Inferior Frontal Sulcal Hyperintensities on Brain MRI Are Associated with Amyloid Positivity beyond Age—Results from the Multicentre Observational DELCODE Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Measurements
2.3. Brain MRI Acquisition and Processing
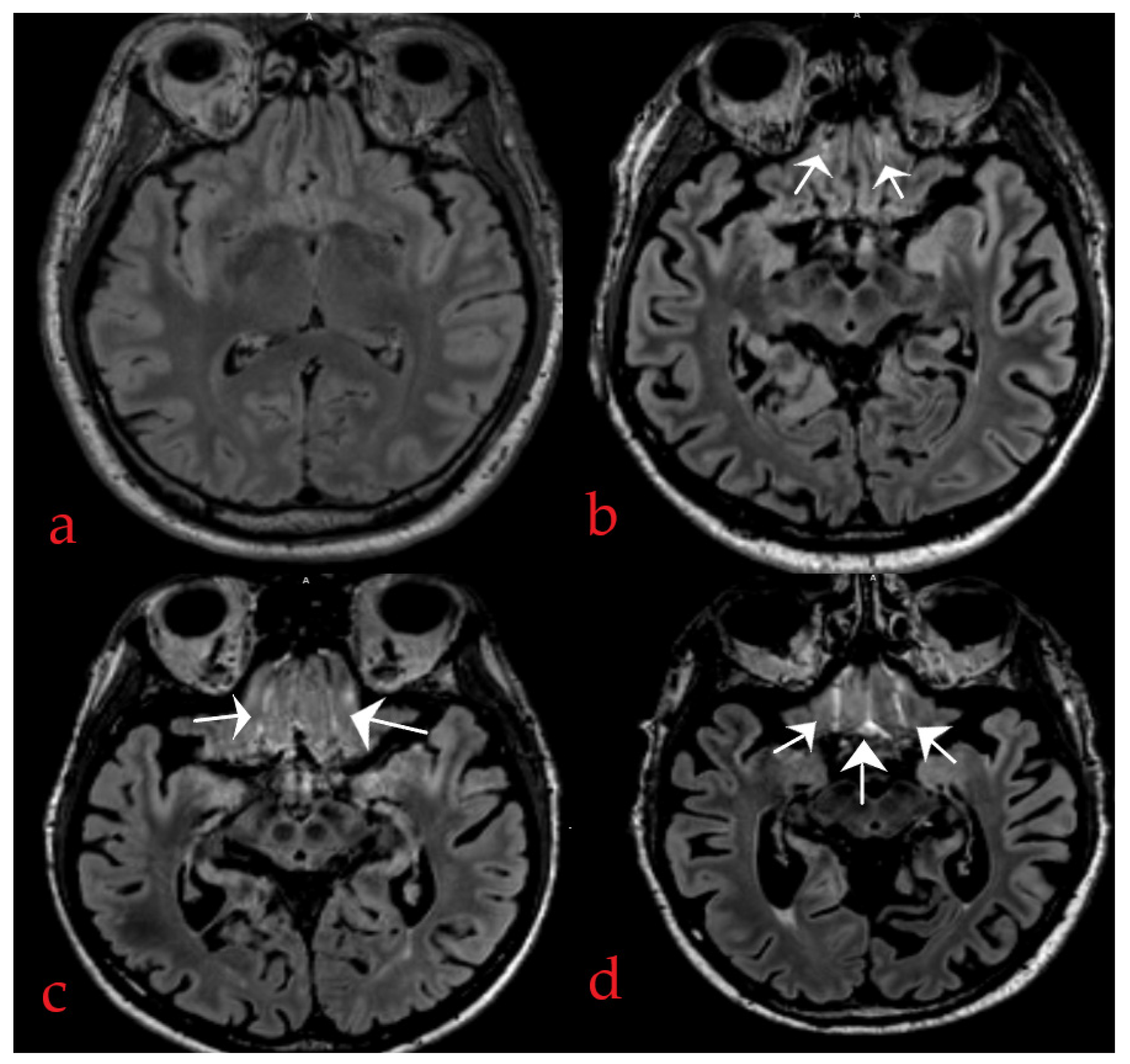
2.4. Method of IFSHs Rating
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Sample
3.2. Associations between IFSHs and AD Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wardlaw, J.M.; Liebeskind, D.S. Not Just Blood: Brain Fluid Systems and Their Relevance to Cerebrovascular Diseases. Stroke 2022, 53, 1399–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Lim, H.F.; Chappell, F.M.; Clancy, U.; Wiseman, S.; Valdés-Hernandéz, M.C.; Garcia, D.J.; Bastin, M.E.; Doubal, F.N.; Hewins, W.; et al. Relationship between inferior frontal sulcal hyperintensities on brain MRI, ageing and cerebral small vessel disease. Neurobiol. Aging 2021, 106, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntyre, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xie, L.; Zhang, Y.; Wu, X.; Hong, H.; Zhang, R.; Zeng, Q.; Li, K.; Luo, X.; Zhang, M.; et al. Inferior Frontal Sulcal Hyperintensity on FLAIR Is Associated with Small Vessel Disease but not Alzheimer’s Pathology. J. Alzheimer’s Dis. 2023, 92, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Rowe, C.C. Long night’s journey into the day: Amyloid-β imaging in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S349–S359. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Spottke, A.; Boecker, H.; Brosseron, F.; Buerger, K.; Catak, C.; Fliessbach, K.; Franke, C.; Fuentes, M.; Heneka, M.T.; et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimer’s Res. Ther. 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging—Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging—Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, J.L.; Rabin, L.A.; Amariglio, R.; Buckley, R.; Dubois, B.; Ellis, K.A.; Ewers, M.; Hampel, H.; Klöppel, S.; Rami, L.; et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. 2017, 13, 296–311. [Google Scholar] [CrossRef]
- Bernal, J.; Schreiber, S.; Menze, I.; Ostendorf, A.; Pfister, M.; Geisendörfer, J.; Nemali, A.; Maass, A.; Yakupov, R.; Peters, O.; et al. Arterial hypertension and β-amyloid accumulation have spatially overlapping effects on posterior white matter hyperintensity volume: A cross-sectional-study. Alzheimer’s Res. Ther. 2023, 15, 97. [Google Scholar] [CrossRef]
- Papp, K.V.; Rentz, D.M.; Orlovsky, I.; Sperling, R.A.; Mormino, E.C. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: The PACC5. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Wolfsgruber, S.; Kleineindam, L.; Guski, J.; Polcher, A.; Frommann, I.; Roeske, S.; Spruth, E.J.; Franke, C.; Priller, J.; Kilimann, I.; et al. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 2020, 95, e1134–e1143. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Wolfsgruber, S.; Kleineindam, L.; Spottke, A.; Altenstein, S.; Bartels, C.; Berger, M.; Brosserion, F.; Daamen, M.; Dichgans, M.; et al. Subjective cognitive decline and stage 2 of Alzheimer disease in patients from memory centers. Alzheimer’s Dement. 2022, 19, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzmann, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.F.; Zhang, J.-F.; Wardlaw, J. User Guide for Inferior Frontal Sulcal Hyperintensity (IFSH) Scale and Related Template; Mendeley Data, V1; Mendeley Ltd.: London, UK, 2021. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Laird, A.R.; Eickhoff, S.B.; Martinez, M.J.; Fox, P.M.; Fox, P.T. Automated regional behavioral analysis for human brain images. Front. Neuroinform. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Kutner, M.H. Applied Linear Statistical Models; McGraw-Hill Irwin: Boston, MA, USA, 2005; p. 410. [Google Scholar]
- Weller, R.O.; Djuanada, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, J.; Zhang, W.; Gong, X.; Yan, S.; Zhang, K.; Luo, Z.; Sun, J.; Jiang, Q.; Lou, M. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann. Neurol. 2020, 87, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Vibha, D.; Tiemeier, H.; Mirza, S.S.; Adams, H.H.H.; Niessen, W.J.; Hofman, A.; Prasad, K.; van der Lugt, A.; Vernooij, M.W.; Ikram, M.A. Brain volumes and longitudinal cognitive change: A population-based study. Alzheimer Dis. Assoc. Disord. 2018, 32, 43–49. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The glymphatic system and waste clearance with brain aging: A review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Ringstad, G.; Vatnehol, S.A.S.; Eide, P.K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017, 140, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- De Leon, M.J.; Li, Y.; Okamura, N.; Tsui, W.H.; Saint-Louis, L.A.; Glodzik, L.; Osorio, R.S.; Fortea, J.; Butler, T.; Pirraglia, E.; et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J. Nucl. Med. 2017, 58, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Palmqvist, S.; Stomrud, E.; Vogel, J.; Hansson, O. Staging β-Amyloid Pathology with Amyloid Positron Emission Tomography. JAMA Neurol. 2019, 76, 1319–1329. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The meningeal lymphatic system: A new player in neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef]
- Goulay, R.; Mena, R.L.; Hol, E.M.; Dijkhuizen, R.M. From stroke to dementia: A comprehensive review exposing tight interactions between stroke and amyloid-β formation. Transl. Stroke Res. 2020, 11, 601–614. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglial responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Herz, J.; Wall, M.; Dykstra, T.; De Lima, K.A.; Norris, G.T.; Dabhi, N.; Kennedy, T.; Baker, W.; Kipnis, J. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and amyloid-β pathology. Sci. Adv. 2021, 7, eabe4601. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.T.; Mielke, M.M. Sex Differences in Alzheimer’s disease. Neurol. Clin. 2023, 41, 343–358. [Google Scholar] [CrossRef]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.L.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C.; et al. Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.A.; Huston, J., III; Ward, H.A. Imaging Artifacts at 3.0T. J. Magn. Reson. Imaging 2006, 24, 735–746. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 361) | AD Dementia (n = 46) | MCI (n = 79) | SCD (n = 156) | NC (n = 80) | p-Value (p < 0.05) | |
|---|---|---|---|---|---|---|
| Age, y | 70.97 (5.78) | 74.78 (5.85) | 71.56 (5.56) | 70.55 (5.79) | 69.0 (4.84) | <0.001 |
| Male, n (%) | 186 (51.50) | 16 (34.8) | 44 (55.7) | 88 (56.4) | 38 (47.5) | 0.052 |
| Years of education | 14.35 (2.97) | 13.11 (3.11) | 13.73 (2.84) | 14.98 (2.96) | 14.43 (2.72) | <0.001 |
| Arterial hypertension, n (%) | 193 (54.51) | 27 (58.7) | 43 (56.57) | 86 (56.57) | 37 (46.3) | 0.404 |
| MMSE total score | 28.13 (2.63) | 23.02 (3.37) | 27.80 (0.34) | 29.15 (1.08) | 29.40 (0.82) | <0.001 |
| Aβ positivity, n (%) | 158 (43.8) | 41 (89.1) | 47 (59.5) | 52 (33.3) | 18 (22.5) | <0.001 |
| p-tau positivity, n (%) | 89 (24.70) | 32 (69.6) | 30 (38.0) | 22 (14.1) | 5 (6.3) | <0.001 |
| AD pathology (yes), n (%) | 79 (21.90) | 32 (69.6) | 28 (35.40) | 16 (10.3) | 3 (3.8) | <0.001 |
| IFSH score | ||||||
| Right sulcus | 1.10 (0.74) | 1.35 (0.76) | 1.08 (0.69) | 1.08 (0.79) | 1.02 (0.67) | 0.106 |
| Central sulcus | 0.80 (0.53) | 0.89 (0.43) | 0.77 (0.45) | 0.79 (0.60) | 0.79 (0.54) | 0.651 |
| Left sulcus | 1.21 (0.80) | 1.39 (0.71) | 1.04 (0.68) | 1.28 (0.87) | 1.14 (0.77) | 0.048 |
| Total IFSH sum score | 3.11 (1.49) | 3.63 (1.25) | 2.87 (1.36) | 3.15 (1.61) | 2.95 (1.42) | 0.034 |
| Step 1 Univariate | Step 2 Multivariable | Step 3 Multivariable | ||||
|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age | 1.07 (1.02 to 1.11) | 0.002 | 1.05 (1.00 to 1.10) | 0.020 | 1.07 (1.02 to 1.11) | 0.003 |
| Male sex | 0.49 (0.30 to 0.80) | 0.005 | 0.44 (0.26 to 0.76) | 0.004 | 0.46 (0.27 to 0.80) | 0.006 |
| Years of education | 0.91 (0.84 to 0.99) | 0.042 | 0.97 (0.89 to 1.06) | 0.641 | 0.96 (0.88 to 1.05) | 0.471 |
| Arterial hypertension | 1.67 (1.02 to 2.74) | 0.041 | 1.55 (0.94 to 2.57) | 0.084 | 1.53 (0.93 to 2.53) | 0.093 |
| Aβ positivity | 2.33 (1.41 to 3.86) | <0.001 | 1.95 (1.05 to 3.59) | 0.032 | ||
| p-tau positivity | 1.99 (1.14 to 3.46) | 0.014 | 1.12 (0.57 to 2.18) | 0.727 | ||
| AD pathology | 1.84 (1.04 to 3.28) | 0.035 | 1.40 (0.76 to 2.59) | 0.276 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörner, M.; Seebach, K.; Heneka, M.T.; Menze, I.; von Känel, R.; Euler, S.; Schreiber, F.; Arndt, P.; Neumann, K.; Hildebrand, A.; et al. Inferior Frontal Sulcal Hyperintensities on Brain MRI Are Associated with Amyloid Positivity beyond Age—Results from the Multicentre Observational DELCODE Study. Diagnostics 2024, 14, 940. https://doi.org/10.3390/diagnostics14090940
Dörner M, Seebach K, Heneka MT, Menze I, von Känel R, Euler S, Schreiber F, Arndt P, Neumann K, Hildebrand A, et al. Inferior Frontal Sulcal Hyperintensities on Brain MRI Are Associated with Amyloid Positivity beyond Age—Results from the Multicentre Observational DELCODE Study. Diagnostics. 2024; 14(9):940. https://doi.org/10.3390/diagnostics14090940
Chicago/Turabian StyleDörner, Marc, Katharina Seebach, Michael T. Heneka, Inga Menze, Roland von Känel, Sebastian Euler, Frank Schreiber, Philipp Arndt, Katja Neumann, Annkatrin Hildebrand, and et al. 2024. "Inferior Frontal Sulcal Hyperintensities on Brain MRI Are Associated with Amyloid Positivity beyond Age—Results from the Multicentre Observational DELCODE Study" Diagnostics 14, no. 9: 940. https://doi.org/10.3390/diagnostics14090940
APA StyleDörner, M., Seebach, K., Heneka, M. T., Menze, I., von Känel, R., Euler, S., Schreiber, F., Arndt, P., Neumann, K., Hildebrand, A., John, A.-C., Tyndall, A., Kirchebner, J., Tacik, P., Jansen, R., Grimm, A., Henneicke, S., Perosa, V., Meuth, S. G., ... Bernal, J. (2024). Inferior Frontal Sulcal Hyperintensities on Brain MRI Are Associated with Amyloid Positivity beyond Age—Results from the Multicentre Observational DELCODE Study. Diagnostics, 14(9), 940. https://doi.org/10.3390/diagnostics14090940







