Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review
Abstract
1. Introduction
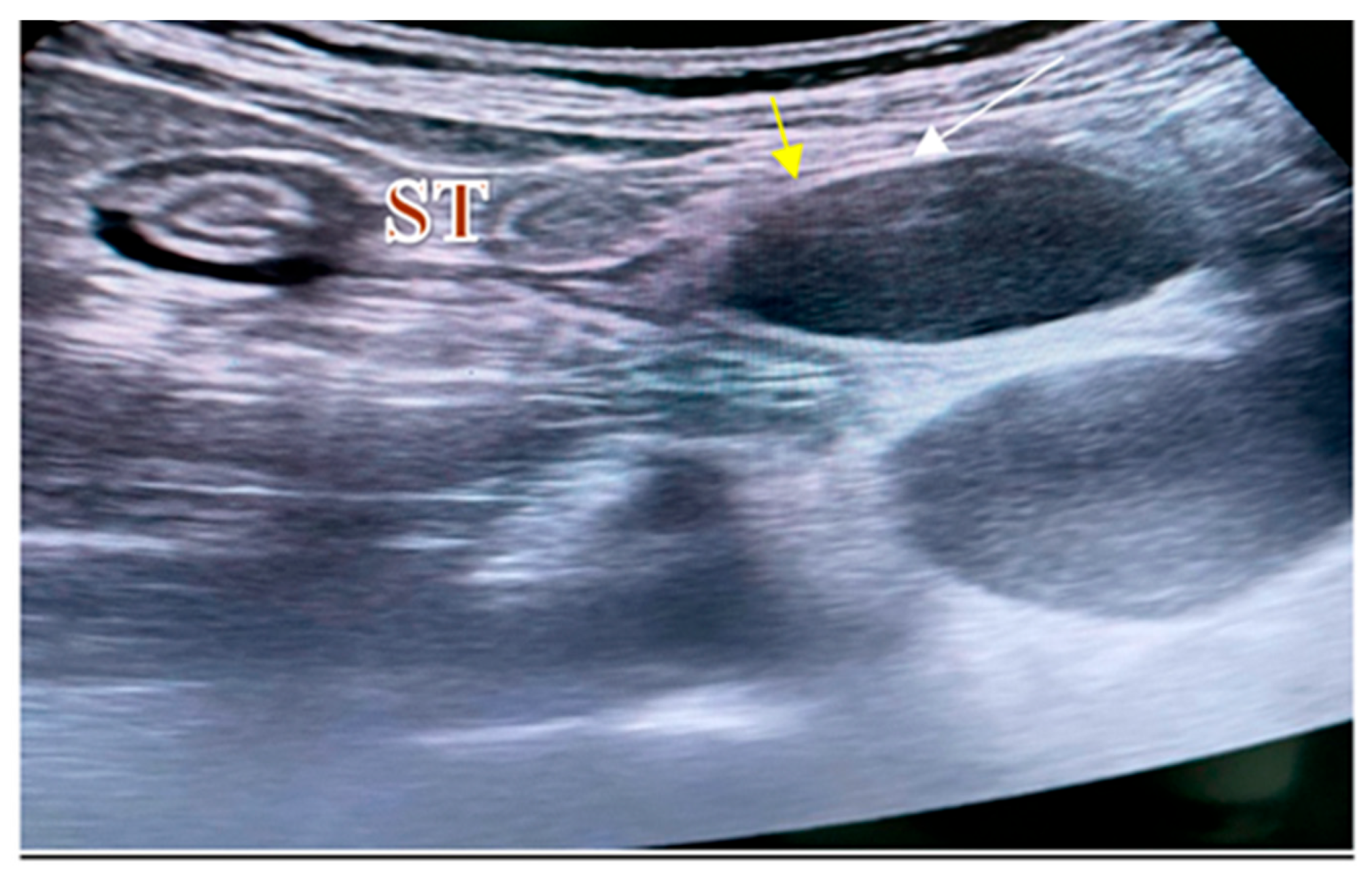
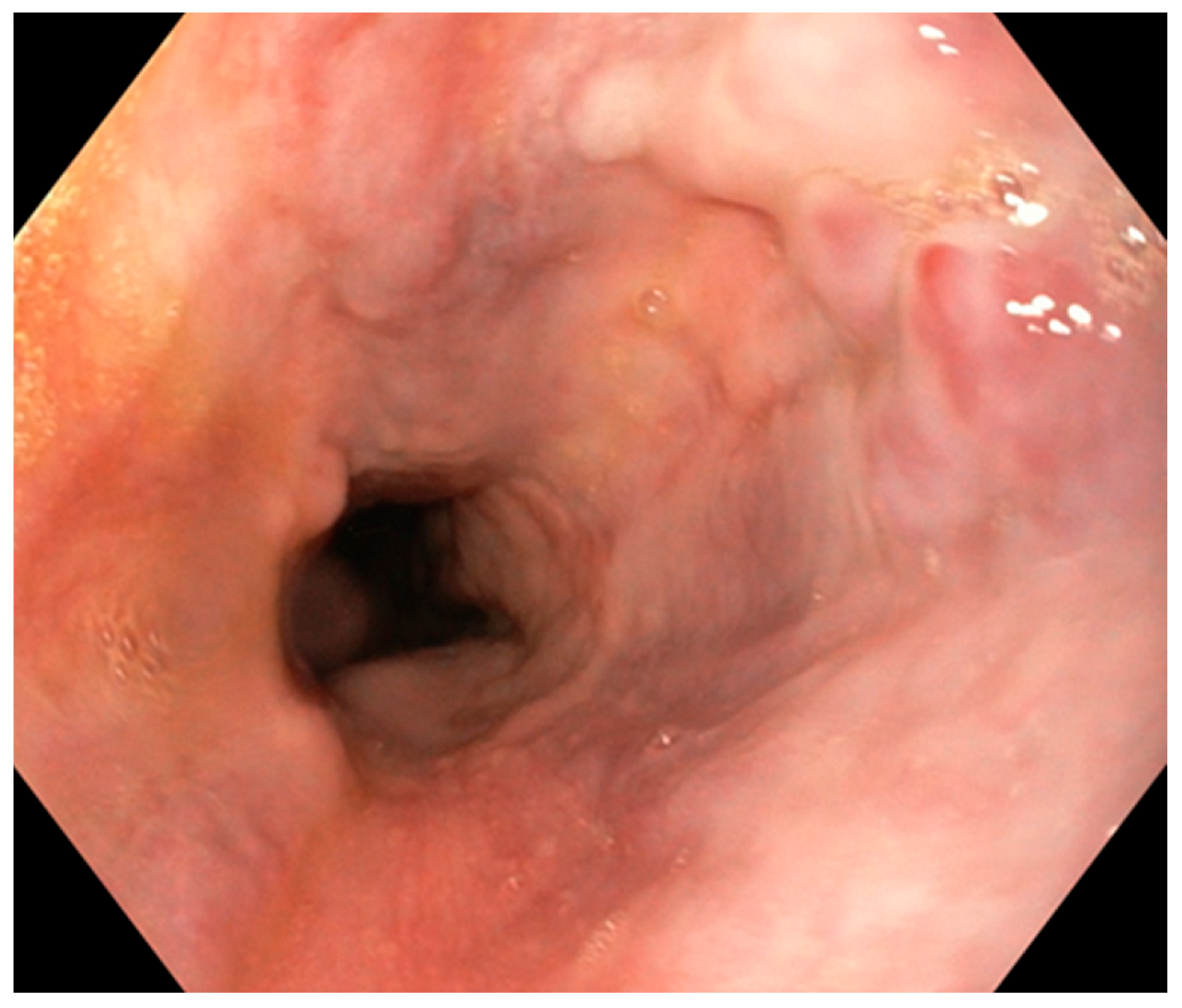
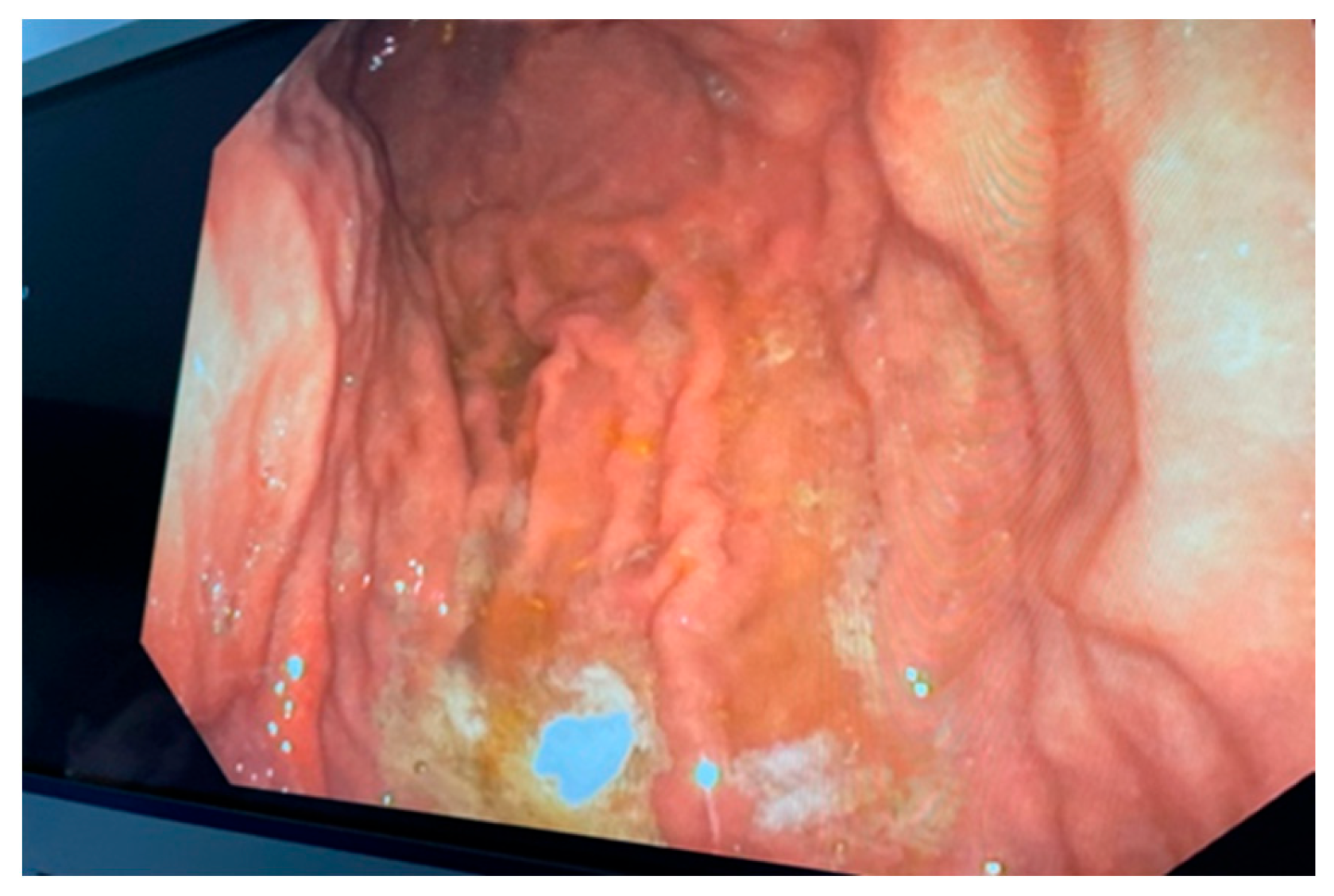
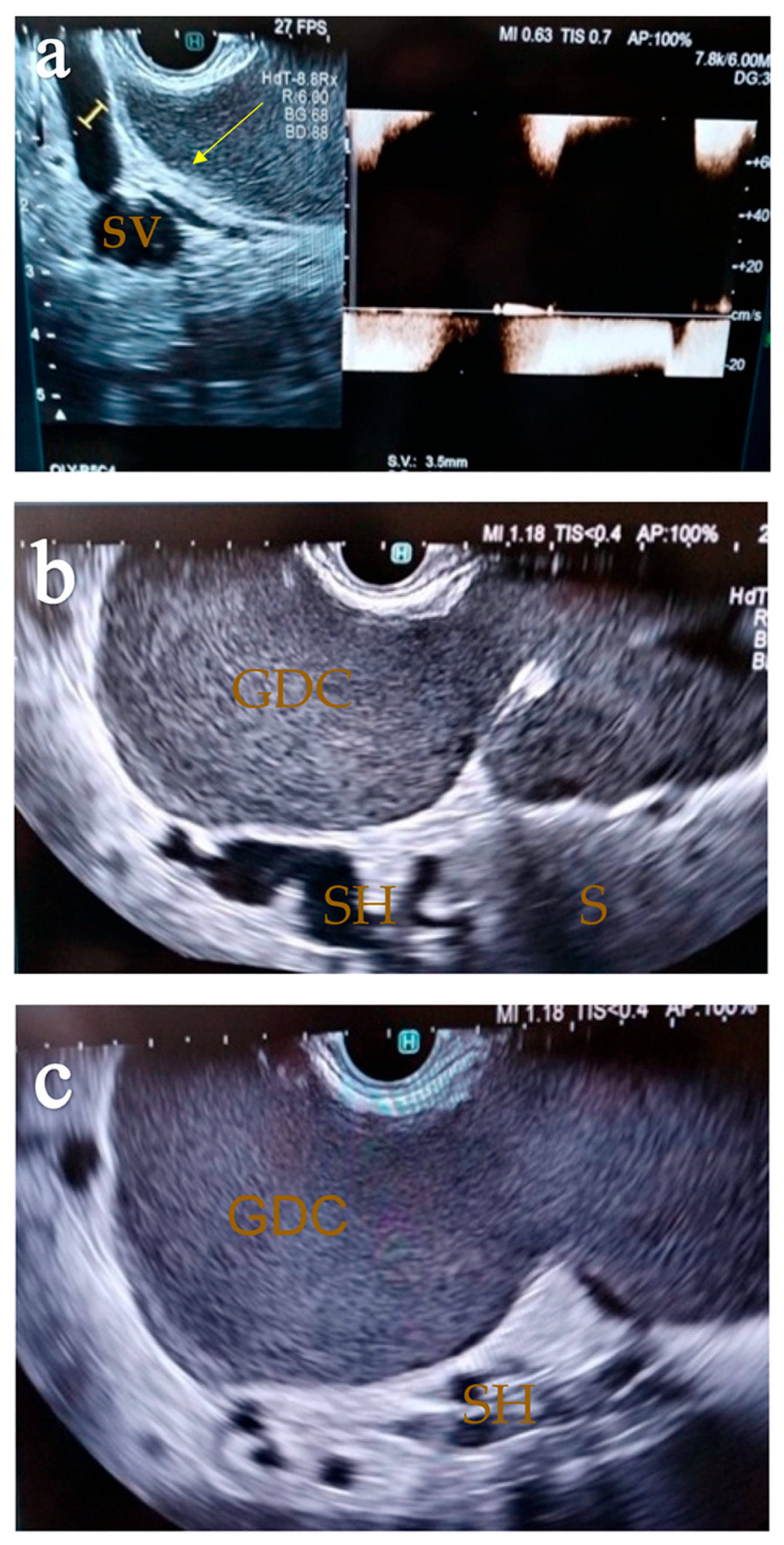
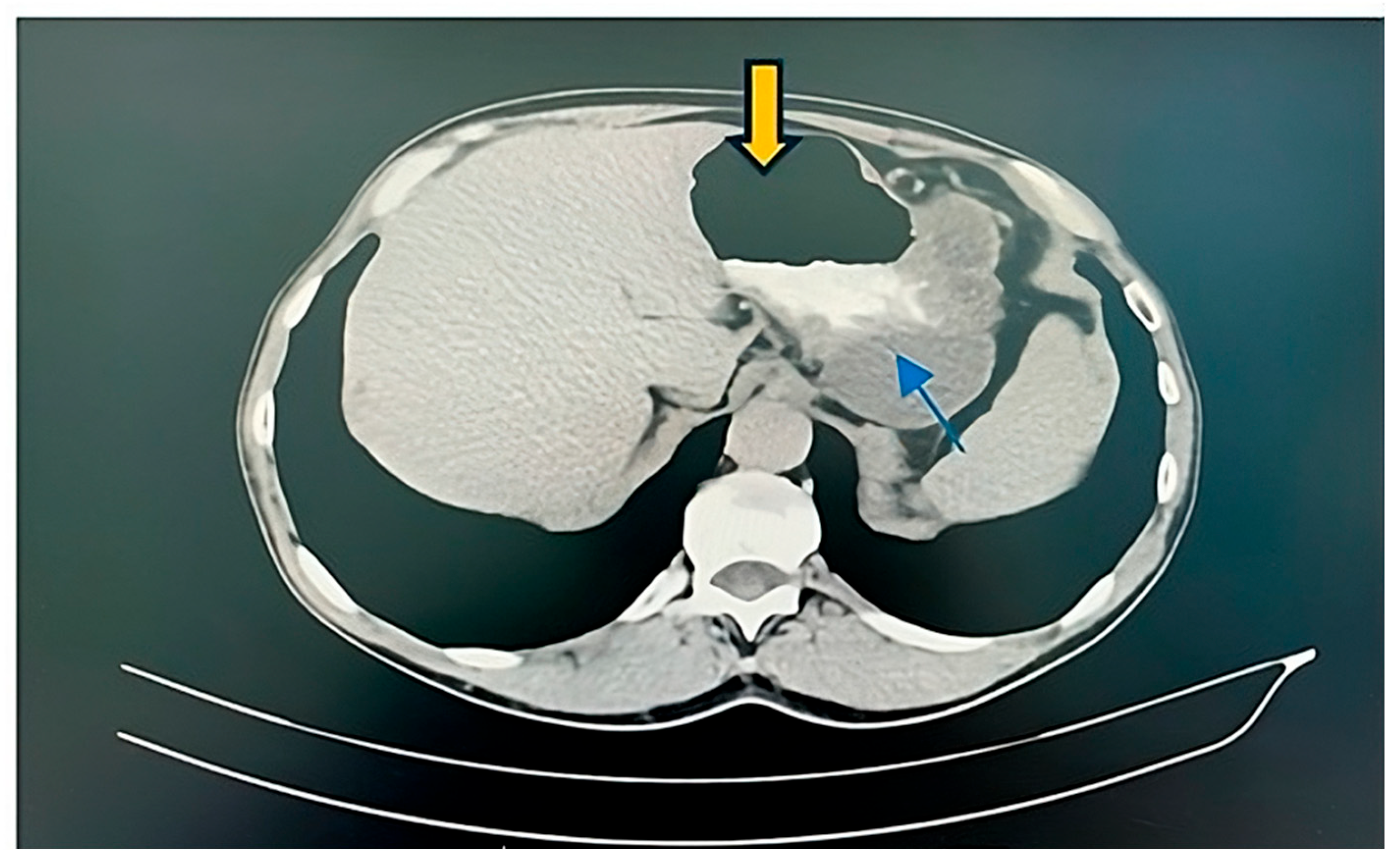
2. Case Report
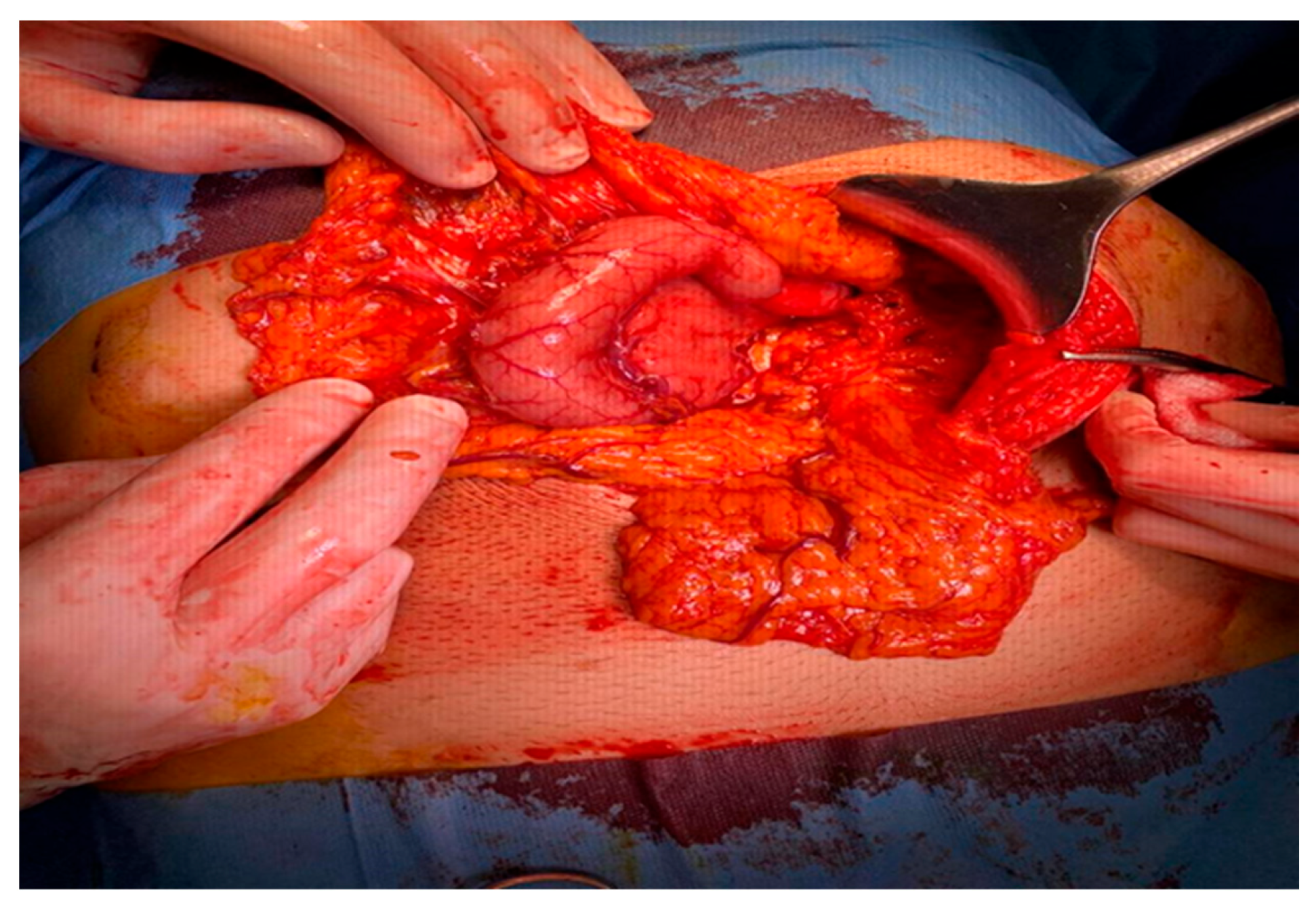
2.1. Surgery
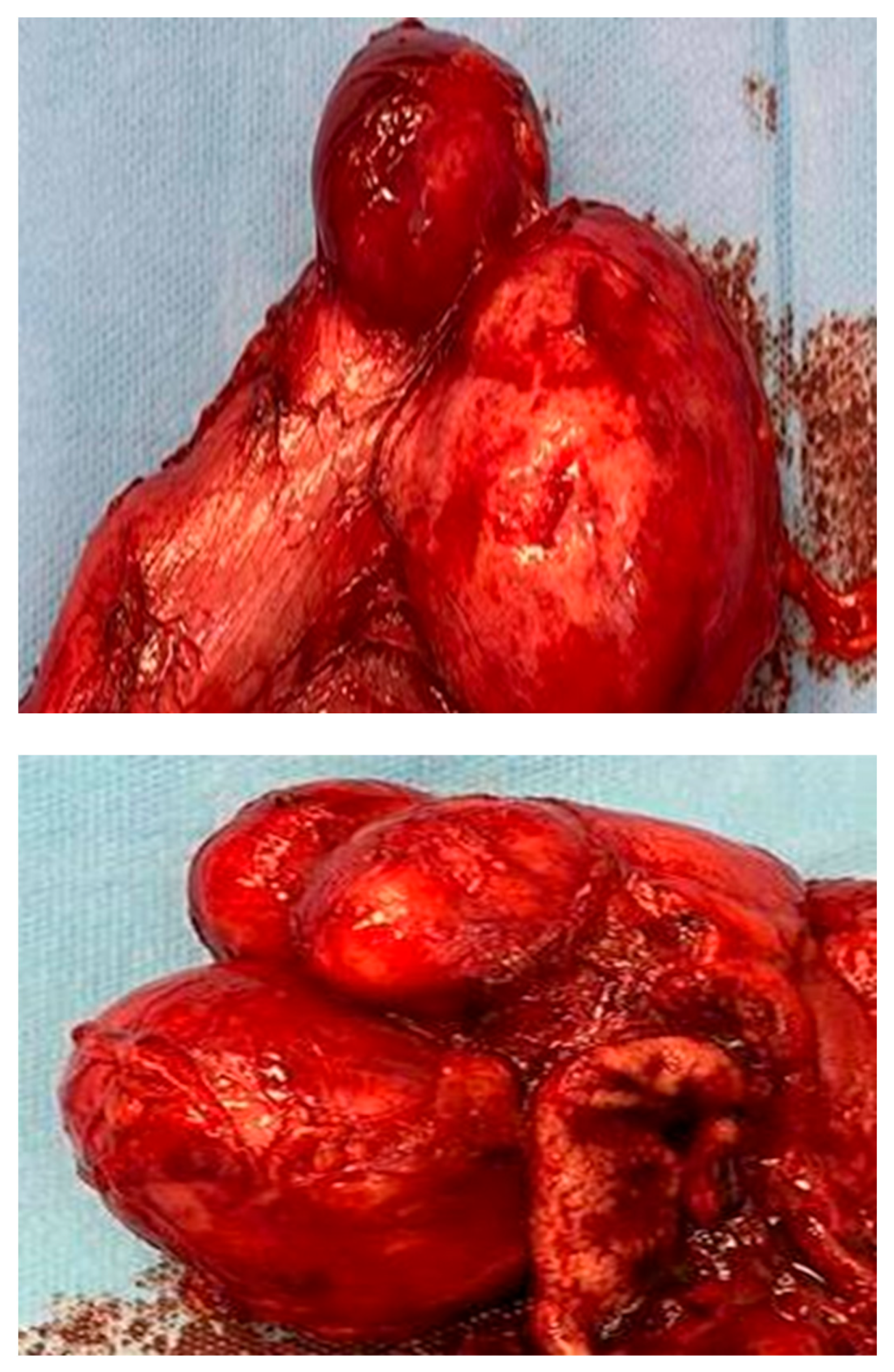
2.2. Histopathological Result
3. Discussion
| Authors | Sex/Age | Symptoms | Location/Size (cm) | CT | US | EUS | Preoperative Diagnosis | Surgery |
|---|---|---|---|---|---|---|---|---|
| [18] | F/28 | Persistent abdominal pain and nausea | Greater curvature of the stomach | NA | Cystic mass divided in two lobules located in the right upper quadrant, having smooth internal surface with dependent echogenic component | Multilayered aspect of the gastric duplication cyst. | GDC | Laparoscopic excision of the cyst (1 year later) |
| [10] | M/27 | Back pain, loss of appetite | Adheres to posterior wall of stomach near to the greater curvature | Nonseptated cystic mass, in relation to pancreatic tail | NA | NA | GDC | Excision of cystic mass with resection of adjacent stomach |
| F/28 | Epigastric pain, vomiting | Medial wall of antrum and pylorus | 4 cystic lesions | NA | Multiple intramural cystic lesions | GDC | Antrectomy and truncal vagotomy | |
| [20] | M/25 | Abdominal pain, epigastric fullness, vomiting, dysphagia, dyspepsia, anemia. | Posterior wall of the stomach | Well-marginated cystic lesion. | NA | NA | GIST | Cyst resected laparoscopically |
| [19] | M/51 | 2 day history of melena | Greater curvature of the stomach | Cystic mass that has a septum dividing it into 2 parts | NA | NA | GDC Adenocarcinoma | Total gastrectomy |
| [21] | F/8 | Abdominal pain and vomiting. | Near the left lobe of the liver and stomach cavity | Cystic mass occupying the epigastric and left hypochondrial area | Upper abdominal cystic mass, which was 12 × 8 × 2.5 cm in size and was located between the left lobe of the liver and stomach cavity | NA | Liver cyst | Cyst resected |
| [34] | F/52 | No | Between the stomach and body of the pancreas | Low density mass without intensification in the arterial phase and portal phase | Anechoic cystic lesion located close to the body of the pancreas | Pancreatic mucinous cystic neoplasm | Laparoscopic resection of the cyst | |
| [22] | F/44 | Abdominal pain, nausea and constipation | Between stomach and body of the pancreas | Cystic lesion in the middle of stomach and body of the pancreas | NA | Normal pancreatic echotexture and a cyst free of internal septations or associated masses but compressed the stomach | Pancreatic mucinos cyst | En bloc resection of the cyst with a portion of the posterior wall of the stomach |
| [23] | M/67 | Anorexia, epigastric pain and severe weight loss | Between the gastric fornix and the body of the pancreas | A cystic lesion located between the gastric fornix and the body of the pancreas. | NO | The cyst, which originated from the demonstrated early diffuse enhancement following the injection of SonoVue. FNA was performed | GDC | Total gastrectomy Histology confirmed the presence of a gastric duplication cyst and malignant degeneration |
| [12] | F/34 | Renal colic | Pancreatic tail | Cystic lesion of the tail of the | Cystic image at the right renal lobe | Cystic dense-walled formation in the tail of the pancreas FNA was performed | GDC | NA |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Li, C.; Wu, H.; Wang, Q.; Gao, Z.-D.; Yang, X.-D.; Jiang, K.-W.; Ye, Y.-J. Clinical Features of Gastric Duplications: Evidence from Primary Case Reports and Published Data. Orphanet J. Rare Dis. 2021, 16, 368. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.-M.; Lu, J.-J.; Ho, L.-C.; Han, L.-L.; Yue, X.; Zhang, H.-H.; Yang, N.-Y. A Huge Completely Isolated Duplication Cyst Complicated by Torsion and Lined by 3 Different Mucosal Epithelial Components in an Adult: A Case Report. Medicine 2018, 97, e13005. [Google Scholar] [CrossRef]
- Alghamdi, A.; Alzaiem, M. Acute Inflammation of Gastric Duplication Cyst in a Toddler. J. Pediatr. Surg. Case Rep. 2020, 58, 101470. [Google Scholar] [CrossRef]
- Bhatti, Z.S.; Anderson, M.A.; Wasnik, A.P. Complete Gastric Duplication in an Adult with Associated Anomalies. Clin. Imaging 2016, 40, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Ibrahim, A.; AlShehabi, Z.; Omran, A.; Sharara, A.I. Gastric Duplication Cyst Presenting as Massive Gastrointestinal Bleeding. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Malekhosseini, S.A.; Moradian, F. Gastric Duplication Cyst in a Man Presenting with Elevated Liver Enzymes and Icterus. Iran. J. Med. Sci. 2014, 39, 228–231. [Google Scholar]
- Sangüesa Nebot, C.; Llorens Salvador, R.; Carazo Palacios, E.; Picó Aliaga, S.; Ibañez Pradas, V. Enteric Duplication Cysts in Children: Varied Presentations, Varied Imaging Findings. Insights Imaging 2018, 9, 1097–1106. [Google Scholar] [CrossRef]
- Anand, S.; Aleem, A. Duplication Cyst. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Inomata, M.; Kai, K.; Ikeda, T.; Ichihara, A.; Masuda, R.; Kiwaki, T.; Tanaka, H.; Kataoka, H.; Nanashima, A. An Adult Case of a Retroperitoneal Isolated Enteric Duplication Cyst with the Imaging Changes over Time. Surg. Case Rep. 2021, 7, 258. [Google Scholar] [CrossRef]
- Singh, J.P.; Rajdeo, H.; Bhuta, K.; Savino, J.A. Gastric Duplication Cyst: Two Case Reports and Review of the Literature. Case Rep. Surg. 2013, 2013, 605059. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.-W.; Han, R.-Q.; Ma, W.-Q.; Wang, G.-N.; Er, L.-M. New Treatment for Gastric Duplication Cyst: Endoscopic Ultrasonography-Guided Fine-Needle Aspiration Combined with Lauromacrogol Sclerotherapy: A Case Report. World J. Clin. Cases 2023, 11, 7905–7910. [Google Scholar] [CrossRef]
- Cherouaqi, Y.; Belabbes, F.; Allaoui, M.; Al Bouzidi, A.; Rouibaa, F. Gastric Duplication Cyst Revealed After an Endoscopic Ultrasound-Guided Fine-Needle Aspiration of a Suspected Mucinous Cystadenoma of the Pancreas. Cureus 2021, 13, e19560. [Google Scholar] [CrossRef]
- Woolfolk, G.M.; McClave, S.A.; Jones, W.F.; Oukrop, R.B.; Mark, M.D. Use of Endoscopic Ultrasound to Guide the Diagnosis and Endoscopic Management of a Large Gastric Duplication Cyst. Gastrointest. Endosc. 1998, 47, 76–79. [Google Scholar] [CrossRef]
- Doepker, M.P.; Ahmad, S.A. Gastric Duplication Cyst: A Rare Entity. J. Surg. Case Rep. 2016, 2016, rjw073. [Google Scholar] [CrossRef][Green Version]
- Glick, Y.; Teixeira, A. Gastric Duplication Cyst. 2012. Available online: https://radiopaedia.org/articles/19579 (accessed on 11 February 2024).
- Lira-Treviño, A.; Carranza Mendoza, I.G.; Borbolla Arizti, J.P.; Soriano-Ríos, A.; Uscanga-Domínguez, L.; Peláez-Luna, M. Pancreatic Cystic Lesions. Differential Diagnosis and Treatment Strategy. Rev. Gastroenterol. México (Engl. Ed.) 2022, 87, 188–197. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The Clinical Utility of CA 19-9 in Pancreatic Adenocarcinoma: Diagnostic and Prognostic Updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef]
- Abdalkader, M.; Al Hassan, S.; Taha, A.; Nica, I. Complicated Gastric Duplication Cyst in an Adult Patient: Uncommon Presentation of an Uncommon Disease. Radiol. Case 2017, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.A.; Al Saeed, M.K.; Ameer AlShaikh, S.A.; Nabar, U.J. Adenocarcinoma Arising from a Gastric Duplication Cyst: A Case Report and Literature Review. Int. Med. Case Rep. J. 2017, 10, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Namdaroglu, O.; Argon, A.; Aydogan, S.; Ozturk, A.; Yakan, S.; Yildirim, M.; Erkan, N. Gastric Duplication Cyst in Adult: Challenge for Surgeons. J. Min. Access Surg. 2017, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.S.; Elhady, H.A.; Elfarargy, M.S. Gastric Duplication Cyst Mimicking a Liver Cyst Case Report and Review of the Literature. Available online: https://pjmhsonline.com/2020/oct_dec/1417.pdf (accessed on 11 February 2024).
- Bailey, C.; Fritz, M.; Webb, L.; Merchant, N.; Parikh, A. Gastric Duplication Cyst Masquerading as a Mucinous Pancreatic Cyst: Case Report and Literature Review. Annals 2014, 96, 88E–90E. [Google Scholar] [CrossRef] [PubMed]
- Togliani, T.; Rinaldi, R.; Pilati, S. Malignant Gastric Duplication Cyst Diagnosed by EUS-FNA. Endosc. Ultrasound 2021, 10, 149. [Google Scholar] [CrossRef]
- Johnston, J.; Wheatley, G.H.; El Sayed, H.F.; Marsh, W.B.; Ellison, E.C.; Bloomston, M. Gastric Duplication Cysts Expressing Carcinoembryonic Antigen Mimicking Cystic Pancreatic Neoplasms in Two Adults. Am. Surg. 2008, 74, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Mardi, K.; Kaushal, V.; Gupta, S. Foregut Duplication Cysts of Stomach Masquerading as Leiomyoma. Indian J. Pathol. Microbiol. 2010, 53, 829. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-B.; Gui, H.-W. Diagnosis of Gastric Duplication Cyst by Positron Emission Tomography/Computed Tomography: A Case Report. World J. Clin. Cases 2019, 7, 3866–3871. [Google Scholar] [CrossRef]
- Bhatia, V.; Garg, P.K.; Gupta, S.D.; Dash, N.R.; Saluja, S.S.; Madan, K. Demonstration of Peristalsis in Gastric Duplication Cyst by EUS: Implications for Diagnosis and Symptomatology (with Videos). Gastrointest. Endosc. 2008, 68, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Seijo Ríos, S.; Lariño Noia, J.; Abdulkader Nallib, I.; Lozano León, A.; Vieites Pérez-Quintela, B.; Iglesias García, J.; Domínguez Muñoz, J.E. Quiste de Duplicación Gástrico: Diagnóstico Por Punción-Aspiración Guiada Por Ecoendoscopia. Rev. Esp. Enferm. Dig. 2008, 100, 586–590. [Google Scholar] [CrossRef][Green Version]
- Mizuide, M.; Ryozawa, S.; Fujita, A.; Ogawa, T.; Katsuda, H.; Suzuki, M.; Noguchi, T.; Tanisaka, Y. Complications of Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Narrative Review. Diagnostics 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Faulx, A.L.; Chak, A. Complications of EUS-FNA. Tech. Gastrointest. Endosc. 2005, 7, 198–199. [Google Scholar] [CrossRef]
- Barr, L.L.; Hayden, C.K.; Stansberry, S.D.; Swischuk, L.E. Enteric Duplication Cysts in Children: Are Their Ultrasonographic Wall Characteristics Diagnostic? Pediatr. Radiol. 1990, 20, 326–328. [Google Scholar] [CrossRef]
- Geller, A.; Wang, K.K.; DiMagno, E.P. Diagnosis of Foregut Duplication Cysts by Endoscopic Ultrasonography. Gastroenterology 1995, 109, 838–842. [Google Scholar] [CrossRef]
- Izumi, H.; Yoshii, H.; Abe, R.; Mukai, M.; Nomura, E.; Ito, H.; Sugiyama, T.; Tajiri, T.; Makuuchi, H. Successful Laparoscopic Resection for Gastric Duplication Cyst: A Case Report. J. Med. Case Rep. 2019, 13, 240. [Google Scholar] [CrossRef]
- Zhan, C.; Zhou, B. Gastric Duplication Cyst Lined by Pseudostratified Columnar Ciliated Epithelium Masquerading as a Pancreatic Mucinous Cystic Neoplasm: A Case Report and Literature Review. Int. J. Clin. Exp. Pathol. 2020, 13, 785–791. [Google Scholar] [PubMed]
- Liu, F.; Xu, X.; Lan, M.; Tao, B.; Liang, Z.; Zeng, J. Clinical Characteristics of Gastric Duplication in Children. Front. Pediatr. 2022, 10, 857056. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gao, T.; Yang, H.; Ma, S.; Li, Q.; Zhou, P.-H. Removal of an Infant’s Gastric Duplication Cyst through Endoscopic Submucosal Dissection: A Case Report. Medicine 2019, 98, e14820. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boicean, A.; Prisca, D.; Bratu, D.G.; Bacila, C.I.; Tanasescu, C.; Chicea, R.; Fleaca, S.R.; Birsan, S.A.; Ichim, C.; Mohor, C.I.; et al. Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics 2024, 14, 675. https://doi.org/10.3390/diagnostics14070675
Boicean A, Prisca D, Bratu DG, Bacila CI, Tanasescu C, Chicea R, Fleaca SR, Birsan SA, Ichim C, Mohor CI, et al. Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics. 2024; 14(7):675. https://doi.org/10.3390/diagnostics14070675
Chicago/Turabian StyleBoicean, Adrian, Diana Prisca, Dan Georgian Bratu, Ciprian Ionut Bacila, Ciprian Tanasescu, Radu Chicea, Sorin Radu Fleaca, Sabrina Andreea Birsan, Cristian Ichim, Calin Ilie Mohor, and et al. 2024. "Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review" Diagnostics 14, no. 7: 675. https://doi.org/10.3390/diagnostics14070675
APA StyleBoicean, A., Prisca, D., Bratu, D. G., Bacila, C. I., Tanasescu, C., Chicea, R., Fleaca, S. R., Birsan, S. A., Ichim, C., Mohor, C. I., Roman, M. D., Cristian, A. N., Todor, S. B., Mohor, C. I., Moisin, A., & Hasegan, A. (2024). Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics, 14(7), 675. https://doi.org/10.3390/diagnostics14070675







