Analysis of OCT Scanning Parameters in AMD and RVO
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Image Preprocessing
2.3. Data Analysis
3. Results
3.1. Frequency of Biomarkers in Data Collection
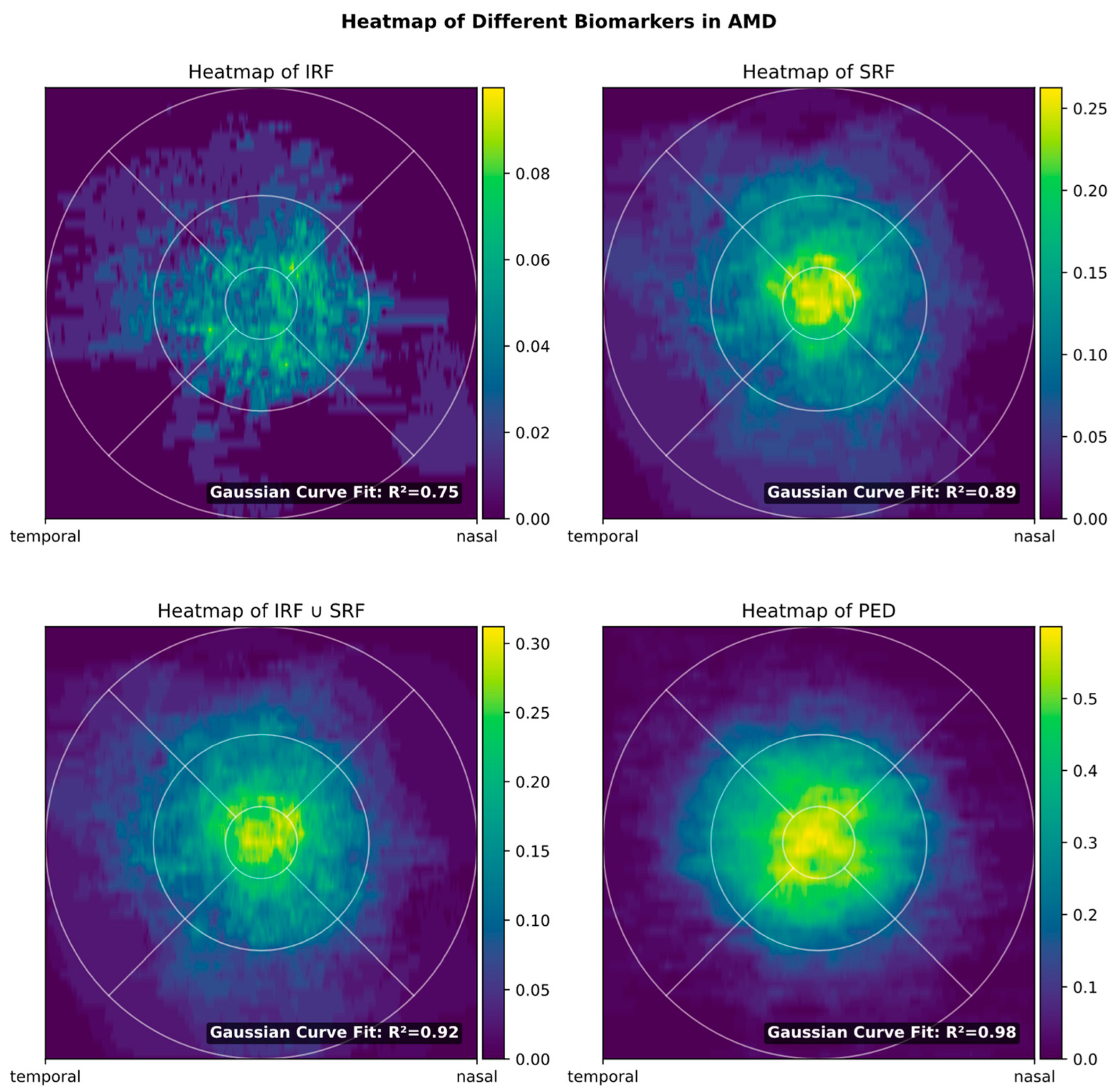
3.2. Biomarker Distribution
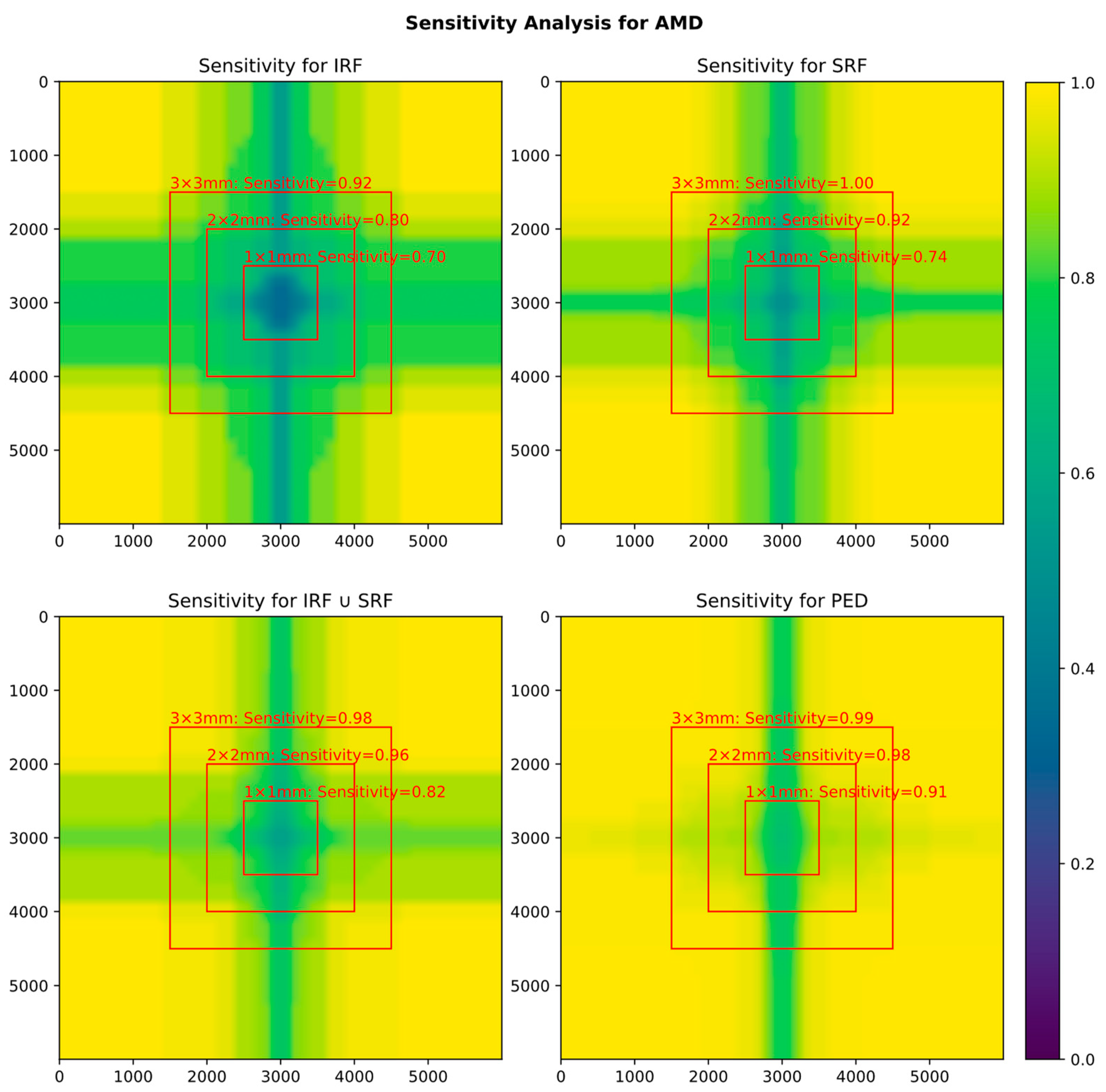
3.3. Influence of FOV on Biomarker Detection
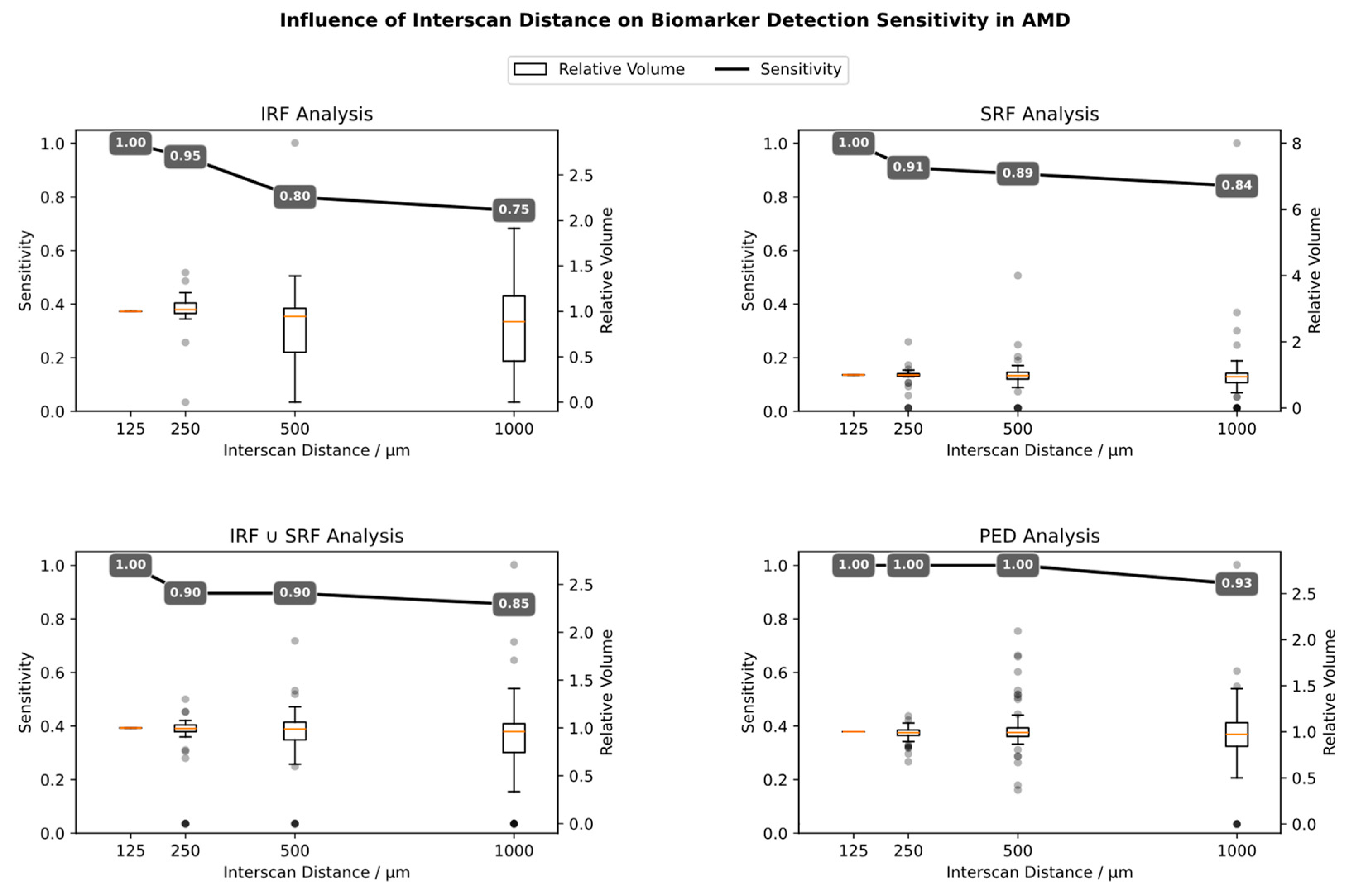
3.4. Influence of Interscan Distance (ISD)
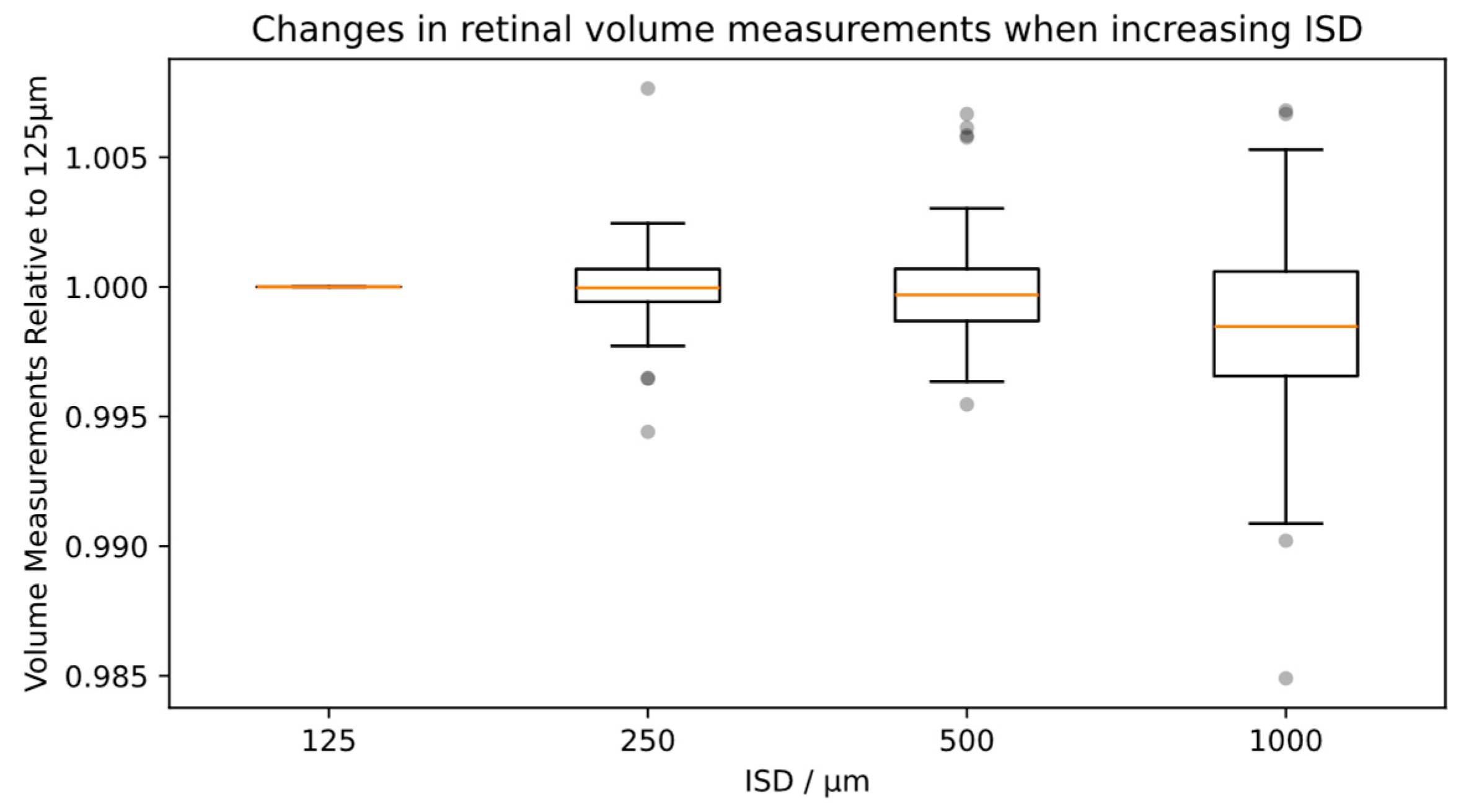
3.5. Influence of ISD on Total Retinal Volume
4. Discussion
4.1. Motivation
- From a patient’s perspective, the acquisition time will increase. Especially in cases of poor fixation because of advanced macular disease, longer acquisition time can quickly become bothersome, especially since the examination potentially must be repeated every other month.
- From the device operator’s perspective, the increased acquisition time is also bothersome and reduces efficiency and productivity. Additionally, not only acquisition but also processing and storage time are prolonged.
- From the ophthalmologist’s perspective, more image material means longer loading time and more data to interpret.
- Also, due to poor fixation or tear film breakup, image quality might shrink with longer acquisition time, so that the advantages of high-resolution widefield OCT might be outweighed by worse image quality.
4.2. Implications for Home Monitoring OCT Devices
4.3. Comparison to Similar Studies
4.4. Authors’ Suggestion for Scanning Parameters
4.5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carnevali, A.; Mastropasqua, R.; Gatti, V.; Vaccaro, S.; Mancini, A.; D’Aloisio, R.; Lupidi, M.; Cerquaglia, A.; Sacconi, R.; Borrelli, E.; et al. Optical Coherence Tomography Angiography in Intermediate and Late Age-Related Macular Degeneration: Review of Current Technical Aspects and Applications. Appl. Sci. 2020, 10, 8865. [Google Scholar] [CrossRef]
- Szeto, S.K.; Lai, T.Y.; Vujosevic, S.; Sun, J.K.; Sadda, S.R.; Tan, G.; Sivaprasad, S.; Wong, T.Y.; Cheung, C.Y. Optical Coherence Tomography in the Management of Diabetic Macular Oedema. Prog. Retin. Eye Res. 2024, 98, 101220. [Google Scholar] [CrossRef]
- Hirano, Y.; Suzuki, N.; Tomiyasu, T.; Kurobe, R.; Yasuda, Y.; Esaki, Y.; Yasukawa, T.; Yoshida, M.; Ogura, Y. Multimodal Imaging of Microvascular Abnormalities in Retinal Vein Occlusion. J. Clin. Med. 2021, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F.; et al. Guidelines for the Management of Neovascular Age-Related Macular Degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P1–P65. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G. Retinal Vein Occlusions Preferred Practice Pattern®. Ophthalmology 2020, 127, P288–P320. [Google Scholar] [CrossRef] [PubMed]
- Joltikov, K.A.; Sesi, C.A.; de Castro, V.M.; Davila, J.R.; Anand, R.; Khan, S.M.; Farbman, N.; Jackson, G.R.; Johnson, C.A.; Gardner, T.W. Disorganization of Retinal Inner Layers (DRIL) and Neuroretinal Dysfunction in Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5481–5486. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; von der Emde, L.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M.; Rubin, D.L. Progression of Photoreceptor Degeneration in Geographic Atrophy Secondary to Age-Related Macular Degeneration. JAMA Ophthalmol. 2020, 138, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Yu, H.J.; Wakatsuki, Y.; Marion, K.M.; Wykoff, C.C.; Sadda, S.R. OCT Risk Factors for Development of Atrophy in Eyes with Intermediate Age-Related Macular Degeneration. Ophthalmol. Retin. 2023, 7, 253–260. [Google Scholar] [CrossRef]
- von der Burchard, C.; Treumer, F.; Ehlken, C.; Koinzer, S.; Purtskhvanidze, K.; Tode, J.; Roider, J. Retinal Volume Change Is a Reliable OCT Biomarker for Disease Activity in Neovascular AMD. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, S.A.; Engelbert, M.; Laud, K.; Margolis, R.; Spaide, R.F.; Freund, K.B. Outer Retinal Tubulation: A Novel Optical Coherence Tomography Finding. Arch. Ophthalmol. 2009, 127, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Parravano, M.; Clemens, A.; Dolz-Marco, R.; Holz, F.G.; Munk, M.R.; Nicolò, M.; Ricci, F.; Silva, R.; Talks, S.J.; et al. Fluid as a Critical Biomarker in Neovascular Age-Related Macular Degeneration Management: Literature Review and Consensus Recommendations. Eye 2021, 35, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Jeng, Y.-T.; Lai, T.-T.; Lin, C.-W.; Chen, T.-C.; Hsieh, Y.-T.; Lin, C.-P.; Ho, T.-C.; Yang, C.-M.; Yang, C.-H. The Impact of Retinal Fluid Tolerance on the Outcomes of Neovascular Age-Related Macular Degeneration Treated Using Aflibercept: A Real-World Study. PLoS ONE 2022, 17, e0271999. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, S.S.; Janakan, V.; Sandhu, S.S.; Amirul-Islam, F.M.; Abedi, F.; Guymer, R.H. Implication of Recurrent or Retained Fluid on Optical Coherence Tomography for Visual Acuity during Active Treatment of Neovascular Age-Related Macular Degeneration with a Treat and Extend Protocol. Retina 2016, 36, 1331–1339. [Google Scholar] [CrossRef]
- Guymer, R.H.; Markey, C.M.; McAllister, I.L.; Gillies, M.C.; Hunyor, A.P.; Arnold, J.J. Fluid Investigators Tolerating Subretinal Fluid in Neovascular Age-Related Macular Degeneration Treated with Ranibizumab Using a Treat-and-Extend Regimen: FLUID Study 24-Month Results. Ophthalmology 2019, 126, 723–734. [Google Scholar] [CrossRef]
- von der Burchard, C.; Sudkamp, H.; Tode, J.; Ehlken, C.; Purtskhvanidze, K.; Moltmann, M.; Heimes, B.; Koch, P.; Münst, M.; Endt, M. vom; et al. Self-Examination Low-Cost Full-Field Optical Coherence Tomography (SELFF-OCT) for Neovascular Age-Related Macular Degeneration: A Cross-Sectional Diagnostic Accuracy Study. BMJ Open 2022, 12, e055082. [Google Scholar] [CrossRef]
- Bogunovic, H.; Venhuizen, F.; Klimscha, S.; Apostolopoulos, S.; Bab-Hadiashar, A.; Bagci, U.; Beg, M.F.; Bekalo, L.; Chen, Q.; Ciller, C.; et al. RETOUCH: The Retinal OCT Fluid Detection and Segmentation Benchmark and Challenge. IEEE Trans. Med. Imaging 2019, 38, 1858–1874. [Google Scholar] [CrossRef]
- Coulibaly, L.M.; Sacu, S.; Fuchs, P.; Bogunovic, H.; Faustmann, G.; Unterrainer, C.; Reiter, G.S.; Schmidt-Erfurth, U. Personalized Treatment Supported by Automated Quantitative Fluid Analysis in Active Neovascular Age-Related Macular Degeneration (nAMD)—A Phase III, Prospective, Multicentre, Randomized Study: Design and Methods. Eye 2023, 37, 1464–1469. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Clemons, T.E.; Domalpally, A.; Elman, M.J.; Havilio, M.; Agrón, E.; Benyamini, G.; Chew, E.Y. Retinal Specialist versus Artificial Intelligence Detection of Retinal Fluid from OCT: Age-Related Eye Disease Study 2: 10-Year Follow-On Study. Ophthalmology 2021, 128, 100–109. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Goldstein, M.; Goldenberg, D.; Zur, D.; Shulman, S.; Loewenstein, A. Prospective, Longitudinal Pilot Study:Daily Self-Imaging with Patient-Operated Home OCT in Neovascular Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100034. [Google Scholar] [CrossRef]
- Kim, J.E.; Tomkins-Netzer, O.; Elman, M.J.; Lally, D.R.; Goldstein, M.; Goldenberg, D.; Shulman, S.; Benyamini, G.; Loewenstein, A. Evaluation of a Self-Imaging SD-OCT System Designed for Remote Home Monitoring. BMC Ophthalmol. 2022, 22, 261. [Google Scholar] [CrossRef]
- Fang, P.P.; Domdei, N.; Herrmann, P.; Schmitz-Valckenberg, S.; Holz, F.G.; Harmening, W.M.; Krohne, T.U. Minimal Optical Coherence Tomography B-Scan Density for Reliable Detection of Intraretinal And Subretinal Fluid in Macular Diseases. Retina 2019, 39, 150. [Google Scholar] [CrossRef] [PubMed]
- Barañano, A.E.; Keane, P.A.; Ruiz-Garcia, H.; Walsh, A.C.; Sadda, S.R. Impact of Scanning Density on Spectral Domain Optical Coherence Tomography Assessments in Neovascular Age-Related Macular Degeneration. Acta Ophthalmol. 2012, 90, e274-80. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.K.; Rayess, N.; Rahimy, E.; Maguire, J.I.; Hsu, J. Radial versus Raster Spectral-Domain Optical Coherence Tomography Scan Patterns for Detection of Macular Fluid in Neovascular Age-Related Macular Degeneration. Br. J. Ophthalmol. 2016, 100, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Nittala, M.G.; Konduru, R.; Ruiz-Garcia, H.; Sadda, S.R. Effect of OCT Volume Scan Density on Thickness Measurements in Diabetic Macular Edema. Eye 2011, 25, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Velaga, S.B.; Nittala, M.G.; Konduru, R.K.; Heussen, F.; Keane, P.A.; Sadda, S.R. Impact of Optical Coherence Tomography Scanning Density on Quantitative Analyses in Neovascular Age-Related Macular Degeneration. Eye 2017, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Barteselli, G.; Bartsch, D.-U.; Kozak, I.; Wang, H.; El-Emam, S.; Doede, A.L.; Cheng, L.; Freeman, W.R. Influence of Scanning Density on Macular Choroidal Volume Measurement Using Spectral-Domain Optical Coherence Tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1303–1309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- von der Burchard, C.; Tode, J.; Ehlken, C.; Roider, J. 2mm Central Macular Volume Scan Is Sufficient to Detect Exudative Age-Related Macular Degeneration Activity in Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 374. [Google Scholar]
- Yanagi, Y.; Foo, V.H.X.; Yoshida, A. Asian Age-Related Macular Degeneration: From Basic Science Research Perspective. Eye 2019, 33, 34–49. [Google Scholar] [CrossRef]

| Mean Absolute Percentage Error (MAPE) When Increasing ISD | |||||
|---|---|---|---|---|---|
| ISD (µm) | Biomarker Volume | ||||
| IRF | SRF | IRF or SRF | PED | Total Retina | |
| AMD Patients | |||||
| 250 | 15.49% | 18.14% | 15.58% | 5.17% | 0.10% |
| 500 | 42.64% | 31.68% | 22.52% | 14.92% | 0.15% |
| 1000 | 45.72% | 53.14% | 36.30% | 26.42% | 0.32% |
| RVO Patients | |||||
| 250 | 9.37% | 1.17% | 9.07% | n/a | n/a |
| 500 | 5.42% | 4.31% | 5.03% | n/a | n/a |
| 1000 | 25.39% | 31.48% | 25.53% | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burchard, C.v.d.; Roider, J.; Kepp, T. Analysis of OCT Scanning Parameters in AMD and RVO. Diagnostics 2024, 14, 516. https://doi.org/10.3390/diagnostics14050516
Burchard Cvd, Roider J, Kepp T. Analysis of OCT Scanning Parameters in AMD and RVO. Diagnostics. 2024; 14(5):516. https://doi.org/10.3390/diagnostics14050516
Chicago/Turabian StyleBurchard, Claus von der, Johann Roider, and Timo Kepp. 2024. "Analysis of OCT Scanning Parameters in AMD and RVO" Diagnostics 14, no. 5: 516. https://doi.org/10.3390/diagnostics14050516
APA StyleBurchard, C. v. d., Roider, J., & Kepp, T. (2024). Analysis of OCT Scanning Parameters in AMD and RVO. Diagnostics, 14(5), 516. https://doi.org/10.3390/diagnostics14050516






