Differences in Histological Subtypes of Invasive Lobular Breast Carcinoma According to Immunohistochemical Molecular Classification
Abstract
1. Introduction
2. Materials and Methods
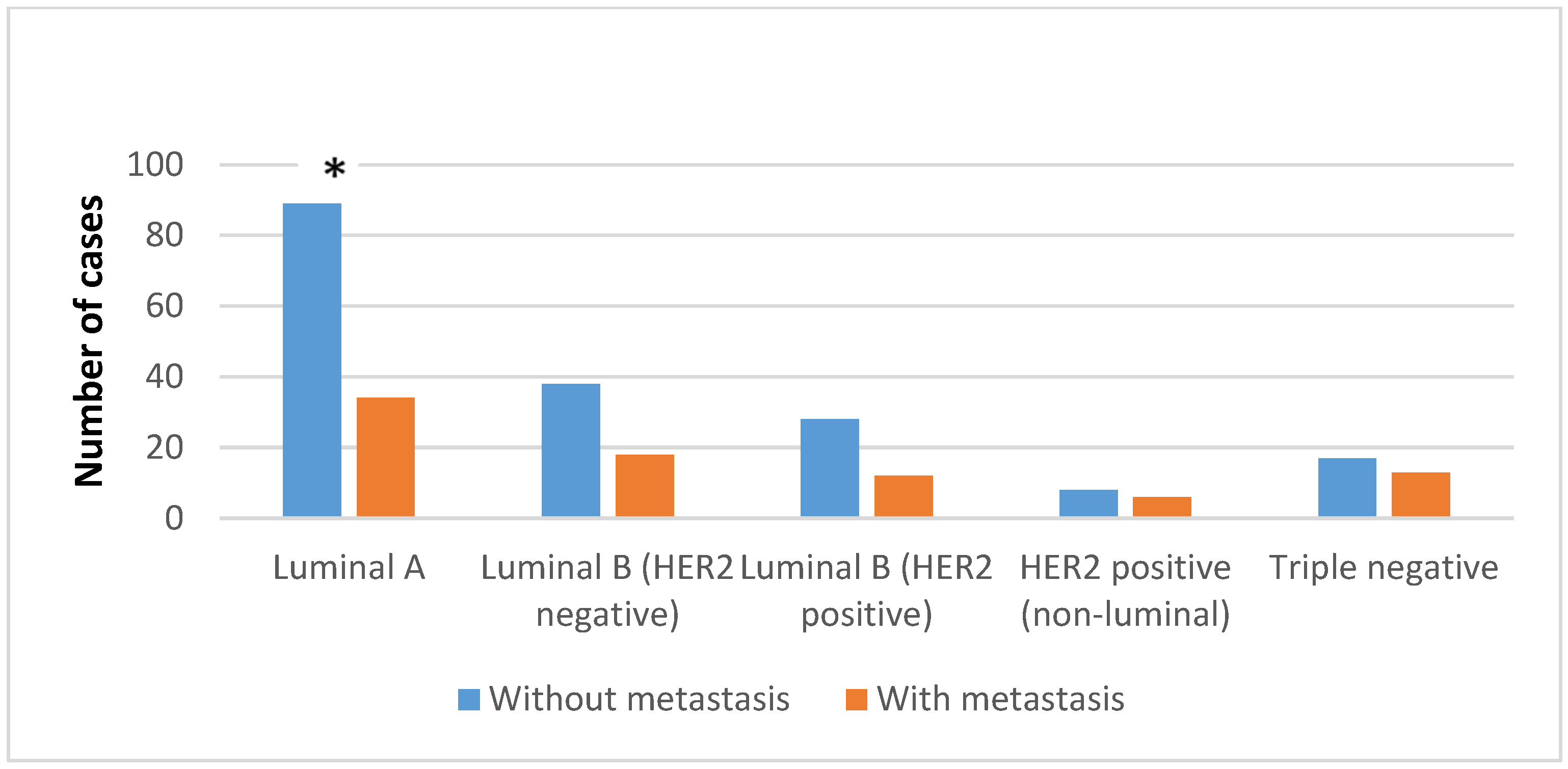
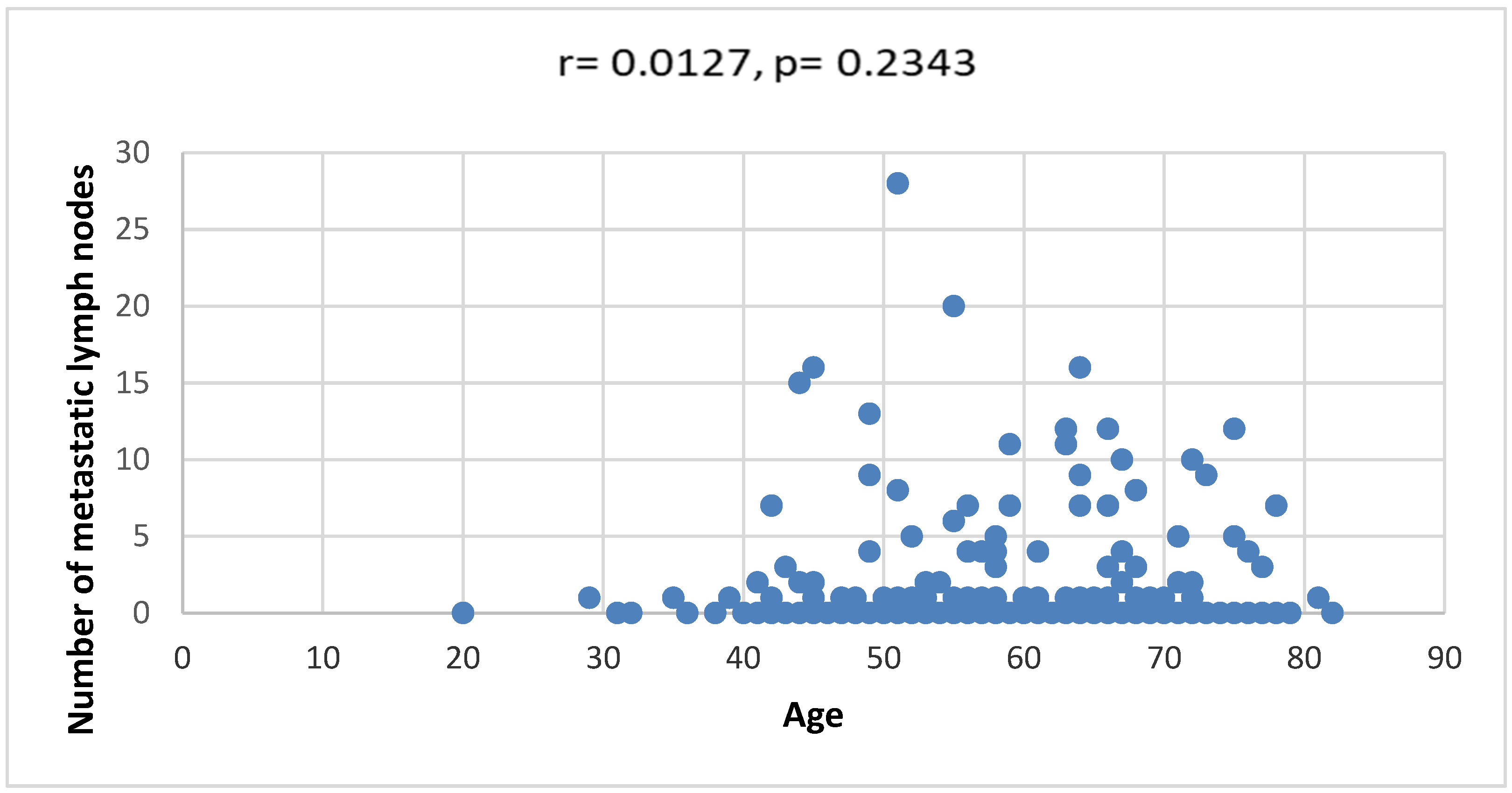
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Classification of Tumours Editorial Board. In Breast Tumours, 5th ed.; IARC: Lyon, France, 2019. [Google Scholar]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Rakha, E.A.; van Deurzen, C.H.M.; Paish, E.C.; Macmillan, R.D.; Ellis, I.O.; Lee, A.H.S. Pleomorphic lobular carcinoma of the breast: Is it a prognostically significant pathological subtype independent of histological grade? Mod. Pathol. 2013, 26, 496–501. [Google Scholar] [CrossRef]
- Dietzel, M.; Baltzer, P.A.; Vag, T.; Gröschel, T.; Gajda, M.; Camara, O.; Kaiser, W.A. Magnetic resonance mammography of invasive lobular versus ductal carcinoma: Systematic comparison of 811 patients reveals high diagnostic accuracy irrespective of typing. J. Comput. Assist. Tomogr. 2010, 34, 587–595. [Google Scholar] [CrossRef]
- Tabar, L.; Dean, P.B.; Lee Tucker, F.; Puchkova, O.; Bozo, R.; Yen, A.M.-F.; Chen, S.L.-S.; Smith, R.A.; Duffy, S.W.; Chen, T.H.-H. The challenging imaging and histopathologic features of diffusely infiltrating breast cancer. Eur. J. Radiol. 2023, 161, 110754. [Google Scholar] [CrossRef]
- Ni, M.; Zhou, X.; Liu, J.; Yu, H.; Gao, Y.; Zhang, X.; Li, Z. Prediction of the clinicopathological subtypes of breast cancer using a fisher discriminant analysis model based on radiomic features of diffusion-weighted MRI. BMC Cancer 2020, 20, 1073. [Google Scholar] [CrossRef]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in Breast Cancer Stage at Diagnosis and Cancer-Specific Survival by Race and Ethnicity in the United States. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Ragazzi, M.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Morganti, S.; Santandrea, G.; Fanelli, G.N.; Despini, L.; Invernizzi, M.; et al. Assessment of estrogen receptor low positive status in breast cancer: Implications for pathologists and oncologists. Histol. Histopathol. 2021, 36, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO–College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, K.H.; Kim, Y.; Park, S.E.; Lee, H.S.; Lim, S.W.; Cho, J.H.; Kim, J.-Y.; Lee, J.E.; Ahn, J.S.; et al. Discordance of the PAM50 Intrinsic Subtypes Compared with Immunohistochemistry-Based Surrogate in Breast Cancer Patients: Potential Implication of Genomic Alterations of Discordance. Cancer Res. Treat. 2019, 51, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Al-thoubaity, F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann. Med. Surg. 2019, 49, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Katerji, H.; Turner, B.M.; Audeh, W.; Hicks, D.G. HER2-low breast cancers: Incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod. Pathol. 2022, 35, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-P.; Wu, Q.-S.; Chen, H.; Wang, X.-X.; Chen, Q.-X.; Zhang, J.; Song, C.-G. Validation of the Prognostic Significance of the Prognostic Stage Group According to the Eighth Edition of American Cancer Joint Committee on Cancer Staging System in Triple-Negative Breast Cancer: An Analysis from Surveillance, Epidemiology, and End Results 18 Database. J. Surg. Res. 2020, 247, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Lillemoe, T.J.; Finkelstein, M.J.; Money, J.E.; Susnik, B.; Grimm, E.; Kang, S.-H.L.; Swenson, K.K. Utility of Oncotype DX risk assessment in patients with invasive lobular carcinoma. Clin. Breast Cancer 2016, 16, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.T.; Le, R.D.; Nguyen, C.V. Breast cancer molecular subtype and relationship with clinicopathological profiles among Vietnamese women: A retrospective study. Pathol.-Res. Pract. 2023, 250, 154819. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Iorfida, M.; Maiorano, E.; Orvieto, E.; Maisonneuve, P.; Bottiglieri, L.; Rotmensz, N.; Montagna, E.; Dellapasqua, S.; Veronesi, P.; Galimberti, V.; et al. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res. Treat. 2012, 133, 713–723. [Google Scholar] [CrossRef]
- Simpson, P.; Reis-Filho, J.; Lambros, M.; Jones, C.; Steele, D.; Mackay, A.; Iravani, M.; Fenwick, K.; Dexter, T.; Jones, A.; et al. Molecular profiling pleomorphic lobular carcinomas of the breast: Evidence for a common molecular genetic pathway with classic lobular carcinomas. J. Pathol. 2008, 215, 231–244. [Google Scholar] [CrossRef]
- Sardanelli, F.; Giuseppetti, G.M.; Panizza, P.; Bazzocchi, M.; Fausto, A.; Simonetti, G.; Lattanzio, V.; Del Maschio, A.; for the Italian Trial for Breast MR in Multifocal/Multicentric Cancer. Sensitivity of MRI Versus Mammography for Detecting Foci of Multifocal, Multicentric Breast Cancer in Fatty and Dense Breasts Using the Whole-Breast Pathologic Examination as a Gold Standard. AJR Am. J. Roentgenol. 2004, 183, 1149–1157. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Bauer, K.R.; Caggiano, V. Variation in breast cancer subtypes with age and race/ethnicity. Crit. Rev. Oncol. Hematol. 2010, 76, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hilleren, D.J.; Andersson, I.T.; Lindholm, K.; Linnell, F.S. Invasive lobular carcinoma: Mammographic findings in a 10-year experience. Radiology 1991, 178, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kwast, A.B.G.; Groothuis-Oudshoorn, K.C.G.M.; Grandjean, I.; Ho, V.K.Y.; Voogd, A.C.; Menke-Pluymers, M.B.E.; van der Sangen, M.J.C.; Tjan-Heijnen, V.C.G.; Kiemeney, L.A.; Siesling, S. Histological type is not an independent prognostic factor for the risk pattern of breast cancer recurrences. Breast Cancer Res. Treat. 2012, 135, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Volz, C.; Mau, C.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Hanusch, C.; Jackisch, C.; et al. Response and prognosis after neoadjuvant chemotherapy in 1,051 patients with infiltrating lobular breast carcinoma. Breast Cancer Res. Treat. 2014, 144, 153–162. [Google Scholar] [CrossRef]
- Abubakar, M.; Figueroa, J.; Ali, H.R.; Blows, F.; Lissowska, J.; Caldas, C.; Easton, D.F.; Sherman, M.E.; Garcia-Closas, M.; Dowsett, M.; et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod. Pathol. 2019, 32, 1244–1256. [Google Scholar] [CrossRef]
- Castellarnau-Visús, M.; Soveral, I.; Regueiro Espín, P.; Pineros Manzano, J.; Del Río Holgado, M. Prognostic factors in Luminal B-like HER2-negative breast cancer tumors. Surg. Oncol. 2023, 49, 101968. [Google Scholar] [CrossRef]
- Segar, J.M.; Pandey, R.; Farr, K.J.; Nagle, R.; LeBeau, L.; Gonzalez, V.J.; Chalasani, P. Clinicopathological and Molecular Characteristics of Pleomorphic Invasive Lobular Carcinoma. Int. J. Breast Cancer 2020, 2020, 8816824. [Google Scholar] [CrossRef]
- Orvieto, E.; Maiorano, E.; Bottiglieri, L.; Maisonneuve, P.; Rotmensz, N.; Galimberti, V.; Luini, A.; Brenelli, F.; Gatti, G.; Viale, G. Clinicopathologic characteristics of invasive lobular carcinoma of the breast: Results of an analysis of 530 cases from a single institution. Cancer 2008, 113, 1511–1520. [Google Scholar] [CrossRef]
- Sheffield, B.S.; Kos, Z.; Asleh-Aburaya, K.; Wang, X.Q.; Leung, S.; Gao, D.; Won, J.; Chow, C.; Rachamadugu, R.; Stijleman, I.; et al. Molecular subtype profiling of invasive breast cancers weakly positive for estrogen receptor. Breast Cancer Res. Treat. 2016, 155, 483–490. [Google Scholar] [CrossRef]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [PubMed]
- Carbognin, L.; Sperduti, I.; Fabi, A.; Dieci, M.V.; Kadrija, D.; Griguolo, G.; Pilotto, S.; Guarneri, V.; Zampiva, I.; Brunelli, M.; et al. Prognostic impact of proliferation for resected early stage ‘pure’ invasive lobular breast cancer: Cut-off analysis of Ki67 according to histology and clinical validation. Breast 2017, 35, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kizy, S.; Huang, J.L.; Marmor, S.; Tuttle, T.M.; Hui, J.Y.C. Impact of the 21-gene recurrence score on outcome in patients with invasive lobular carcinoma of the breast. Breast Cancer Res. Treat. 2017, 165, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Green, I.; McCormick, B.; Cranor, M.; Rosen, P.P. A Comparative Study of Pure Tubular and Tubulolobular Carcinoma of the Breast. Am. J. Surg. Pathol. 1997, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, C.; Zoppoli, G.; Gundem, G.; Pruneri, G.; Larsimont, D.; Fornili, M.; Fumagalli, D.; Brown, D.; Rothé, F.; Vincent, D.; et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J. Clin. Oncol. 2016, 34, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
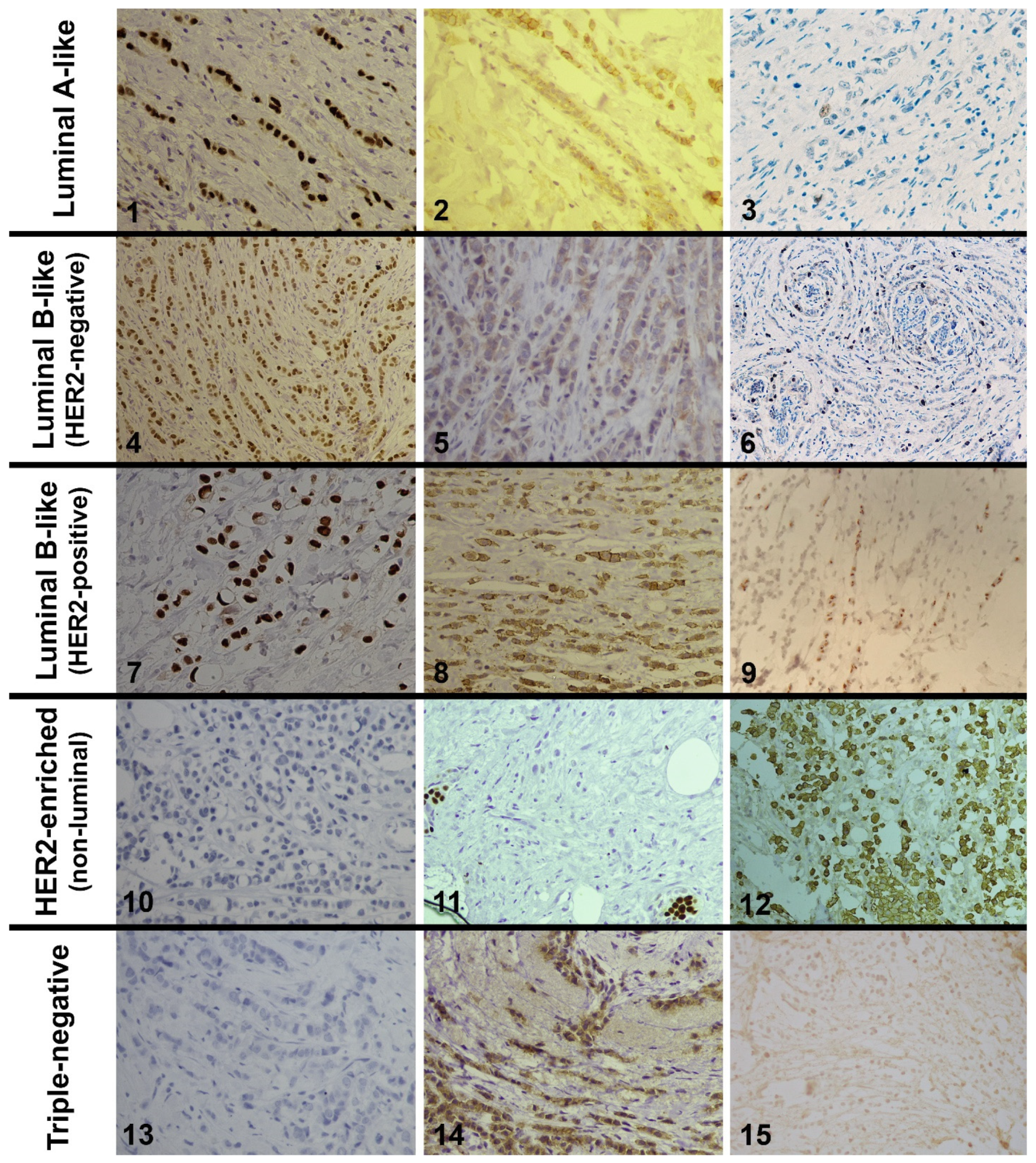

| Average Age | Classic | Alveolar | Solid | Tubulolobular | Pleomorphic | Mixed Lobular | |
|---|---|---|---|---|---|---|---|
| Luminal A-like | 58.7 ± 11.5 | 77 | 43 ± 4.2 | 48.7 ± 5.5 | 62.9 ± 13.4 | 63.5 ± 10.4 | 58.9 ± 11.8 |
| Luminal B-like (HER2 negative) | 61 ± 10 | - | 62 | - | 55.5 ± 13.4 | 56.2 ± 16 | 58.5 ± 11.4 |
| Luminal B-like (HER2 positive) | 56.6 ± 11.8 | 54 | 46 ± 14.1 | - | 68 ± 14.1 | 81 | 57.2 ± 12.5 |
| HER2 positive (non-luminal) | 56 ± 5 | 64 | - | - | 53 ± 22.5 | - | 55.5 ± 13.3 |
| Triple negative | 60.9 ± 10 | - | 36 | - | 54.1 ± 10.5 | 65.7 ± 6.5 | 58.3 ± 11.1 |
| 58.7 ± 11 | 65 ± 11.5 | 46 ± 10.9 * | 48.7 ± 5.5 | 57.1 ± 14.1 | 62.7 ± 12.6 | 58.3 ± 11.8 |
| pT1 | pT2 | pT3 | pT4 | pTx | ∑ | |
|---|---|---|---|---|---|---|
| Luminal A-like | 35 (28.45%) | 55 (44.72%) | 4 (3.25%) | 10 (8.13%) | 19 (15.45%) | 123 |
| Luminal B-like (HER2 negative) | 15 (26.8%) | 30 (53.57%) * | 1 (1.78%) | 2 (3.57%) | 8 (14.28%) | 56 |
| Luminal B-like (HER2 positive) | 5 (12.5%) * | 13 (32.5%) | 5 (12.5%) * | 6 (15%) | 11 (27.5%) * | 40 |
| HER2 positive (non-luminal) | 2 (14.28%) | 4 (28.58%) | 2 (14.28%) | 4 (28.58%) * | 2 (14.28%) | 14 |
| Triple negative | 9 (30%) | 13 (43.33%) | 2 (6.67%) | 2 (6.67%) | 4 (13.33%) | 30 |
| ∑ | 66 | 115 | 14 | 24 | 44 | 263 |
| Classic | Alveolar | Solid | Tubulolobular | Pleomorphic | Mixed Lobular | ∑ | |
|---|---|---|---|---|---|---|---|
| Luminal A-like | 102 (82.93%) * | 1 (0.81%) | 2 (1.63%) | 3 (2.42%) | 9 (7.33%) * | 6 (4.88%) | 123 |
| Luminal B-like (HER2 negative) | 35 (62.5%) | 0 (0%) | 1 (1.79%) | 0 (0%) | 15 (26.78%) | 5 (8.93%) | 56 |
| Luminal B-like (HER2 positive) | 34 (85%) | 1 (2.5%) | 2 (5%) | 0 (0%) | 2 (5%) | 1 (2.5%) | 40 |
| HER2 positive (non-luminal) | 8 (57.14%) | 1 (7.15%) | 0 (0%) | 0 (0%) | 5 (35.71%) | 0 (0%) | 14 |
| Triple negative | 16 (53.33%) * | 0 (0%) | 1 (3.34%) | 0 (0%) | 10 (33.33%) * | 3 (10%) | 30 |
| ∑ | 195 | 3 | 6 | 3 | 41 | 15 | 263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić, I.; Cvetković, J.; Ilić, R.; Cvetković, L.; Milićević, A.; Todorović, S.; Ranđelović, P. Differences in Histological Subtypes of Invasive Lobular Breast Carcinoma According to Immunohistochemical Molecular Classification. Diagnostics 2024, 14, 660. https://doi.org/10.3390/diagnostics14060660
Ilić I, Cvetković J, Ilić R, Cvetković L, Milićević A, Todorović S, Ranđelović P. Differences in Histological Subtypes of Invasive Lobular Breast Carcinoma According to Immunohistochemical Molecular Classification. Diagnostics. 2024; 14(6):660. https://doi.org/10.3390/diagnostics14060660
Chicago/Turabian StyleIlić, Ivan, Jana Cvetković, Ratko Ilić, Ljubiša Cvetković, Aleksandar Milićević, Stefan Todorović, and Pavle Ranđelović. 2024. "Differences in Histological Subtypes of Invasive Lobular Breast Carcinoma According to Immunohistochemical Molecular Classification" Diagnostics 14, no. 6: 660. https://doi.org/10.3390/diagnostics14060660
APA StyleIlić, I., Cvetković, J., Ilić, R., Cvetković, L., Milićević, A., Todorović, S., & Ranđelović, P. (2024). Differences in Histological Subtypes of Invasive Lobular Breast Carcinoma According to Immunohistochemical Molecular Classification. Diagnostics, 14(6), 660. https://doi.org/10.3390/diagnostics14060660








