Evaluation of Retinal and Optic Nerve Parameters in Recovered COVID-19 Patients: Potential Neurodegenerative Impact on the Ganglion Cell Layer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ophthalmic Examination
- Best-Corrected Visual Acuity (BCVA), assessed using a standard Snellen chart.
- Intraocular pressure (IOP), measured with a Goldmann applanation tonometer.
- Anterior Segment Examination, performed using slit-lamp biomicroscopy to evaluate the cornea, anterior chamber, iris, and lens.
- Dilated Fundus Examination, conducted with a 90-diopter lens to assess the vitreous, retina, and optic nerve head.
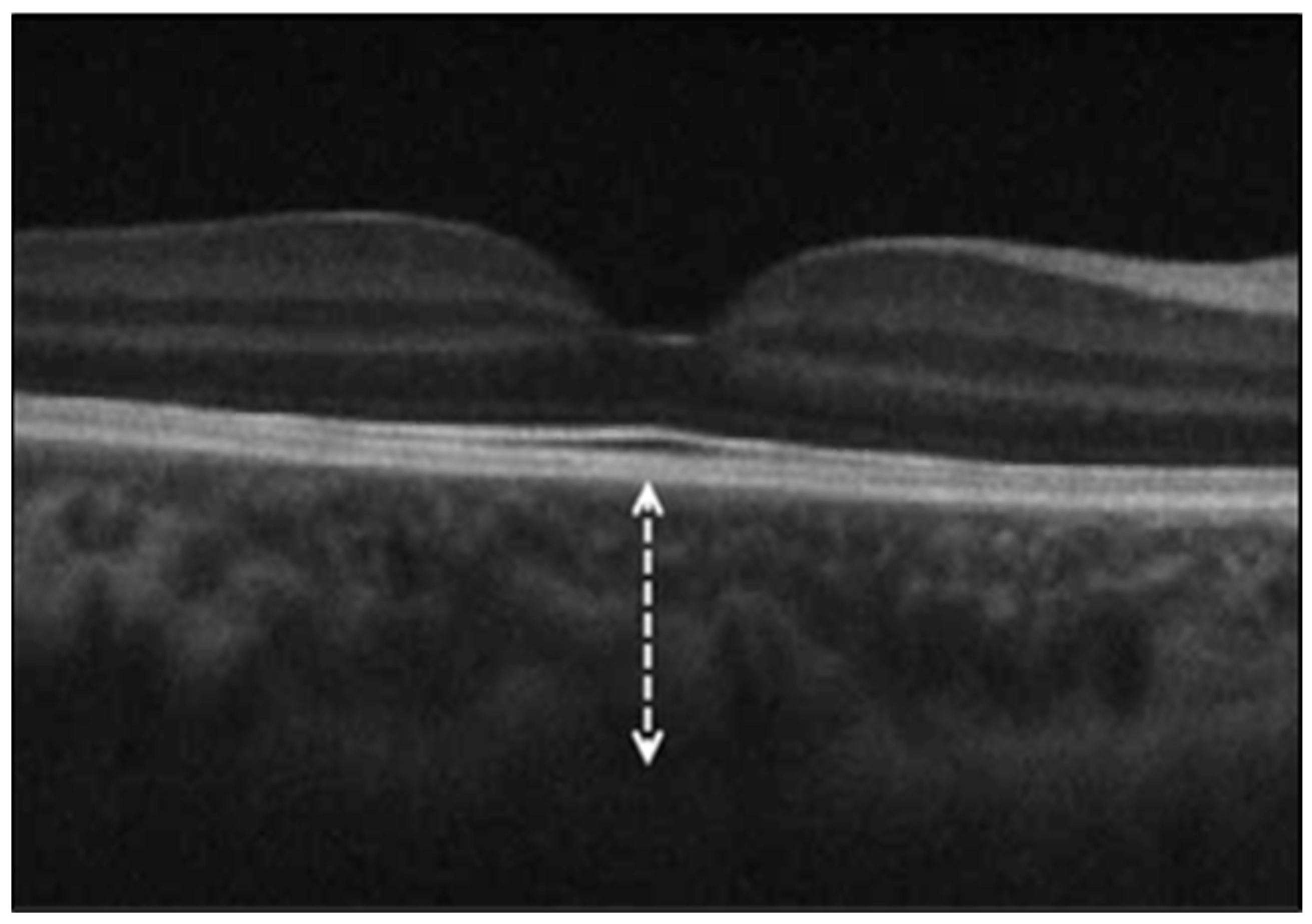
2.2. Optical Coherence Tomography (OCT) Measurements
- Peripapillary retinal nerve fiber layer thickness (RNFLT), measured using the Optic Disc Cube 200 × 200 protocol, which provides average thickness and sectoral measurements (temporal, superior, nasal, and inferior quadrants).
- Optic nerve head (ONH) parameters, evaluated using the same protocol, including rim area, disc area, average cup-to-disc ratio, and vertical cup-to-disc ratio.
- Ganglion cell–inner plexiform layer (GC-IPL) thickness, assessed with the Macular Cube 512 × 128 protocol, providing average and sectoral thicknesses.
2.3. Statistical Analyses
3. Results
3.1. Optic Nerve Head (ONH) Parameters and Retinal Nerve Fiber Layer Thickness (RNFLT)
3.2. Ganglion Cell–Inner Plexiform Layer (GC-IPL) Thickness
3.3. Choroidal Thickness (ChT)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R., Jr. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef] [PubMed]
- Riotto, E.; Mégevand, V.; Mégevand, A.; Marti, C.; Pugin, J.; Stangos, A.N.; Marconi Archinto, L.; Sunaric Mégevand, G. Retinal Manifestations in Patients with COVID-19: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 1828. [Google Scholar] [CrossRef]
- Invernizzi, A.; Torre, A.; Parrulli, S.; Zicarelli, F.; Schiuma, M.; Colombo, V.; Giacomelli, A.; Cigada, M.; Milazzo, L.; Ridolfo, A.; et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. eClinicalMedicine 2020, 27, 100550. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.; Kawasaki, A.; Eandi, C.M. Pathogenesis of Vascular Retinal Manifestations in COVID-19 Patients: A Review. Biomedicines 2022, 10, 2710. [Google Scholar] [CrossRef]
- Gold, D.M.; Galetta, S.L. Neuro-ophthalmologic complications of coronavirus disease 2019 (COVID-19). Neurosci. Lett. 2021, 742, 135531. [Google Scholar] [CrossRef]
- David, J.A.; Fivgas, G.D. Acute macular neuroretinopathy associated with COVID-19 infection. Am. J. Ophthalmol. Case Rep. 2021, 24, 101232. [Google Scholar] [CrossRef] [PubMed]
- Insausti-García, A.; Reche-Sainz, J.A.; Ruiz-Arranz, C.; López Vázquez, Á.; Ferro-Osuna, M. Papillophlebitis in a COVID-19 patient: Inflammation and hypercoagulable state. Eur. J. Ophthalmol. 2022, 32, NP168–NP172. [Google Scholar] [CrossRef] [PubMed]
- Benito-Pascual, B.; Gegúndez, J.A.; Díaz-Valle, D.; Arriola-Villalobos, P.; Carreño, E.; Culebras, E.; Rodríguez-Avial, I.; Benitez-Del-Castillo, J.M. Panuveitis and Optic Neuritis as a Possible Initial Presentation of the Novel Coronavirus Disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 2020, 28, 922–925. [Google Scholar] [CrossRef]
- Ahmad Mulyadi Lai, H.I.; Chou, S.-J.; Chien, Y.; Tsai, P.-H.; Chien, C.-S.; Hsu, C.-C.; Jheng, Y.-C.; Wang, M.-L.; Chiou, S.-H.; Chou, Y.-B.; et al. Expression of Endogenous Angiotensin-Converting Enzyme 2 in Human Induced Pluripotent Stem Cell-Derived Retinal Organoids. Int. J. Mol. Sci. 2021, 22, 1320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Y.; Wang, Q.Y.; Li, J. Ocular neuroinflammatory response secondary to SARS-CoV-2 infection-a review. Front. Immunol. 2025, 16, 1515768. [Google Scholar] [CrossRef]
- Lin, T.P.H.; Ko, C.-N.; Zheng, K.; Lai, K.H.W.; Wong, R.L.M.; Lee, A.; Zhang, S.; Huang, S.S.; Wan, K.H.; Lam, D.S.C. COVID-19: Update on Its Ocular Involvements, and Complications from Its Treatments and Vaccinations. Asia-Pac. J. Ophthalmol. 2021, 10, 521–529. [Google Scholar] [CrossRef]
- Alonso, R.; Gonzalez-Moron, D.; Garcea, O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: A review. Mult. Scler. Relat. Disord. 2018, 22, 77–82. [Google Scholar] [CrossRef]
- Monu, M.; Ahmad, F.; Olson, R.M.; Balendiran, V.; Singh, P.K. SARS-CoV-2 infects cells lining the blood-retinal barrier and induces a hyperinflammatory immune response in the retina via systemic exposure. PLoS Pathog. 2024, 20, e1012156. [Google Scholar] [CrossRef]
- Menuchin-Lasowski, Y.; Schreiber, A.; Lecanda, A.; Mecate-Zambrano, A.; Brunotte, L.; Psathaki, O.E.; Ludwig, S.; Rauen, T.; Schöler, H.R. SARS-CoV-2 infects and replicates in photoreceptor and retinal ganglion cells of human retinal organoids. Stem Cell Rep. 2022, 17, 789–803. [Google Scholar] [CrossRef]
- Ashander, L.M.; Lumsden, A.L.; Ma, Y.; Tan, A.C.R.; Appukuttan, B.; Daniel, S.; Michael, M.Z.; Smith, J.R. Brief research report: Transcriptional blockade of angiotensin converting enzyme 2 modelled in human retinal pigment epithelial cells. Front. Drug Discov. 2024, 4, 1416728. [Google Scholar] [CrossRef]
- McQuaid, C.; Montagne, A. SARS-CoV-2 and vascular dysfunction: A growing role for pericytes. Cardiovasc. Res. 2022, 119, 2591–2593. [Google Scholar] [CrossRef] [PubMed]
- Raony, Í.; Saggioro de Figueiredo, C. Retinal outcomes of COVID-19: Possible role of CD147 and cytokine storm in infected patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 165, 108280. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Ara, J.R.; Martin, J.; Almarcegui, C.; Dolz, I.; Vilades, E.; Gil-Arribas, L.; Fernandez, F.J.; Polo, V.; Larrosa, J.M.; et al. Retinal and Optic Nerve Degeneration in Patients with Multiple Sclerosis Followed up for 5 Years. Ophthalmology 2017, 124, 688–696. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Li, Z.; Zhang, X.; Wu, Y.; Yang, H.; Min, B.; Zhang, X.; Ma, D.; Lu, Y. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Kal, M.; Brzdek, M.; Karska-Basta, I.; Rzymski, P.; Pinna, A.; Mackiewicz, J.; Odrobina, D.; Winiarczyk, M.; Zarebska-Michaluk, D. Changes in macular ganglion cell and retinal nerve fiber layer thickness during recovery from infection with the B.1.1.7 variant of SARS-CoV-2 in previously hospitalized patients with COVID-19 bilateral pneumonia. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2024, 75, 489–498. [Google Scholar] [CrossRef]
- Gedik, B.; Yuksel, O.; Kazim Erol, M.; Duman, F.; Dogan, B.; Suren, E.; Yavuz, S. Evaluation of the retina, choroid and optic disc vascular structures in individuals with a history of COVID-19. J. Francais D Ophtalmol. 2024, 47, 104014. [Google Scholar] [CrossRef]
- Sumer, F.; Subasi, S. Effects of COVID-19 on Retinal and Choroidal Thickness by Optical Coherence Tomography. Eur. J. Gastroenterol. Hepatol. 2023, 32, 569–574. [Google Scholar] [CrossRef]
- Abrishami, M.; Hassanpour, K.; Hosseini, S.; Shoeibi, N.; Ansari-Astaneh, M.R.; Emamverdian, Z.; Gharib, B.; Amini, N.; Abrishami, M. Peripapillary Nerve Fiber Layer Thickness and Optic Nerve Head Parameters in Patients Recovered from COVID-19: A Longitudinal Study. J. Ophthalmol. 2022, 2022, 4643973. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Donate-Lopez, J.; Vidal-Villegas, B.; García-Feijóo, J. Optic nerve analysis in COVID-19 patients. J. Med. Virol. 2021, 93, 190–191. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Vidal-Villegas, B.; Martinez-de-la-Casa, J.M.; Donate-Lopez, J.; Martín-Sánchez, F.J.; González-Armengol, J.J.; Porta-Etessam, J.; Martin, J.L.R.; Garcia-Feijoo, J. Optic nerve and macular optical coherence tomography in recovered COVID-19 patients. Eur. J. Ophthalmol. 2022, 32, 628–636. [Google Scholar] [CrossRef]
- Dağ Şeker, E.; Timur, İ.E.E. Assessment of early and long-COVID related retinal neurodegeneration with optical coherence tomography. Int. Ophthalmol. 2023, 43, 2073–2081. [Google Scholar] [CrossRef]
- Ozmen, S.; Cakir, B.; Okan, H.D.; Aksoy, N.O.; Guclu, E. The effect of COVID-19 infection on retinal nerve fiber layer and ganglion cell complex layer thicknesses. Exp. Biomed. Res. 2021, 4, 175–180. [Google Scholar] [CrossRef]
- Meagan, S.; Saadeh-Jackson, S.; Kim, L.A.; Tripathy, K.; Randolph, J.; Ichlangod, A.M. Retinal Manifestations of COVID-19. 2025. Available online: https://eyewiki.org/Retinal_Manifestations_of_COVID-19 (accessed on 15 March 2025).
- Li, J.; Mao, N.; Wang, Y.; Deng, S.; Chen, K. Novel insights into the ROCK-JAK-STAT signaling pathway in upper respiratory tract infections and neurodegenerative diseases. Mol. Ther. 2025, 33, 32–50. [Google Scholar] [CrossRef]
- Enright, J.; Van Stavern, G. Application of optical coherence tomography in hereditary, toxic and metabolic optic neuropathies. Ann. Eye Sci. 2020, 5, 17. [Google Scholar] [CrossRef]
- Sachdeva, M.M. Retinal Neurodegeneration in Diabetes: An Emerging Concept in Diabetic Retinopathy. Curr. Diabetes Rep. 2021, 21, 65. [Google Scholar] [CrossRef]
- Dag Seker, E.; Erbahceci Timur, I.E. COVID-19: More than a respiratory virus, an optical coherence tomography study. Int. Ophthalmol. 2021, 41, 3815–3824. [Google Scholar] [CrossRef]
- Wang, Y.; Detrick, B.; Yu, Z.X.; Zhang, J.; Chesky, L.; Hooks, J.J. The role of apoptosis within the retina of coronavirus-infected mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3011–3018. [Google Scholar]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Girkin, C.A.; Leung, C.K.; Liebmann, J.M.; Peace, J.H.; Werner, J.S.; Wollstein, G. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7872–7879. [Google Scholar] [CrossRef]
- Sen, S.; Saxena, R.; Tripathi, M.; Vibha, D.; Dhiman, R. Neurodegeneration in Alzheimer’s disease and glaucoma: Overlaps and missing links. Eye 2020, 34, 1546–1553. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, S.; Chen, H.; Yang, J.; Chen, Y. New findings on retinal microvascular changes in patients with primary COVID-19 infection: A longitudinal study. Front. Immunol. 2024, 15, 1404785. [Google Scholar] [CrossRef]
- Casagrande, M.; Fitzek, A.; Püschel, K.; Aleshcheva, G.; Schultheiss, H.P.; Berneking, L.; Spitzer, M.S.; Schultheiss, M. Detection of SARS-CoV-2 in Human Retinal Biopsies of Deceased COVID-19 Patients. Ocul. Immunol. Inflamm. 2020, 28, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Özer, Ö.; Güçlü, E.S. Optical Coherence Tomography Analysis of Retinal Thickness in COVID-19 Patients. Kocatepe Tıp Derg. 2024, 25, 241–245. [Google Scholar] [CrossRef]
- Gündoğan, M.; Vural, E.; Bayram, N.; Altunel, O.; Gündoğan, F.; Göktaş, S. Change in retinal vessel diameter and choroidal thickness in patients with severe COVID-19: Change in Retinal Parameters in Patients with Severe COVID-19. Photodiagnosis Photodyn. Ther. 2022, 37, 102674. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.; Karahan, M.; Ava, S.; Dursun, M.E.; Dursun, B.; Hazar, L.; Bozarslan Senol, B.; Keklikci, U. Evaluation of choroidal thickness in patients who have recovered from COVID-19. Int. Ophthalmol. 2022, 42, 841–846. [Google Scholar] [CrossRef]
- Hepokur, M.; Gunes, M.; Durmus, E.; Aykut, V.; Esen, F.; Oguz, H. Long-term follow-up of choroidal changes following COVID-19 infection: Analysis of choroidal thickness and choroidal vascularity index. Can. J. Ophthalmol. 2023, 58, 59–65. [Google Scholar] [CrossRef]
| Control Group | COVID-19 Group | p Value | |
|---|---|---|---|
| ONH parameters | |||
| Rim area (mm2) | 1.48 ± 0.29 | 1.45 ± 0.26 | 0.433 |
| Disc area (mm2) | 1.89 ± 0.27 | 1.87 ± 0.32 | 0.606 |
| Average C/D ratio | 0.42 ± 0.15 | 1.41 ± 0.18 | 0.741 |
| Vertical C/D ratio | 0.40 ± 0.14 | 1.38 ± 0.18 | 0.596 |
| Control Group | COVID-19 Group | p Value | |
| RNFL Thickness (μm) | |||
| Average | 96.42 ± 10.19 | 93.16 ± 9.89 | 0.071 |
| Superior | 119.66 ± 13.30 | 122.69 ± 17.96 | 0.267 |
| Inferior | 122.28 ± 17.11 | 127.10 ± 15.44 | 0.093 |
| Nasal | 68.07 ± 9.38 | 70.75 ± 13.03 | 0.172 |
| Temporal | 67.08 ± 15.88 | 65.90 ± 11.57 | 0.622 |
| Control Group | COVID-19 Group | p Value | |
|---|---|---|---|
| GC-IPL Thickness (μm) | |||
| Average | 78.38 ± 16.21 | 84.61 ± 8.34 | 0.012 |
| Minimum | 74.36 ± 18.41 | 81.42 ± 10.31 | 0.014 |
| Superior | 78.50 ± 17.68 | 85.82 ± 8.84 | 0.007 |
| Superonasal | 79.50 ± 16.00 | 86.12 ± 10.13 | 0.010 |
| Superotemporal | 76.83 ± 16.91 | 84.03 ± 8.24 | 0.005 |
| Inferior | 77.00 ± 16.13 | 82.71 ± 8.89 | 0.022 |
| Inferonasal | 79.49 ± 15.54 | 84.73 ± 8.93 | 0.030 |
| Inferotemporal | 78.74 ± 16.77 | 84.53 ± 8.37 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaim, M.; Kır, M.B.; Uzun, F.; Findik, H. Evaluation of Retinal and Optic Nerve Parameters in Recovered COVID-19 Patients: Potential Neurodegenerative Impact on the Ganglion Cell Layer. Diagnostics 2025, 15, 1195. https://doi.org/10.3390/diagnostics15101195
Kaim M, Kır MB, Uzun F, Findik H. Evaluation of Retinal and Optic Nerve Parameters in Recovered COVID-19 Patients: Potential Neurodegenerative Impact on the Ganglion Cell Layer. Diagnostics. 2025; 15(10):1195. https://doi.org/10.3390/diagnostics15101195
Chicago/Turabian StyleKaim, Muhammet, Muhammet Bahattin Kır, Feyzahan Uzun, and Hüseyin Findik. 2025. "Evaluation of Retinal and Optic Nerve Parameters in Recovered COVID-19 Patients: Potential Neurodegenerative Impact on the Ganglion Cell Layer" Diagnostics 15, no. 10: 1195. https://doi.org/10.3390/diagnostics15101195
APA StyleKaim, M., Kır, M. B., Uzun, F., & Findik, H. (2025). Evaluation of Retinal and Optic Nerve Parameters in Recovered COVID-19 Patients: Potential Neurodegenerative Impact on the Ganglion Cell Layer. Diagnostics, 15(10), 1195. https://doi.org/10.3390/diagnostics15101195






