 |
| Hemiabdomens | 50 |
| age (mean) | 51.1 |
| Range | 41−66 |
| Sex-female | 25 |
| BMI (mean) | 21.6 |
| Range | 18.4−30.4 |
| Hemiabdomen width (mm, from midline to ASIS) | 109.65 ± 7.50 * |
| Smoker | 5/25, 20% |
| No detectable superficical arteries on both sides | 0/25, 0% |
| Detectable SCIA or SIEA on one side only | 1/25, 4% |
| Detectable SCIA or SIEA on both sides | 24/25, 96% |
| Detectable SCIA and SIEA on one side | 8/25, 32% |
| Detectable SCIA and SIEA on both sides | 1/25, 4% |
| sSCIA | SCIA VC | SCIV | |
|---|---|---|---|
| detectable at ASIS level | 48/50, 96% | 43/50, 86% | 48/50, 96% |
| Average diameter at ASIS (mean ± SD, mm) | 0.76 ± 0.25 | 0.94 ± 0.37 | 1.72 ± 0.60 |
| Average depth at ASIS (mean ± SD, mm) | 5.42 ± 2.13 | 5.42 ± 2.13 | 3.66 ± 3.29 * |
| Average diameter when traced proximal (mean ± SD, mm) | 0.97 ± 0.25 | 1.34 ± 0.58 * | 2.07 ± 0.48 * |
| Average distance to midline (mean ± SD, mm) | 91.02 ± 15.89 | 101.58 ± 17.95 | |
| Average distance to ASIS (mean ± SD, mm) | 18.71 ± 15.44 (3.27−34.15 ǂ) | 10.46 ± 14.71 * (−4.25–25.17 ǂ) | |
| Superior-lateral pedicle course | 47/48, 98% | 48/48, 100% | |
| Superior pedicle course | 1/48, 2% | 0/48, 0% | |
| Superior-medial pedicle course | 0/48, 0% | 0/48, 0% | |
| Average SCIA-SCIV distance (mm) | 15.47 ± 19.10 * |
| SIEA | SIEA VC | SIEV | |
|---|---|---|---|
| detectable at ASIS level | 11/50, 22% | 8/50, 16% | 50/50, 100% |
| Average diameter at ASIS (mean ± SD, mm) | 0.63 ± 0.22 * | 0.64 ± 0.28 * | 2.18 ± 0.48 |
| Average depth at ASIS (mean ± SD, mm) | 4.94 ± 3.60 * | 4.94 ± 3.60 * | 3.46 ± 2.80 * |
| Average diameter when traced proximal (mean ± SD, mm) | 1.05 ± 0.20 * | 1.35 ± 1.06 * | 2.39 ± 0.57 |
| Average distance to midline (mean ± SD, mm) | 50.92 ± 22.26 * | 47.94 ± 10.11 (37.83−58.05 ǂ) | |
| Average distance to ASIS (mean ± SD, mm) | 51.32 ± 20.57 * | 62.63 ± 12.44 | |
| Superior-lateral pedicle course | 2/11, 18% | 2/50, 4% | |
| Superior pedicle course | 3/11, 27% | 10/50, 20% | |
| Superior-medial pedicle course | 6/11, 55% | 38/50, 76% | |
| Average SIEA-SIEV distance | 13.65 ± 14.88 * |
| Vessel Diameter (mm) * | Smoker (5 Patients) | Non-Smoker (20 Patients) | p Value (Mann-Whitney U Test) |
|---|---|---|---|
| sSCIA, ASIS | 0.63 ± 0.44 | 0.74 ± 0.32 | 0.78 |
| sSCIA, proximal | 0.90 ± 0.29 | 0.96 ± 0.45 | 0.96 |
| SCIV, ASIS | 1.97 ± 0.39 | 1.62 ± 1.03 | 0.35 |
| SCIV, proximal | 2.16 ± 0.28 | 2.01 ± 0.47 | 0.24 |
| SIEA, ASIS | 0.53 ± 0.12 | 0.71 ± 0.14 | 0.08 |
| SIEA, proximal | 1.08 ± 0.21 | 1.04 ± 0.14 | 0.93 |
| SIEV, ASIS | 2.30 ± 0.72 | 2.17 ± 0.79 | 0.25 |
| SIEV, proximal | 2.53 ± 0.65 | 2.25 ± 0.83 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, S.C.-H.; Karakawa, R.; Imai, H.; Kagimoto, S.; Seki, Y.; Suesada, N.; Yoshimatsu, H.; Yano, T. Ultra-High-Frequency Ultrasound Mapping of the Superficial Circumflex Iliac and Superficial Inferior Epigastric Vessels: An Anatomical Study. Diagnostics 2025, 15, 1210. https://doi.org/10.3390/diagnostics15101210
Kuo SC-H, Karakawa R, Imai H, Kagimoto S, Seki Y, Suesada N, Yoshimatsu H, Yano T. Ultra-High-Frequency Ultrasound Mapping of the Superficial Circumflex Iliac and Superficial Inferior Epigastric Vessels: An Anatomical Study. Diagnostics. 2025; 15(10):1210. https://doi.org/10.3390/diagnostics15101210
Chicago/Turabian StyleKuo, Spencer Chia-Hao, Ryo Karakawa, Hirofumi Imai, Shintaro Kagimoto, Yukio Seki, Nobuko Suesada, Hidehiko Yoshimatsu, and Tomoyuki Yano. 2025. "Ultra-High-Frequency Ultrasound Mapping of the Superficial Circumflex Iliac and Superficial Inferior Epigastric Vessels: An Anatomical Study" Diagnostics 15, no. 10: 1210. https://doi.org/10.3390/diagnostics15101210
APA StyleKuo, S. C.-H., Karakawa, R., Imai, H., Kagimoto, S., Seki, Y., Suesada, N., Yoshimatsu, H., & Yano, T. (2025). Ultra-High-Frequency Ultrasound Mapping of the Superficial Circumflex Iliac and Superficial Inferior Epigastric Vessels: An Anatomical Study. Diagnostics, 15(10), 1210. https://doi.org/10.3390/diagnostics15101210








