Updates on the Applications of Spectral Computed Tomography for Musculoskeletal Imaging
Abstract
1. Introduction
2. Methods
3. Overview of Spectral CT
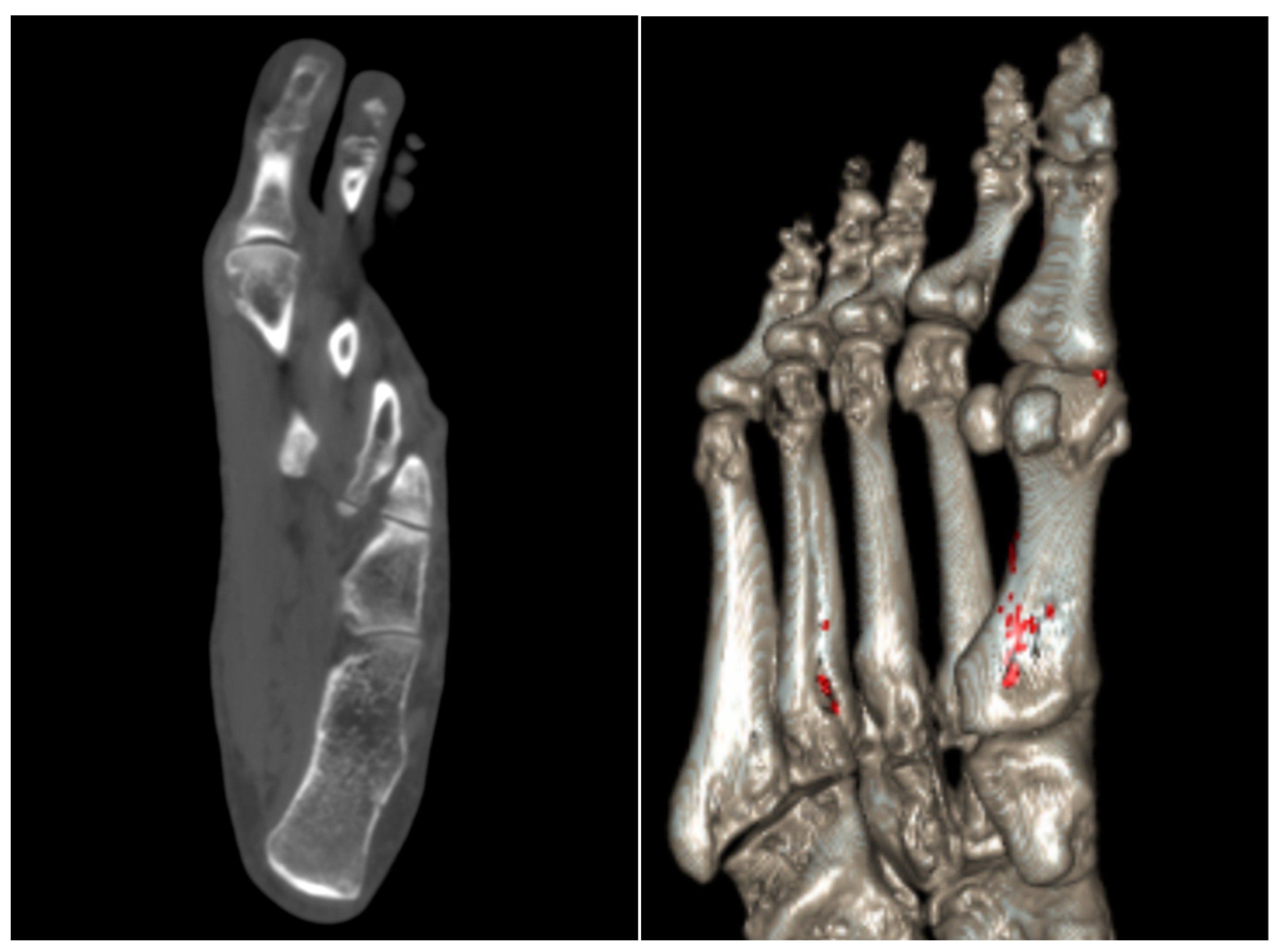
4. Gout
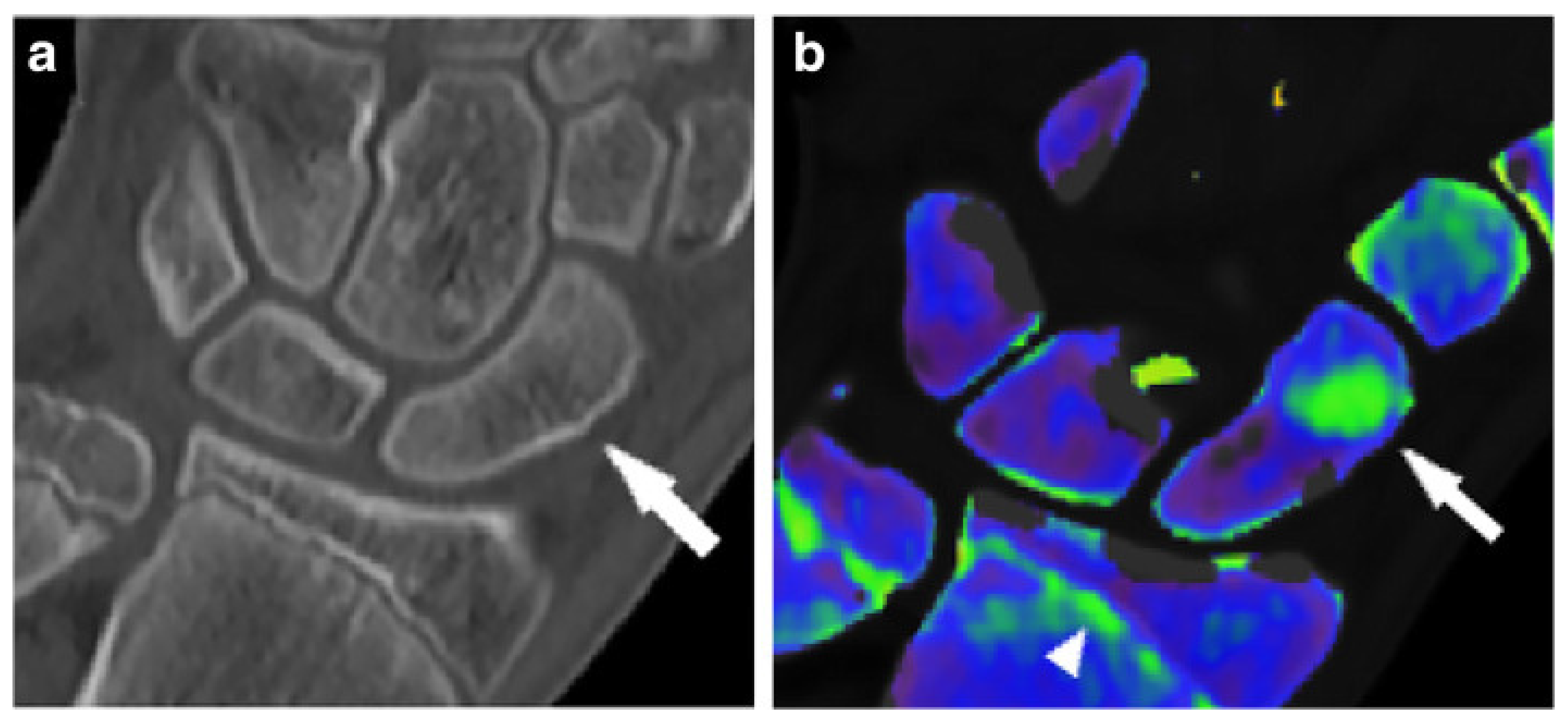
5. Inflammatory Arthropathies
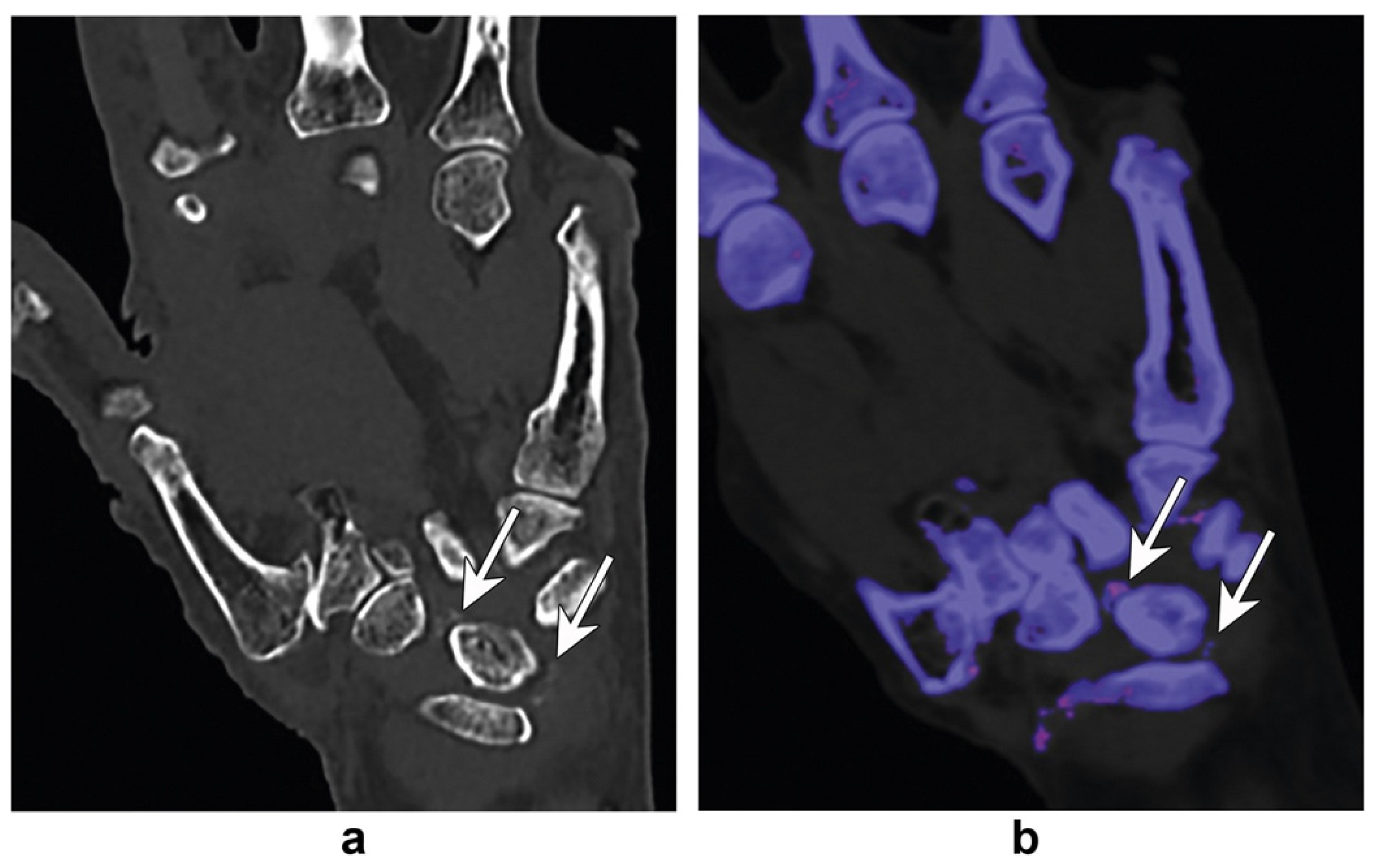
6. Bone-Marrow Edema and Fracture Identification
7. Acute Knee Trauma
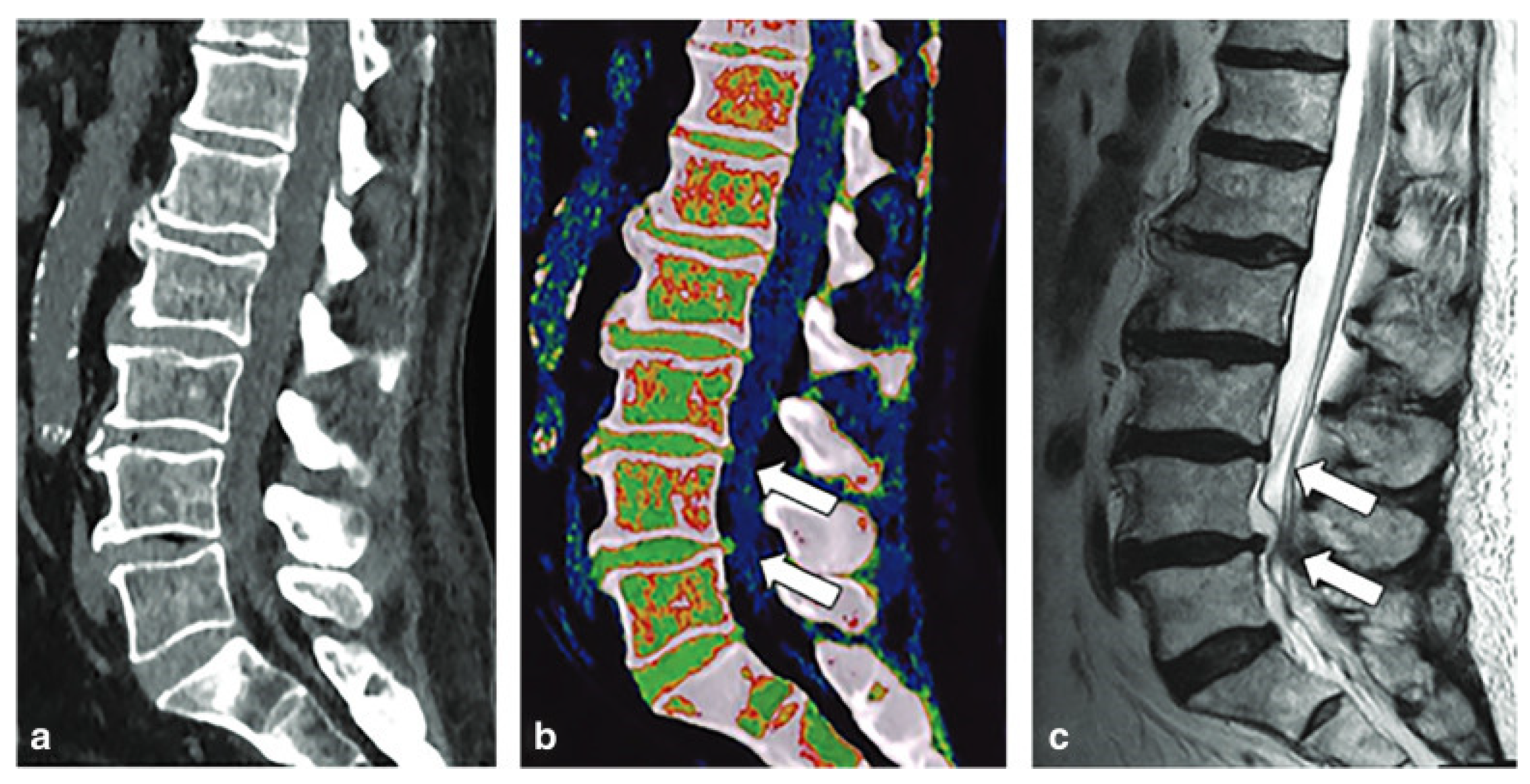
8. Degenerative Disc Disease
9. Osteoporosis
10. Primary Malignant Bone Cancers
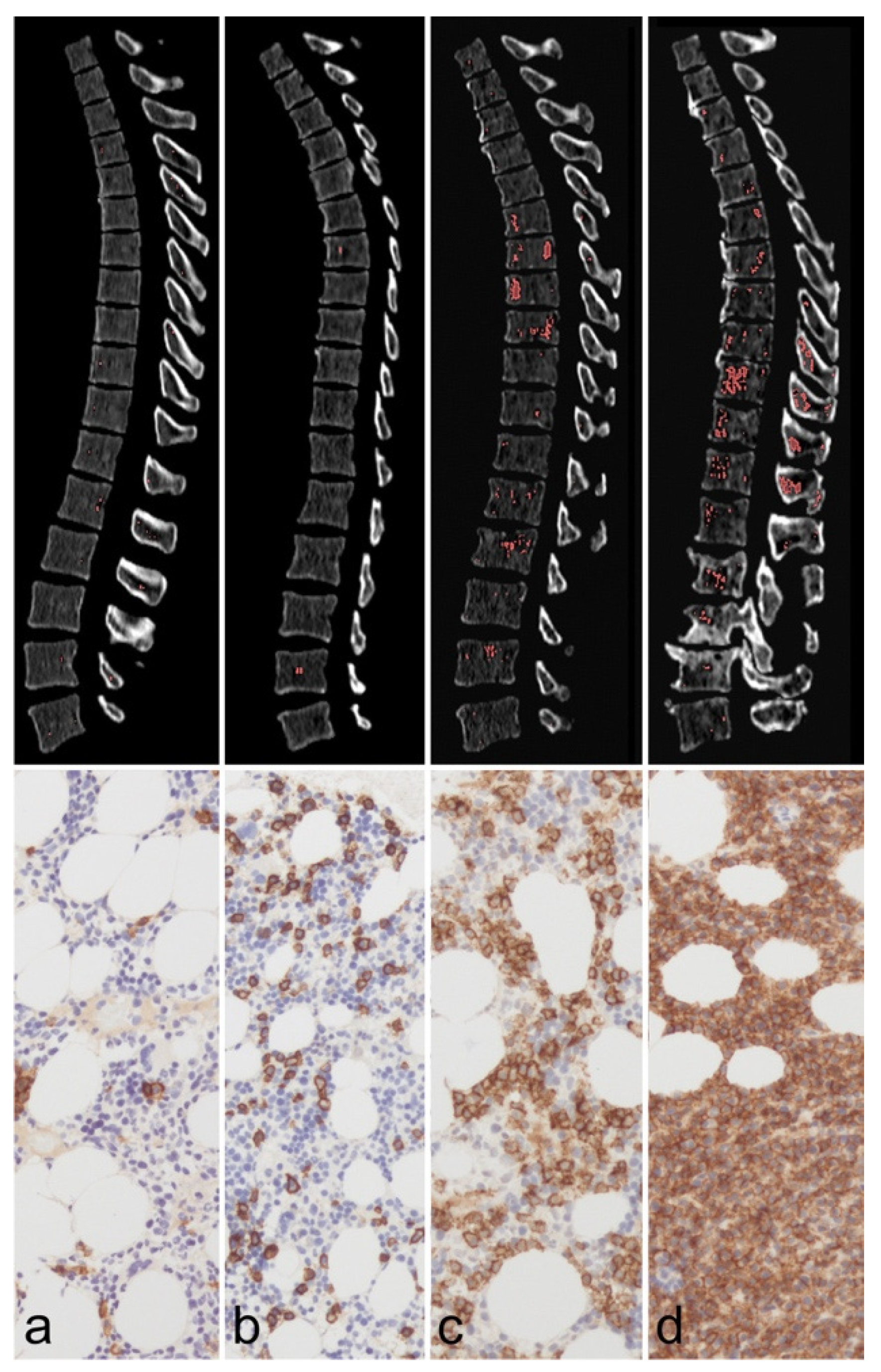
11. Bone Metastases
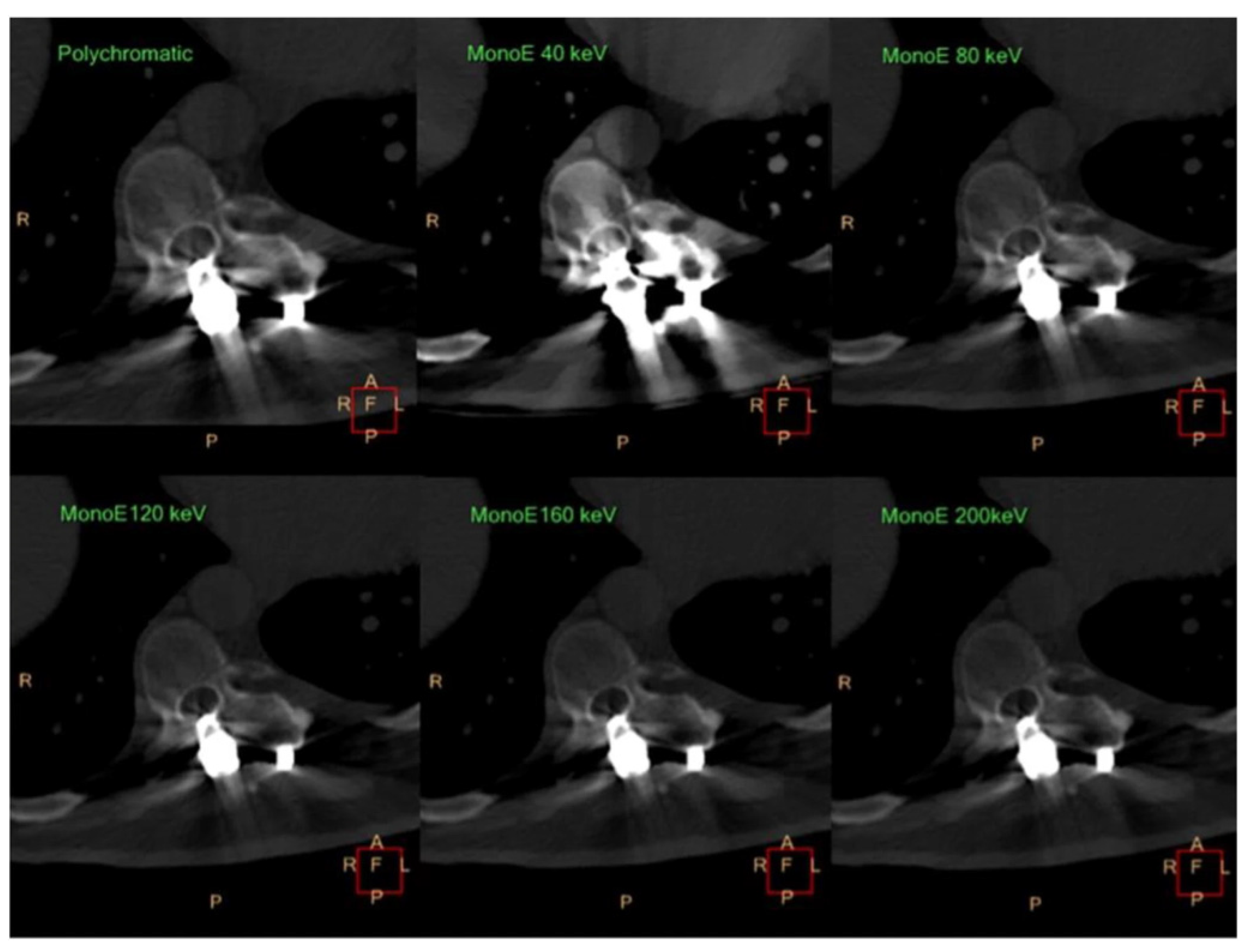
12. Metal-Artifact Reduction
13. Limitations of DECT
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- So, A.; Nicolaou, S. Spectral Computed Tomography: Fundamental Principles and Recent Developments. Korean J. Radiol. 2021, 22, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Feger, J.; Sharma, R.; et al. Dual energy CT. Reference Article, Radiopaedia.org. Available online: https://radiopaedia.org/articles/dual-energy-ct-2 (accessed on 25 March 2024).
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G.; Jacobsen, M.C.; Schellingerhout, D.; Wood, C.A.; Tamm, E.P.; Godoy, M.C.; Sun, J.; et al. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. Radiographics 2021, 41, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, L.S.; Vogl, T.J.; Waldeck, S.S.; Overhoff, D.; D’angelo, T.; Martin, S.S.; Yel, I.; Gruenewald, L.D.; Koch, V.; Fulisch, F.; et al. Dual-Energy CT in Cardiothoracic Imaging: Current Developments. Diagnostics 2023, 13, 2116. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Yokose, C.; McCormick, N.; Lu, N.; Tanikella, S.; Lin, K.; Joshi, A.D.; Raffield, L.M.; Warner, E.; Merriman, T.; Hsu, J.; et al. Trends in Prevalence of Gout Among US Asian Adults, 2011–2018. JAMA Netw. Open 2023, 6, e239501. [Google Scholar] [CrossRef] [PubMed]
- Fenando, A.; Rednam, M.; Gujarathi, R.; Widrich, J. Gout. [Updated 2022 Dec 27]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546606/ (accessed on 27 December 2023).
- Dehlin, M.; Sandström, T.Z.; Jacobsson, L.T. Incident Gout: Risk of Death and Cause-Specific Mortality in Western Sweden: A Prospective, Controlled Inception Cohort Study. Front. Med. 2022, 9, 802856. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, D.; Sehra, S.T.; Anand, S.; Stallings, G.W.; Danve, A. Role of Dual Energy Computed Tomography Imaging in the Diagnosis of Gout. Cureus 2017, 9, e985. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Chin, T.Y.; Peh, W.C. Dual-energy CT in gout—A review of current concepts and applications. J. Med. Radiat. Sci. 2017, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gamala, M.; Jacobs, J.W.G.; van Laar, J.M. The diagnostic performance of dual-energy CT for diagnosing gout: A systematic literature review and meta-analysis. Rheumatology 2019, 58, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Bongartz, T.; Glazebrook, K.N.; Kavros, S.J.; Murthy, N.S.; Merry, S.P.; Franz, W.B., 3rd; Michet, C.J.; Veetil, B.M.; Davis, J.M.; Mason, T.G., 2nd; et al. Dual-energy CT for the diagnosis of gout: An accuracy and diagnostic yield study. Ann. Rheum. Dis. 2015, 74, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.J.; Kondro, D.A.; Kuczynski, M.T.; Pauchard, Y.; Veljkovic, A.; Holdsworth, D.W.; Frasson, V.; Manske, S.L.; MacMullan, P.; Salat, P. Assessing the Sensitivity of Dual-Energy Computed Tomography 3-Material Decomposition for the Detection of Gout. Investig. Radiol. 2022, 57, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Vellarackal, A.J.; Kaim, A.H. Metal artefact reduction of different alloys with dual energy computed tomography (DECT). Sci. Rep. 2021, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; McCollough, C.H.; Yu, L. Three-Material Decomposition in Multi-energy CT: Impact of Prior Information on Noise and Bias. In Proceedings of the Medical Imaging 2018: Physics of Medical Imaging, Houston, TX, USA, 10–15 February 2018; Volume 10573. [Google Scholar]
- Dalbeth, N.; Nicolaou, S.; Baumgartner, S.; Hu, J.; Fung, M.; Choi, H.K. Presence of monosodium urate crystal deposition by dual-energy CT in patients with gout treated with allopurinol. Ann. Rheum. Dis. 2018, 77, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.G.; Bayat, S.; Petsch, C.; Englbrecht, M.; Faustini, F.; Kleyer, A.; Hueber, A.J.; Cavallaro, A.; Lell, M.; Dalbeth, N.; et al. Tophus resolution with pegloticase: A prospective dual-energy CT study. RMD Open 2015, 1, e000075. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Haydar, S.M.A.; Shojania, K.; Ouellette, H.; Nicolaou, S.; Murray, N. Assessing tophaceous spinal gout treatment response using dual-energy CT as a point-of-care imaging modality: Case report. Skelet. Radiol. 2023, 52, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Song, Y.S.; Choi, Y.; Yoo, W.H.; Kummel, F.; Park, E.H. Clumpy artifacts can be differentiated from tophi with DECT: Comparison between gout-free and gouty patients. Br. J. Radiol. 2022, 95, 20210990. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Yoo, W.H.; Song, Y.S.; Byon, J.H.; Pak, J.; Choi, Y. Not All Green Is Tophi: The Importance of Optimizing Minimum Attenuation and Using a Tin Filter to Minimize Clumpy Artifacts on Foot and Ankle Dual-Energy CT. AJR Am. J. Roentgenol. 2020, 214, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Zhang, D.; Levine, B.D.; Dalbeth, N.; Pool, B.; Ranganath, V.K.; Benhaim, P.; Nelson, S.D.; Hsieh, S.S.; FitzGerald, J.D. Limitations of dual-energy CT in the detection of monosodium urate deposition in dense liquid tophi and calcified tophi. Skelet. Radiol. 2021, 50, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Jia, E.; Zhu, J.; Huang, W.; Chen, X.; Li, J. Dual-energy computed tomography has limited diagnostic sensitivity for short-term gout. Clin. Rheumatol. 2018, 37, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.N.; Kurano, T.; Thakur, U.J.; Thawait, G.K.; Fuld, M.K.; Maynard, J.W.; McAdams-DeMarco, M.; Fishman, E.K.; Carrino, J.A. Dual-energy computed tomography has limited sensitivity for non-tophaceous gout: A comparison study with tophaceous gout. BMC Musculoskelet. Disord. 2016, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, M.; Mews, J.; Ulas, S.T.; Ziegeler, K.; Hamm, B.; Diekhoff, T. Influence of contrast medium on tophus detection using dual-energy CT: Phantom study and clinical illustration. Eur. Radiol. Exp. 2023, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, J.F.; Cavalcante, V.D.A.; Sarmento, M.T.R.; Braz, A.D.S.; Freire, E.A. Chronic tophaceous gout mimicking rheumatoid arthritis. Rev. Bras. Reumatol. 2009, 49, 741–746. [Google Scholar] [CrossRef]
- Sanghavi, N.; Korem, S.; Dey, S.; Wasserman, A.; Ash, J. Dual-Energy Computed Tomography (DECT) Resolves the Diagnostic Dilemma in an Atypically Presenting Case of Gout. Cureus 2023, 15, e38247. [Google Scholar] [CrossRef] [PubMed]
- Jans, L.; De Kock, I.; Herregods, N.; Verstraete, K.; Van den Bosch, F.; Carron, P.; Oei, E.H.; Elewaut, D.; Jacques, P. Dual-energy CT: A new imaging modality for bone marrow oedema in rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? AJR. Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Gandikota, G.; Fakuda, T.; Finzel, S. Computed tomography in rheumatology—From DECT to high-resolution peripheral quantitative CT. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101641. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, K.; Richter, S.T.; Hermann, S.; Hermann, K.G.A.; Hamm, B.; Diekhoff, T. Dual-energy CT collagen density mapping of wrist ligaments reveals tissue remodeling in CPPD patients: First results from a clinical cohort. Skelet. Radiol. 2021, 50, 417–423. [Google Scholar] [CrossRef] [PubMed]
- National Psoriasis Foundation. (n.d.). Psoriasis Statistics. Available online: https://www.psoriasis.org/psoriasis-statistics/ (accessed on 2 January 2024).
- Kayama, R.; Fukuda, T.; Ogiwara, S.; Momose, M.; Tokashiki, T.; Umezawa, Y.; Asahina, A.; Fukuda, K. Quantitative analysis of therapeutic response in psoriatic arthritis of digital joints with Dual-energy CT iodine maps. Sci. Rep. 2020, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Umezawa, Y.; Tojo, S.; Yonenaga, T.; Asahina, A.; Nakagawa, H.; Fukuda, K. Initial Experience of Using Dual-Energy CT with an Iodine Overlay Image for Hand Psoriatic Arthritis: Comparison Study with Contrast-enhanced MR Imaging. Radiology 2017, 284, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Asahina, A.; Fukuda, T.; Ishiuji, Y.; Yaginuma, A.; Yanaba, K.; Umezawa, Y.; Nakagawa, H. Usefulness of dual-energy computed tomography for the evaluation of early-stage psoriatic arthritis only accompanied by nail psoriasis. J. Dermatol. 2017, 44, e326–e327. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Yanaba, K.; Asahina, A.; Nakagawa, H.; Fukuda, T.; Fukuda, K. Usefulness of dual-energy computed tomography for the evaluation of psoriatic arthritis accompanied by knee osteoarthritis. J. Dermatol. 2019, 46, e30–e32. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Umezawa, Y.; Asahina, A.; Nakagawa, H.; Furuya, K.; Fukuda, K. Dual energy CT iodine map for delineating inflammation of inflammatory arthritis. Eur. Radiol. 2017, 27, 5034–5040. [Google Scholar] [CrossRef] [PubMed]
- Gosangi, B.; Mandell, J.C.; Weaver, M.J.; Uyeda, J.W.; Smith, S.E.; Sodickson, A.D.; Khurana, B. Bone Marrow Edema at Dual-Energy CT: A Game Changer in the Emergency Department. Radiographics 2020, 40, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Chung, B.M.; Kim, W.T.; Gil, J.R. Nondisplaced fractures on hip CT: Added value of dual-energy CT virtual non-calcium imaging for detection of bone marrow edema using visual and quantitative analyses. Acta Radiologica 2019, 60, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.T.; Wong, W.D.; Liang, T.; Khosa, F.; Mian, M.; Jalal, S.; Nicolaou, S. Clinical Utility of Dual-Energy CT Analysis of Bone Marrow Edema in Acute Wrist Fractures. AJR. Am. J. Roentgenol. 2018, 210, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Yun, S.J.; Jin, W.; Lee, S.H.; Park, S.Y.; Ryu, C.W. Diagnostic performance of dual-energy CT for the detection of bone marrow oedema: A systematic review and meta-analysis. Eur. Radiol. 2018, 28, 4182–4194. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Sherbaf, F.; Sair, H.I.; Shakoor, D.; Fritz, J.; Schwaiger, B.J.; Johnson, M.H.; Demehri, S. DECT in Detection of Vertebral Fracture-associated Bone Marrow Edema: A Systematic Review and Meta-Analysis with Emphasis on Technical and Imaging Interpretation Parameters. Radiology 2021, 300, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, G.; Chang, X. Diagnostic accuracy of dual-energy computed tomography in bone marrow edema with vertebral compression fractures: A meta-analysis. Eur. J. Radiol. 2018, 99, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Karaca, L.; Yuceler, Z.; Kantarci, M.; Çakır, M.; Sade, R.; Calıkoglu, C.; Ogul, H.; Bayrakturan, U. The feasibility of dual-energy CT in differentiation of vertebral compression fractures. Br. J. Radiol. 2016, 89, 20150300. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Wang, H.; Ji, X.; Fu, Z.; Zhang, K.; Su, W.; Zhang, J.; Ni, D. Spectral computed tomography-guided photothermal therapy of osteosarcoma by bismuth sulfide nanorods. Nano Res. 2023, 16, 9885–9893. [Google Scholar] [CrossRef]
- Cavallaro, M.; D’angelo, T.; Albrecht, M.H.; Yel, I.; Martin, S.S.; Wichmann, J.L.; Lenga, L.; Mazziotti, S.; Blandino, A.; Ascenti, G.; et al. Comprehensive comparison of dual-energy computed tomography and magnetic resonance imaging for the assessment of bone marrow edema and fracture lines in acute vertebral fractures. Eur. Radiol. 2022, 32, 561–571. [Google Scholar] [CrossRef]
- Booz, C.; Nöske, J.; Lenga, L.; Martin, S.S.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Huizinga, N.; Albrecht, M.H.; Vogl, T.J.; et al. Color-coded virtual non-calcium dual-energy CT for the depiction of bone marrow edema in patients with acute knee trauma: A multireader diagnostic accuracy study. Eur. Radiol. 2020, 30, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.P.; Lui, K.; Nobbee, D.; Murad, M.H.; Katlariwala, P.; Low, G. Diagnostic accuracy of dual-energy CT for detecting bone marrow edema in patients with acute knee injuries: A systematic review and meta-analysis. Skelet. Radiol. 2021, 50, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Sauer, B.; Detreille, R.; Zabel, J.P.; Pierrucci, F.; Witte, Y.; Dap, F. The diagnosis of recent scaphoid fractures: Review of the literature. J. Radiol. 2007, 88, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.C.; Escobedo, E.M.; Wilson, A.J.; Hanel, D.P.; Zink-Brody, G.C.; Mann, F.A. MR imaging of clinically suspected scaphoid fractures. AJR. Am. J. Roentgenol. 1997, 168, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Albrecht, M.H.; Caudo, D.; Mazziotti, S.; Vogl, T.J.; Wichmann, J.L.; Martin, S.; Yel, I.; Ascenti, G.; Koch, V.; et al. Virtual non-calcium dual-energy CT: Clinical applications. Eur. Radiol. Exp. 2021, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Fang, H.; Li, C.; Shi, L.; Fan, X.; Xu, X.; Gao, F.; Sun, W.; Qing, J. Diagnostic accuracy of dual-energy CT for bone marrow edema in patients with acute knee injury: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 826. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Mahmoudi, S.; Bernatz, S.; Eichler, K.; Alizadeh, L.S.; D’Angelo, T.; Mazziotti, S.; et al. Diagnostic value of DECT-based colored collagen maps for the assessment of cruciate ligaments in patients with acute trauma. Eur. Radiol. 2023, 33, 6339–6350. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, P.; Cai, Z.-J.; Lu, W.-H.; Pan, L.-Y.; Liu, X.; Peng, X.-J.; Li, Y.-S.; Xiao, W.-F. Valid and reliable diagnostic performance of dual-energy CT in anterior cruciate ligament rupture. Eur. Radiol. 2023, 33, 7769–7778. [Google Scholar] [CrossRef] [PubMed]
- Hickle, J.; Walstra, F.; Duggan, P.; Ouellette, H.; Munk, P.; Mallinson, P. Dual-energy CT characterization of winter sports injuries. Br. J. Radiol. 2020, 93, 20190620. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Sasaki, F.; Ohmura, T.; Itoh, T.; Endo, T.; Kinoshita, T. Evaluation of lumbar intervertebral disc degeneration using dual energy CT virtual non-calcium imaging. Eur. J. Radiol. 2020, 124, 108817. [Google Scholar] [CrossRef] [PubMed]
- IOF International Osteoporosis Foundation. Epidemiology: International Osteoporosis Foundation. Available online: https://www.osteoporosis.foundation/health-professionals/about-osteoporosis/epidemiology#:~:text=Osteoporosis%20is%20a%20major%20non,age%20of%2050%20worldwide%20%5B1%5D (accessed on 25 March 2024).
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, A.L.; Franks, P.; Robbins, J.A.; Xing, G.; Fenton, J.J. Underuse and Overuse of Osteoporosis Screening in a Regional Health System: A Retrospective Cohort Study. J. Gen. Intern. Med. 2015, 30, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yao, Y.; Shang, A.-L.; Du, T.; Zhang, J.; Yang, Q.; Li, J.; Wang, Q.; Li, X. Opportunistic screening for osteoporosis using hydroxyapatite measurements of the vertebral by thorax dual-energy spectral CT in postmenopausal females. Sci. Rep. 2022, 12, 21642. [Google Scholar] [CrossRef] [PubMed]
- Nowak, T.; Eberhard, M.; Schmidt, B.; Frey, D.; Distler, O.; Saltybaeva, N.; Alkadhi, H.; Euler, A. Bone Mineral Density Quantification from Localizer Radiographs: Accuracy and Precision of Energy-integrating Detector CT and Photon-counting Detector CT. Radiology 2021, 298, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Wesarg, S.; Kirschner, M.; Becker, M.; Erdt, M.; Kafchitsas, K.; Khan, M.F. Dual-energy CT-based assessment of the trabecular bone in vertebrae. Methods Inf. Med. 2012, 51, 398–405. [Google Scholar] [CrossRef] [PubMed]
- van Hamersvelt, R.W.; Schilham, A.M.R.; Engelke, K.; Harder, A.M.D.; de Keizer, B.; Verhaar, H.J.; Leiner, T.; de Jong, P.A.; Willemink, M.J. Accuracy of bone mineral density quantification using dual-layer spectral detector CT: A phantom study. Eur. Radiol. 2017, 27, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Lenga, L.; Wichmann, J.L.; Alizadeh, L.S.; Albrecht, M.H.; et al. Diagnostic accuracy of quantitative dual-energy CT-based volumetric bone mineral density assessment for the prediction of osteoporosis-associated fractures. Eur. Radiol. 2022, 32, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, H.; Wu, S.; Li, S.; Sun, J.; Ren, T.; Li, Z.; Zhou, J. Diagnostic Value of Dual-Energy CT Virtual Non-Calcium and Rho/Z Images for Bone Marrow Infiltration in Primary Malignant Bone Tumors. Acad. Radiol. 2023, 30, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, K.; Wang, H.; Li, Y.; Zhang, J.; Zhou, J.; Hu, J.; Xie, D.; Ni, D. Spectral computed tomography-guided radiotherapy of osteosarcoma utilizing BiOI nanosheets. Acta Biomater. 2023, 166, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.F.; Murray, J.G.; Eustace, S.J.; Madewell, J.; O’Gorman, P.J.; O’Sullivan, P. Multiple myeloma: A review of imaging features and radiological techniques. Bone Marrow Res. 2011, 2011, 583439. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krauss, B.; Horger, M. Dual-Energy CT-Based Bone Marrow Imaging in Multiple Myeloma: Assessment of Focal Lesions in Relation to Disease Status and MRI Findings. Acad. Radiol. 2022, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Schabel, C.; Krauss, B.; Weisel, K.; Bongers, M.; Claussen, C.D.; Horger, M. Dual-energy CT: Virtual calcium subtraction for assessment of bone marrow involvement of the spine in multiple myeloma. AJR. Am. J. Roentgenol. 2015, 204, W324–W331. [Google Scholar] [CrossRef]
- Fervers, P.; Fervers, F.; Kottlors, J.; Lohneis, P.; Pollman-Schweckhorst, P.; Zaytoun, H.; Rinneburger, M.; Maintz, D.; Große Hokamp, N. Feasibility of artificial intelligence-supported assessment of bone marrow infiltration using dual-energy computed tomography in patients with evidence of monoclonal protein—A retrospective observational study. Eur. Radiol. 2022, 32, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, R.; Vallee, J.N.; Lafitte, F.; Menuel, C.; Duverneuil, N.M.; Chiras, J. Spine metastasis imaging: Review of the literature. J. Neuroradiol. 2007, 34, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Kong, L.; Deng, X. Dual-energy computed tomography for differentiation between osteoblastic metastases and bone Islands. Front. Oncol. 2022, 12, 815955. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, S.; Machida, H.; Wang, B.; Liu, A.; Liu, Y.; Zhang, X. Differential diagnosis of osteoblastic metastases from bone islands in patients with lung cancer by single-source dual-energy CT: Advantages of spectral CT imaging. Eur. J. Radiol. 2015, 84, 901–907. [Google Scholar] [CrossRef]
- Burke, M.C.; Garg, A.; Youngner, J.M.; Deshmukh, S.D.; Omar, I.M. Initial experience with dual-energy computed tomography-guided bone biopsies of bone lesions that are occult on monoenergetic CT. Skeletal Radiol. 2019, 48, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, N.; Hokamp, N.G.; Lennartz, S.; Holz, J.A.; Romman, Z.; Pahn, G.; Neuhaus, V.; Maintz, D.; Krug, B.; Borggrefe, J. Improvements of diagnostic accuracy and visualization of vertebral metastasis using multi-level virtual non-calcium reconstructions from dual-layer spectral detector computed tomography. Eur. Radiol. 2019, 29, 5941–5949. [Google Scholar] [CrossRef]
- Wu, H.; Dong, S.; Li, X.; Shi, L.; Shao, D.; Zhang, Q.; Chen, M.; Cao, Y.; Thant, M.; Huang, X. Clinical utility of dual-energy CT used as an add-on to 18F FDG PET/CT in the preoperative staging of resectable NSCLC with suspected single osteolytic metastases. Lung Cancer 2020, 140, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.; Davis, D.; Mulligan, M.E. The Ability of Dual-Energy Computed Tomography to Distinguish Normal Bone Marrow from Metastases Using Bone Marrow Color Maps. J. Comput. Assist. Tomogr. 2018, 42, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.; Hojjati, M.; Young, P.C.; Abedi, A.; Gholamrezanezhad, A.; Rajiah, P. Dual-layer spectral computerized tomography for metal artifact reduction: Small versus large orthopedic devices. Skelet. Radiol. 2019, 48, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Lennartz, S.; Neuhaus, V.-F.; Hokamp, N.G.; Rau, R.; Le Blanc, M.; Abudullayev, N.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. CT metal artifacts in patients with total hip replacements: For artifact reduction monoenergetic reconstructions and post-processing algorithms are both efficient but not similar. Eur. Radiol. 2018, 28, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Boas, F.E.; Fleischmann, D. CT artifacts: Causes and reduction techniques. Imaging Med. 2012, 4, 229–240. [Google Scholar] [CrossRef]
- Wellenberg, R.H.H.; Donders, J.C.E.; Kloen, P.; Beenen, L.F.M.; Kleipool, R.P.; Maas, M.; Streekstra, G.J. Exploring metal artifact reduction using dual-energy CT with pre-metal and post-metal implant cadaver comparison: Are implant specific protocols needed? Skelet. Radiol. 2018, 47, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, V.; Hokamp, N.G.; Abdullayev, N.; Rau, R.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. Metal artifact reduction by dual-layer computed tomography using virtual monoenergetic images. Eur. J. Radiol. 2017, 93, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cheng, Q.; Liu, C.; Wang, Q.; Lv, Y.; Tang, Z.; Luo, Y.; Yang, H. Optimal combination periprosthetic vasculature visualization and metal artifact reduction by spectral computed tomography using virtual monoenergetic images in total hip arthroplasty. Insights Imaging 2023, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Wichtmann, H.M.; Laukamp, K.R.; Manneck, S.; Appelt, K.; Stieltjes, B.; Boll, D.T.; Benz, M.R.; Obmann, M.M. Metal implants on abdominal CT: Does split-filter dual-energy CT provide additional value over iterative metal artifact reduction? Abdom. Radiol. 2023, 48, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, P.; Baffour, F.I.; Adkins, M.C.; Yu, L.; McCollough, C.H.; Fletcher, J.G.; Glazebrook, K.N. Benefits of iterative metal artifact reduction and dual-energy CT towards mitigating artifact in the setting of total shoulder prostheses. Skeletal Radiol. 2021, 50, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.N.; Schabel, C.; Thomas, C.; Raupach, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Comparison and Combination of Dual-Energy- and Iterative-Based Metal Artefact Reduction on Hip Prosthesis and Dental Implants. PLoS ONE 2015, 10, e0143584. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; DeLone, D.R.; Kotsenas, A.L.; Lehman, V.T.; Nagelschneider, A.A.; Michalak, G.J.; Fletcher, J.G.; McCollough, C.H.; Yu, L. Clinical Assessment of Metal Artifact Reduction Methods in Dual-Energy CT Examinations of Instrumented Spines. AJR Am. J. Roentgenol. 2019, 212, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.J.; Lee, S.J.; Kim, D.W.; Lee, Y.J.; Baek, J.W.; Han, J.Y.; Heo, Y.J. Comparison of the Quality of Various Polychromatic and Monochromatic Dual-Energy CT Images with or without a Metal Artifact Reduction Algorithm to Evaluate Total Knee Arthroplasty. Korean J. Radiol. 2021, 22, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Hong, S.H.; Chung, B.M.; Moon, S.J.; Choi, J.Y.; Chae, H.D.; Chang, M.Y. Metal Artifact Reduction in Virtual Monoenergetic Spectral Dual-Energy CT of Patients With Metallic Orthopedic Implants in the Distal Radius. AJR Am. J. Roentgenol. 2018, 211, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Layer, Y.C.; Mesropyan, N.; Kupczyk, P.A.; Luetkens, J.A.; Isaak, A.; Dell, T.; Attenberger, U.I.; Kuetting, D. Combining iterative metal artifact reduction and virtual monoenergetic images severely reduces hip prosthesis-associated artifacts in photon-counting detector CT. Sci. Rep. 2023, 13, 8955. [Google Scholar] [CrossRef] [PubMed]
- Subhas, N.; Primak, A.N.; Obuchowski, N.A.; Gupta, A.; Polster, J.M.; Krauss, A.; Iannotti, J.P. Iterative metal artifact reduction: Evaluation and optimization of technique. Skelet. Radiol. 2014, 43, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagnostic and Interventional Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Euler, A.; Laqua, F.C.; Cester, D.; Lohaus, N.; Sartoretti, T.; Pinto Dos Santos, D.; Alkadhi, H.; Baessler, B. Virtual Monoenergetic Images of Dual-Energy CT-Impact on Repeatability, Reproducibility, and Classification in Radiomics. Cancers 2021, 13, 4710. [Google Scholar] [CrossRef] [PubMed]



| Modality | Image Acquisition Approach | Strengths | Weaknesses |
|---|---|---|---|
| Dual Energy: | |||
| (a) Dual Spin |
| Least complex hardware requirements |
|
| (b) Dual Source |
|
|
|
| Rapid Kilovolt Peak Switching |
| Near-simultaneous acquisition of high- and low-energy images | Alternating of energy levels leads to low spectral separation |
| Split-Filter Twin Beam |
| Many existing CT scanners can be retrofitted with a split filter. | Limited spectral separation |
| Dual-Layer Computed Tomography |
|
| Low spectral separation |
| Photon-Counting Computed Tomography | Directly counts individual photons at varying energy levels by converting X-ray energy to an electronic signal |
| Limited availability |
| Reference | Condition Being Diagnosed | Dual-Energy CT Type | Sample Size (Patients) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Jang et al., 2019 [39] | Nondisplaced fractures of the hip (proximal femur and pelvic bones) | Dual-Source CT System (SOMATOM Definition Flash, Siemens Healthcare) | 35 | 100% | 94% |
| Ali et al., 2018 [40] | Acute fractures of the wrist (carpal bones) | Dual-Source CT System (SOMATOM Definition Flash, Siemens Healthcare) | 37 (24 visually interpreted to develop an attenuation value cutoff; 13 to validate the cutoff) | 100% (develop-mental set); 100% (validation set) | 99.5% (develop-mental set); 100% (validation set) |
| Suh et al., 2018 [41] | Bone-marrow edema of the spine, ankle, hip, and knee | Various (Meta-analysis) | 450 (Meta-analysis) | 85% (overall performance) | 97% (overall performance) |
| Sherbaf et al., 2021 [42] | Vertebral fractures | Various (Meta-analysis) | 606 (Meta-analysis) | 89% (overall performance) | 96% (overall performance) |
| Yang et al., 2018 [43] | Vertebral compression fractures | Various (Meta-analysis) | 510 vertebrae (Meta-analysis) | 82% | 98% |
| Karaca et al., 2016 [44] | Vertebral compression fractures | Dual-Source CT System (SOMATOM Definition Flash, Siemens Healthcare) | 23 | 89.3% | 98.7% |
| Li et al., 2023 [45] | Acute knee injury | Various (Meta-analysis) | 290 (Meta-analysis) | 85% (overall performance) | 96% (overall performance) |
| Cavallaro et al., 2022 [46] | Acute vertebral fractures | Dual-Source CT System (SOMATOM Force, Siemens Healthcare) | 88 | 89% (injury presence); 84% (injury extent) | 98% (injury presence); 98% (injury extent) |
| Booz et al., 2020 [47] | Acute knee injury | Dual-Source CT System (SOMATOM Force, Siemens Healthcare) | 57 | 94% (qualitative analysis); 95% (quantitative analysis with cutoff of −42 Hounsfield units); 96% (quantitative analysis with cutoff of −51 Hounsfield units) | 95% (qualitative analysis); 95% (quantitative analysis with cutoff of −42 Hounsfield units); 97% (quantitative analysis with cutoff of −51 Hounsfield units) |
| Wilson et al., 2021 [48] | Acute knee injury | Various (Meta-analysis) | 267 (Meta-analysis) | 84% (overall performance) | 96% (overall performance) |
| Reference | Study Aims | Dual-Energy CT Type | Key Findings Surrounding Metal-Artifact Reduction |
|---|---|---|---|
| Kosmas et al., 2019 [78] | Comparison of the utility of dual-layer CT vs. conventional polychromatic CT in reduction of artifacts from metal implants | Dual-Layer CT System (Prototype, Phillips Healthcare) |
|
| Wellenberg et al., 2018 [81] | Quantification and optimization of metal-artifact reduction with virtual monochromatic dual-energy CT for various metal implants, with non-metal scans as a reference for comparison | Dual-Source CT System (SOMATOM Force, Siemens Healthcare) |
|
| Laukamp et al., 2018 [79] | Comparison of metal-artifact reduction of three imaging methods (VMI; metal-artifact-reduction-specialized reconstructions, an iterative algorithm; and conventional images from dual-layer CT) in imaging of total hip replacements | Dual-Layer CT System (IQon, Phillips Healthcare) |
|
| Neuhaus et al., 2017 [82] | Examination of metal-artifact reduction performance in virtual monoenergetic images from dual-layer CT | Dual-Layer CT System (IQon, Phillips Healthcare) |
|
| Zhao et al., 2023 [83] | Examination of the image quality of VMI as well as optimization of visualization of orthopedic MAR and the periprosthetic vasculature in THA patients | Dual-Layer CT System (IQon, Phillips Healthcare) |
|
| Wichtmann et al., 2023 [84] | Evaluation of split-filter abdominal CT image quality and metal-artifact reduction using VMI or iMAR in patients with hip or spinal implants | Split-Filter CT System (SOMATOM Definition Edge, Siemens, and SOMATOM Definition AS+, Siemens Healthcare) |
|
| Mohammedinejad et al., 2021 [85] | Evaluation of iMAR and VMI in DECT of patients with total shoulder prosthesis | Dual-Source CT System (SOMATOM Force, Siemens Healthcare) | VMI and iMAR independently improved image quality and reduced artifacts, but the strongest performance was achieved by using both |
| Bongers et al. 2015 [86] | Evaluation of VMI and iMAR in metal-artifact reduction for imaging of hip prosthesis and dental implants | Dual-Source CT System (SOMATOM Definition Flash, Siemens Healthcare) | VMI and iMAR independently reduced artifacts, but the strongest performance was achieved by using both |
| Long et al., 2019 [87] | Evaluation of VMI, iMAR, and the combination of VMI and iMAR in DECT imaging of instrumented spines | Dual-Source CT System (SOMATOM Definition Flash, Siemens Healthcare) | The combination of VMI and iMAR demonstrated stronger metal-artifact reduction compared to either algorithm individually |
| Choo et al., 2021 [88] | Evaluation of the use of VMI and iMAR in DECT imaging of total knee arthroplasty | Dual-Source CT System (SOMATOM Drive, Siemens Healthcare) | Non-VMI images combined with iMAR resulted in the strongest artifact reduction |
| Yoo et al., 2018 [89] | Evaluation of VMI in patients with metallic implants of the distal radius | Dual-Layer CT System (IQon, Phillips Healthcare) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eibschutz, L.S.; Matcuk, G.; Chiu, M.K.-J.; Lu, M.Y.; Gholamrezanezhad, A. Updates on the Applications of Spectral Computed Tomography for Musculoskeletal Imaging. Diagnostics 2024, 14, 732. https://doi.org/10.3390/diagnostics14070732
Eibschutz LS, Matcuk G, Chiu MK-J, Lu MY, Gholamrezanezhad A. Updates on the Applications of Spectral Computed Tomography for Musculoskeletal Imaging. Diagnostics. 2024; 14(7):732. https://doi.org/10.3390/diagnostics14070732
Chicago/Turabian StyleEibschutz, Liesl S., George Matcuk, Michael Kuo-Jiun Chiu, Max Yang Lu, and Ali Gholamrezanezhad. 2024. "Updates on the Applications of Spectral Computed Tomography for Musculoskeletal Imaging" Diagnostics 14, no. 7: 732. https://doi.org/10.3390/diagnostics14070732
APA StyleEibschutz, L. S., Matcuk, G., Chiu, M. K.-J., Lu, M. Y., & Gholamrezanezhad, A. (2024). Updates on the Applications of Spectral Computed Tomography for Musculoskeletal Imaging. Diagnostics, 14(7), 732. https://doi.org/10.3390/diagnostics14070732






