Progress of Multiparameter Magnetic Resonance Imaging in Bladder Cancer: A Comprehensive Literature Review
Abstract
1. Introduction
2. The Role of DWI in Bladder Cancer
2.1. The Role of DWI in Staging and Grading of Bladder Cancer
2.2. The Role of DWI in the Evaluation of Biological Invasiveness and Prognosis of Bladder Cancer
2.3. Intravoxel Incoherent Motion (IVIM) and Diffusion Kurtosis Imaging (DKI)
2.4. The Role of DWI Radiomics in Bladder Cancer
2.5. Diagnostic Value of DWI Imaging Features in Bladder Cancer
3. The Role of DCE in Bladder Cancer
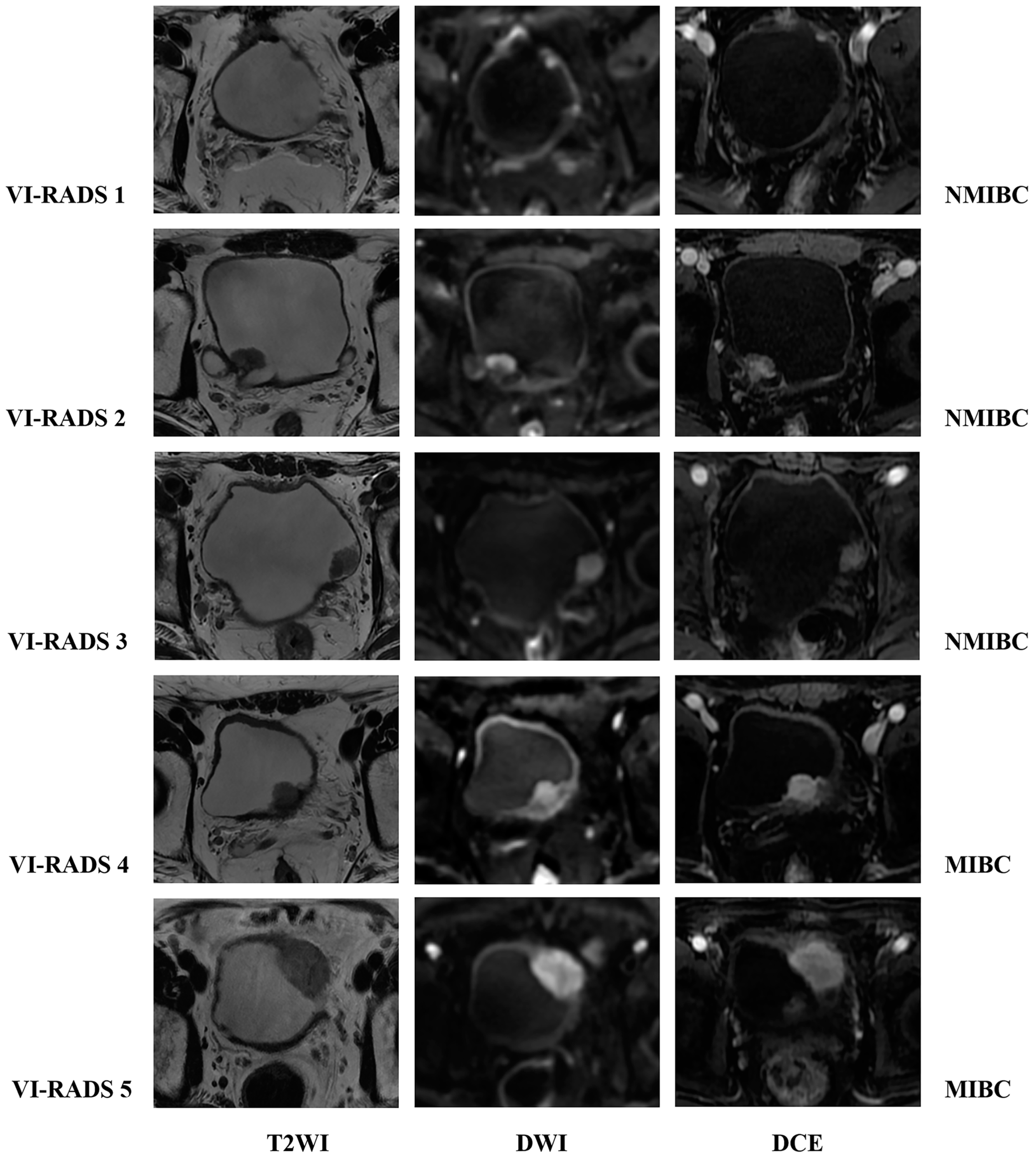
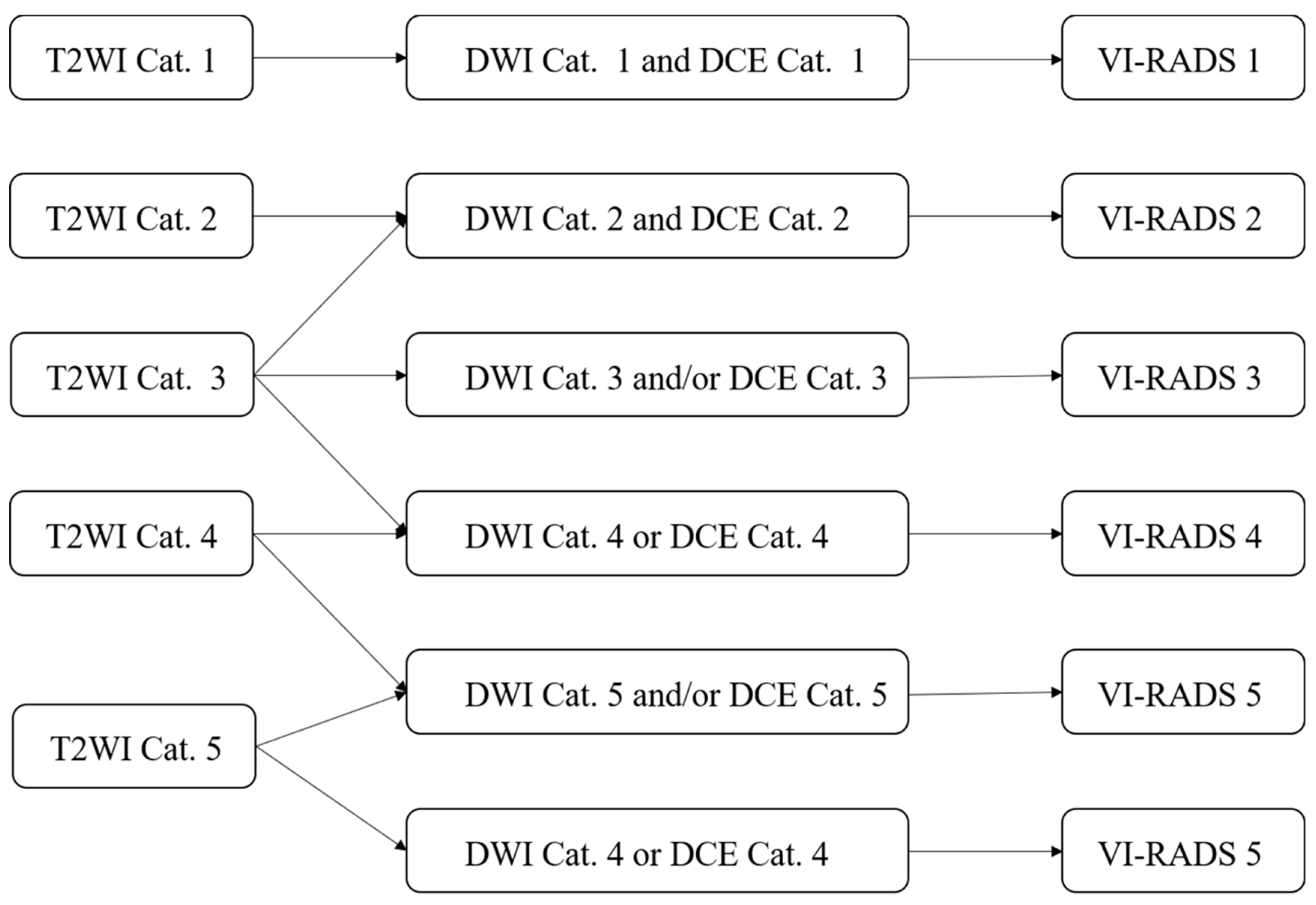
4. The VI-RADS
4.1. The Value of VI-RADS in Evaluating Muscle Invasion in Bladder Cancer
4.2. Optimization of VI-RADS Sequence and Diagnostic Accuracy of Biparametric MRI (bpMRI) VI-RADS
4.3. The Influence of Reader Experience on the Accuracy of VI-RADS and Consistency Analysis
4.4. The Additional Value of Quantitative Imaging Parameters for VI-RADS
4.5. VI-RADS and Efficacy Evaluation of Bladder Cancer
5. Application of Other MRI Functional Sequences in Bladder Cancer
6. The Role of Machine Learning and Artificial Intelligence (AI) Based on MRI Radiomics in Bladder Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Mshs Bladder Cancer. JAMA-J. Am. Med. Assoc. 2020, 324, 1980. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Caglic, I.; Panebianco, V.; Vargas, H.A.; Bura, V.; Woo, S.; Pecoraro, M.; Cipollari, S.; Sala, E.; Barrett, T. MRI of Bladder Cancer: Local and Nodal Staging. J. Magn. Reson. Imaging 2020, 52, 649–667. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Cathomas, R.; Lorch, A.; Bruins, H.M.; Compérat, E.M.; Cowan, N.C.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Hernández, V.; Espinós, E.L.; et al. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur. Urol. 2022, 81, 95–103. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Zapała, P.; Dybowski, B.; Poletajew, S.; Białek, A.; Niewczas, A.; Radziszewski, P. Clinical rationale and safety of restaging transurethral resection in indication-stratified patients with high-risk non-muscle-invasive bladder cancer. World J. Surg. Oncol. 2018, 16, 6. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef]
- Awiwi, M.O.; Vikram, R. Radiologic Diagnosis and Staging of Bladder Cancer: An Update. J. Comput. Assist. Tomogr. 2022, 46, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, S.W.E.; Veenboer, P.W.; Wessels, F.J.; Meijer, R.P. Diagnostic Accuracy of Multiparametric MRI for Local Staging of Bladder Cancer: A Systematic Review and Meta-Analysis. Urology 2020, 145, 22–29. [Google Scholar] [CrossRef]
- Yoshida, S.; Koga, F.; Masuda, H.; Fujii, Y.; Kihara, K. Role of diffusion-weighted magnetic resonance imaging as an imaging biomarker of urothelial carcinoma. Int. J. Urol. 2014, 21, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Inada, Y.; Tatsugami, F.; Tanikake, M.; Narabayashi, I.; Katsuoka, Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: Initial results. Eur. Radiol. 2007, 17, 201–204. [Google Scholar] [CrossRef]
- Suo, S.; Chen, X.; Fan, Y.; Wu, L.; Yao, Q.; Cao, M.; Liu, Q.; Xu, J. Histogram Analysis of Apparent Diffusion Coefficient at 3.0 T in Urinary Bladder Lesions. Acad. Radiol. 2014, 21, 1027–1034. [Google Scholar] [CrossRef]
- Kobayashi, S.; Koga, F.; Kajino, K.; Yoshita, S.; Ishii, C.; Tanaka, H.; Saito, K.; Masuda, H.; Fujii, Y.; Yamada, T.; et al. Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J. Magn. Reson. Imaging 2014, 39, 172–178. [Google Scholar] [CrossRef]
- Yoshida, S.; Takahara, T.; Kwee, T.C.; Waseda, Y.; Kobayashi, S.; Fujii, Y. DWI as an Imaging Biomarker for Bladder Cancer. Am. J. Roentgenol. 2017, 208, 1218–1228. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sasaki, S.; Ito, M.; Okada, S.; Takahashi, S.; Kawai, T.; Suzuki, K.; Oshima, H.; Hara, M.; Shibamoto, Y. Urinary Bladder Cancer: Diffusion-weighted MR Imaging—Accuracy for Diagnosing T Stage and Estimating Histologic Grade. Radiology 2009, 251, 112–121. [Google Scholar] [CrossRef]
- Kobayashi, S.; Koga, F.; Yoshida, S.; Masuda, H.; Ishii, C.; Tanaka, H.; Komai, Y.; Yokoyama, M.; Saito, K.; Fujii, Y.; et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: Potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur. Radiol. 2011, 21, 2178–2186. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Y.; Dan, G.; Zhong, Z.; Karaman, M.M.; Li, Z.; Hu, D.; Zhou, X.J. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur. Radiol. 2022, 32, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pui, M.H.; Guo, Y.; Yang, D.; Pan, B.; Zhou, X. Diffusion-weighted MRI in bladder carcinoma: The differentiation between tumor recurrence and benign changes after resection. Abdom. Imaging 2014, 39, 135–141. [Google Scholar] [CrossRef]
- van der Pol, C.B.; Shinagare, A.B.; Tirumani, S.H.; Preston, M.A.; Vangel, M.G.; Silverman, S.G. Bladder cancer local staging: Multiparametric MRI performance following transurethral resection. Abdom. Radiol. 2018, 43, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Yoshida, S.; Koga, F.; Tanaka, H.; Kaga, M.; Watanabe, K.; Fukushima, H.; Nakanishi, Y.; Yokoyama, M.; Ishioka, J.; et al. Standardization of the apparent diffusion coefficient value of bladder cancer across different centers: Applicability in predicting aggressive pathologic phenotypes. Clin. Imaging 2017, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Gu, Z.; Xu, F.; Maskey, N.; He, Y.; Yan, Y.; Xu, T.; Liu, S.; Yao, X. Magnetic resonance imaging-based radiomics signature for preoperative prediction of Ki67 expression in bladder cancer. Cancer Imaging 2021, 21, 65. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Mussi, T.C.; Spieler, B.; Melamed, J.; Taneja, S.S.; Huang, W.C. High-grade bladder cancer: Association of the apparent diffusion coefficient with metastatic disease: Preliminary results. J. Magn. Reson. Imaging 2012, 35, 1478–1483. [Google Scholar] [CrossRef]
- Funatsu, H.; Imamura, A.; Takano, H.; Ueda, T.; Uno, T. Can pretreatment ADC values predict recurrence of bladder cancer after transurethral resection? Eur. J. Radiol. 2012, 81, 3115–3119. [Google Scholar] [CrossRef]
- Sevcenco, S.; Maj-Hes, A.B.; Hruby, S.; Ponhold, L.; Heinz-Peer, G.; Rauchenwald, M.; Marszalek, M.; Klingler, H.C.; Polanec, S.; Baltzer, P.A.T. Apparent diffusion coefficient values obtained by unenhanced MRI predicts disease-specific survival in bladder cancer. Clin. Radiol. 2018, 73, 881–885. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, K.J.; Lee, G.; Kim, M.; Kim, J.K. Urothelial phase CT for assessment of pathologic complete response after neoadjuvant chemotherapy in muscle-invasive bladder cancer. Eur. J. Radiol. 2020, 126, 108902. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Taher, M.G.A.; Ali, W.A.; Ebrahem, M.A.E.S. Diagnostic performance of contrast-enhanced dynamic and diffusion-weighted MR imaging in the assessment of tumor response to neoadjuvant therapy in muscle-invasive bladder cancer. Abdom. Radiol. 2021, 46, 2712–2721. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhang, J.; Xu, X.; Zhang, L.; Zhang, M.; Xie, L.; Shou, J.; Chen, Y. Muscle-invasive bladder cancer: Pretreatment prediction of response to neoadjuvant chemotherapy with diffusion-weighted MR imaging. Abdom. Radiol. 2022, 47, 2148–2157. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Shah, Z.K.; Mortazavi, A.; Pohar, K.S.; Wei, L.; Jia, G.; Zynger, D.L.; Knopp, M.V. Non-invasive quantification of tumour heterogeneity in water diffusivity to differentiate malignant from benign tissues of urinary bladder: A phase I study. Eur. Radiol. 2017, 27, 2146–2152. [Google Scholar] [CrossRef][Green Version]
- Nguyen, H.T.; Mortazavi, A.; Pohar, K.S.; Zynger, D.L.; Wei, L.; Shah, Z.K.; Jia, G.; Knopp, M.V. Quantitative Assessment of Heterogeneity in Bladder Tumor MRI Diffusivity: Can Response be Predicted Prior to Neoadjuvant Chemotherapy? Bladder Cancer 2017, 3, 237–244. [Google Scholar] [CrossRef]
- Necchi, A.; Bandini, M.; Calareso, G.; Raggi, D.; Pederzoli, F.; Farè, E.; Colecchia, M.; Marandino, L.; Bianchi, M.; Gallina, A.; et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pembrolizumab in Muscle-invasive Bladder Cancer: Preliminary Findings from the PURE-01 Study. Eur. Urol. 2020, 77, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, S.H.; Kang, B.J.; Kang, Y.J.; Yoo, H.; Yoo, J.; Lee, J.; Son, Y.H.; Grimm, R. Intravoxel incoherent motion (IVIM)-derived parameters in diffusion-weighted MRI: Associations with prognostic factors in invasive ductal carcinoma. J. Magn. Reson. Imaging 2017, 45, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Y.; Cong, X.; Zhao, X. Utility of intravoxel incoherent motion MRI derived parameters for prediction of aggressiveness in urothelial bladder carcinoma. J. Magn. Reson. Imaging 2018, 48, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, D.; Yu, H.; Shen, Y.; Tang, H.; Kamel, I.R.; Li, Z. Comparison of the Diagnostic Value of Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MRI in Differentiating Tumor Stage and Histological Grade of Bladder Cancer. Acad. Radiol. 2019, 26, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, L.M.; Hua, X.L.; Zhao, Z.Z.; Chen, X.X.; Xu, J.R. Intravoxel incoherent motion diffusion-weighted imaging in assessing bladder cancer invasiveness and cell proliferation. J. Magn. Reson. Imaging 2018, 47, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiao, Q.; Hu, F.; Fu, C.; Jia, H.; Yan, X.; Xin, C.; Cai, S.; Peng, W.; Wang, X.; et al. Diffusion kurtosis imaging in the characterisation of rectal cancer: Utilizing the most repeatable region-of-interest strategy for diffusion parameters on a 3T scanner. Eur. Radiol. 2018, 28, 5211–5220. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Padhani, A.R.; Chenevert, T.L.; Koh, D.; De Keyzer, F.; Taouli, B.; Le Bihan, D. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J. Magn. Reson. Imaging 2015, 42, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, H.; Zhang, R.; Jin, D.; Xu, S.; Wu, G.; Xu, J. Diffusion kurtosis imaging to assess correlations with clinicopathologic factors for bladder cancer: A comparison between the multi-b value method and the tensor method. Eur. Radiol. 2019, 29, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, B.; Tan, Q.; Liu, K.; Jiang, S.; Zhou, J. Prediction of muscle invasion of bladder cancer: A comparison between DKI and conventional DWI. Eur. J. Radiol. 2021, 136, 109522. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Shanbhogue, A.K.; Wang, A.; Kong, M.X.; Babb, J.S.; Taneja, S.S. Length of capsular contact for diagnosing extraprostatic extension on prostate MRI: Assessment at an optimal threshold. J. Magn. Reson. Imaging 2016, 43, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Hwang, S.I.; Lee, H.J.; Choe, G.; Oh, J.J.; Jeong, S.J.; Byun, S.; Kim, J.K. Quantitation of bladder cancer for the prediction of muscle layer invasion as a complement to the vesical imaging-reporting and data system. Eur. Radiol. 2021, 31, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, B.; Liu, K.; Sun, H.; Ding, Y.; Yan, C.; Wu, P.; Dai, C.; Rao, S.; Zeng, M.; et al. Detecting the muscle invasiveness of bladder cancer: An application of diffusion kurtosis imaging and tumor contact length. Eur. J. Radiol. 2022, 151, 110329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, N.; Sun, F.; Wen, Z.; Lan, X.; Lei, Y.; Cui, E.; Lin, F. Detecting Muscle Invasion of Bladder Cancer Using a Proposed Magnetic Resonance Imaging Strategy. J. Magn. Reson. Imaging 2021, 54, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, D.; Yao, H.; Chen, M.; Li, S.; Chen, H.; Luo, J.; Feng, Y.; Guo, Y. Radiomics analysis of multiparametric MRI for the preoperative evaluation of pathological grade in bladder cancer tumors. Eur. Radiol. 2019, 29, 6182–6190. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Tian, Q.; Wang, H.; Cui, L.; Li, S.; Tang, X.; Li, B.; Dolz, J.; Ayed, I.B.; et al. Quantitative Identification of Nonmuscle-Invasive and Muscle-Invasive Bladder Carcinomas: A Multiparametric MRI Radiomics Analysis. J. Magn. Reson. Imaging 2019, 49, 1489–1498. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Zhang, X.; Liu, Y.; Ouyang, L.; Du, P.; Li, S.; Tian, Q.; Ling, J.; Guo, Y.; et al. Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: A double-center study. Eur. Radiol. 2020, 30, 4816–4827. [Google Scholar] [CrossRef]
- Xu, S.; Yao, Q.; Liu, G.; Jin, D.; Chen, H.; Xu, J.; Li, Z.; Wu, G. Combining DWI radiomics features with transurethral resection promotes the differentiation between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Eur. Radiol. 2020, 30, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Wang, H.; Liu, Y.; Wang, Y.; Dong, Q.; Li, Z.; Guo, Y.; Lu, H. The Additional Value of Tri-parametric MRI in Identifying Muscle-invasive Status in Bladder Cancer. Acad. Radiol. 2023, 30, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Du, P.; Zhang, F.; Li, S.; Zhang, Z.; Yuan, J.; Liang, Z.; Zhang, X.; Guo, Y.; et al. A predictive nomogram for individualized recurrence stratification of bladder cancer using multiparametric MRI and clinical risk factors. J. Magn. Reson. Imaging 2019, 50, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, M.; Zhao, Y.; Xu, S.; Sun, Q.; Zhai, G.; Liang, D.; Wu, G.; Li, Z. Radiomics nomogram for preoperative prediction of progression-free survival using diffusion-weighted imaging in patients with muscle-invasive bladder cancer. Eur. J. Radiol. 2020, 131, 109219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Tian, Q.; Li, B.; Wu, Y.; Yang, Z.; Liang, Z.; Liu, Y.; Cui, G.; Lu, H. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J. Magn. Reson. Imaging 2017, 46, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yoshida, S.; Tsuchiya, J.; Yamada, I.; Tanaka, H.; Yokoyama, M.; Matsuoka, Y.; Yoshimura, R.; Tateishi, U.; Fujii, Y. Usefulness of texture features of apparent diffusion coefficient maps in predicting chemoradiotherapy response in muscle-invasive bladder cancer. Eur. Radiol. 2022, 32, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kong, J.; Wu, S.; Li, Y.; Cai, J.; Yu, H.; Xie, W.; Qin, H.; Wu, Z.; Huang, J.; et al. Development of a noninvasive tool to preoperatively evaluate the muscular invasiveness of bladder cancer using a radiomics approach. Cancer 2019, 125, 4388–4398. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Lan, X.; Deng, J.; Lei, Y.; Lin, F. The role of radiomics with machine learning in the prediction of muscle-invasive bladder cancer: A mini review. Front. Oncol. 2022, 12, 990176. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhang, J.; Zhang, L.; Wang, S.; Chen, Y. Development of a MRI-Based Radiomics Nomogram for Prediction of Response of Patients With Muscle-Invasive Bladder Cancer to Neoadjuvant Chemotherapy. Front. Oncol. 2022, 12, 878499. [Google Scholar] [CrossRef]
- Arévalo, N.; Méndez, R.; Barrera, J. “Inchworm sign” in urinary bladder cancer. Abdom. Radiol. 2018, 43, 3509–3510. [Google Scholar] [CrossRef]
- Razik, A.; Das, C.J.; Sharma, S.; Seth, A.; Srivastava, D.N.; Mathur, S.; Kumar, R.; Gupta, A.K. Diagnostic performance of diffusion-weighted MR imaging at 3.0 T in predicting muscle invasion in urinary bladder cancer: Utility of evaluating the morphology of the reactive tumor stalk. Abdom. Radiol. 2018, 43, 2431–2441. [Google Scholar] [CrossRef]
- Wang, H.; Pui, M.H.; Guan, J.; Li, S.; Lin, J.; Pan, B.; Guo, Y. Comparison of Early Submucosal Enhancement and Tumor Stalk in Staging Bladder Urothelial Carcinoma. Am. J. Roentgenol. 2016, 207, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Yajima, S.; Yoshida, S.; Takahara, T.; Arita, Y.; Tanaka, H.; Waseda, Y.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Saito, K.; et al. Usefulness of the inchworm sign on DWI for predicting pT1 bladder cancer progression. Eur. Radiol. 2019, 29, 3881–3888. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Shin, S.; Oh, Y.T.; Jung, D.C.; Cho, N.H.; Choi, Y.D.; Park, S.Y. Non-contrast magnetic resonance imaging for bladder cancer: Fused high b value diffusion-weighted imaging and T2-weighted imaging helps evaluate depth of invasion. Eur. Radiol. 2017, 27, 3752–3758. [Google Scholar] [CrossRef]
- Yuan, L.; Li, D.; Mu, D.; Zhang, X.; Kong, W.; Cheng, L.; Shu, X.; Zhang, B.; Wang, Z. Combined T2 SPAIR, Dynamic Enhancement and DW Imaging Reliably Detect T Staging and Grading of Bladder Cancer With 3.0T MRI. Front. Oncol. 2020, 10, 582532. [Google Scholar] [CrossRef] [PubMed]
- Tuncbilek, N.; Kaplan, M.; Altaner, S.; Atakan, I.H.; Süt, N.; Inci, O.; Demir, M.K. Value of dynamic contrast-enhanced MRI and correlation with tumor angiogenesis in bladder cancer. Am. J. Roentgenol. 2009, 192, 949. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pohar, K.S.; Jia, G.; Shah, Z.K.; Mortazavi, A.; Zynger, D.L.; Wei, L.; Clark, D.; Yang, X.; Knopp, M.V. Improving Bladder Cancer Imaging Using 3-T Functional Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Investig. Radiol. 2014, 49, 390–395. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, X.; Zhang, J.; Zhu, J.; Zong, G.; Wang, Z. Contrast-enhanced dynamic and diffusion-weighted MR imaging at 3.0T to assess aggressiveness of bladder cancer. Eur. J. Radiol. 2014, 83, 2013–2018. [Google Scholar] [CrossRef]
- Chakiba, C.; Cornelis, F.; Descat, E.; Gross-Goupil, M.; Sargos, P.; Roubaud, G.; Houédé, N. Dynamic contrast enhanced MRI-derived parameters are potential biomarkers of therapeutic response in bladder carcinoma. Eur. J. Radiol. 2015, 84, 1023–1028. [Google Scholar] [CrossRef]
- Panebianco, V.; De Berardinis, E.; Barchetti, G.; Simone, G.; Leonardo, C.; Grompone, M.D.; Del Monte, M.; Carano, D.; Gallucci, M.; Catto, J.; et al. An evaluation of morphological and functional multi-parametric MRI sequences in classifying non-muscle and muscle invasive bladder cancer. Eur. Radiol. 2017, 27, 3759–3766. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, X.; Wang, C.; Chen, S.; Wu, J.; Zhang, G.; Zhu, W.; Liu, J.; Xu, B.; Du, M.; et al. Diagnostic Accuracy of Multi-Parametric Magnetic Resonance Imaging for Tumor Staging of Bladder Cancer: Meta-Analysis. Front. Oncol. 2019, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Pecoraro, M.; Del Giudice, F.; Takeuchi, M.; Muglia, V.F.; Messina, E.; Cipollari, S.; Giannarini, G.; Catalano, C.; Narumi, Y. VI-RADS for Bladder Cancer: Current Applications and Future Developments. J. Magn. Reson. Imaging 2022, 55, 23–36. [Google Scholar] [CrossRef]
- Ueno, Y.; Takeuchi, M.; Tamada, T.; Sofue, K.; Takahashi, S.; Kamishima, Y.; Hinata, N.; Harada, K.; Fujisawa, M.; Murakami, T. Diagnostic Accuracy and Interobserver Agreement for the Vesical Imaging-Reporting and Data System for Muscle-invasive Bladder Cancer: A Multireader Validation Study. Eur. Urol. 2019, 76, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, C.; Zhang, F.; Guan, J.; Li, S.; Yao, H.; Chen, J.; Luo, J.; Chen, L.; Guo, Y. Multiparametric MRI for Bladder Cancer: Validation of VI-RADS for the Detection of Detrusor Muscle Invasion. Radiology 2019, 291, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pui, M.H.; Guo, Y.; Li, S.; Guan, J.; Zhang, X.; Cai, H. Multiparametric 3-T MRI for Differentiating Low-Versus High-Grade and Category T1 Versus T2 Bladder Urothelial Carcinoma. Am. J. Roentgenol. 2015, 204, 330–334. [Google Scholar] [CrossRef]
- Kim, S.H. Validation of vesical imaging reporting and data system for assessing muscle invasion in bladder tumor. Abdom. Radiol. 2020, 45, 491–498. [Google Scholar] [CrossRef]
- Del Giudice, F.; Barchetti, G.; De Berardinis, E.; Pecoraro, M.; Salvo, V.; Simone, G.; Sciarra, A.; Leonardo, C.; Gallucci, M.; Catalano, C.; et al. Prospective Assessment of Vesical Imaging Reporting and Data System (VI-RADS) and Its Clinical Impact on the Management of High-risk Non–muscle-invasive Bladder Cancer Patients Candidate for Repeated Transurethral Resection. Eur. Urol. 2020, 77, 101–109. [Google Scholar] [CrossRef]
- Marchioni, M.; Primiceri, G.; Delli Pizzi, A.; Basilico, R.; Berardinelli, F.; Mincuzzi, E.; Castellucci, R.; Sessa, B.; Di Nicola, M.; Schips, L. Could Bladder Multiparametric MRI Be Introduced in Routine Clinical Practice? Role of the New VI-RADS Score: Results from a Prospective Study. Clin. Genitourin. Cancer 2020, 18, 409–415.e1. [Google Scholar] [CrossRef]
- Woo, S.; Panebianco, V.; Narumi, Y.; Del Giudice, F.; Muglia, V.F.; Takeuchi, M.; Ghafoor, S.; Bochner, B.H.; Goh, A.C.; Hricak, H.; et al. Diagnostic Performance of Vesical Imaging Reporting and Data System for the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Huang, B.; Wu, Y.; Chen, J.; Chen, L. Use of Vesical Imaging-Reporting and Data System (VI-RADS) for detecting the muscle invasion of bladder cancer: A diagnostic meta-analysis. Eur. Radiol. 2020, 30, 4606–4614. [Google Scholar] [CrossRef] [PubMed]
- Juri, H.; Narumi, Y.; Panebianco, V.; Osuga, K. Staging of bladder cancer with multiparametric MRI. Br. J. Radiol. 2020, 93, 20200116. [Google Scholar] [CrossRef]
- Gmeiner, J.; Garstka, N.; Helbich, T.H.; Shariat, S.F.; Baltzer, P.A. Vesical Imaging Reporting and Data System (VI-RADS): Are the individual MRI sequences equivalent in diagnostic performance of high grade NMIBC and MIBC? Eur. J. Radiol. 2021, 142, 109829. [Google Scholar] [CrossRef]
- Hickling, D.R.; Sun, T.; Wu, X. Anatomy and Physiology of the Urinary Tract: Relation to Host Defense and Microbial Infection. Microbiol. Spectr. 2015, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Shigeta, K.; Akita, H.; Suzuki, T.; Kufukihara, R.; Kwee, T.C.; Ishii, R.; Mikami, S.; Okuda, S.; Kikuchi, E.; et al. Clinical utility of the Vesical Imaging-Reporting and Data System for muscle-invasive bladder cancer between radiologists and urologists based on multiparametric MRI including 3D FSE T2-weighted acquisitions. Eur. Radiol. 2021, 31, 875–883. [Google Scholar] [CrossRef]
- Meng, X.; Hu, H.; Wang, Y.; Hu, D.; Li, Z.; Feng, C. Application of bi-planar reduced field-of-view DWI (rFOV DWI) in the assessment of muscle-invasiveness of bladder cancer. Eur. J. Radiol. 2021, 136, 109486. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, A.; Mastrodicasa, D.; Taraschi, A.; Civitareale, N.; Mincuzzi, E.; Censi, S.; Marchioni, M.; Primiceri, G.; Castellan, P.; Castellucci, R.; et al. Conspicuity and muscle-invasiveness assessment for bladder cancer using VI-RADS: A multi-reader, contrast-free MRI study to determine optimal b-values for diffusion-weighted imaging. Abdom. Radiol. 2022, 47, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Di Campli, E.; Delli Pizzi, A.; Seccia, B.; Cianci, R.; D Annibale, M.; Colasante, A.; Cinalli, S.; Castellan, P.; Navarra, R.; Iantorno, R.; et al. Diagnostic accuracy of biparametric vs multiparametric MRI in clinically significant prostate cancer: Comparison between readers with different experience. Eur. J. Radiol. 2018, 101, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, A.; Basilico, R.; Cianci, R.; Seccia, B.; Timpani, M.; Tavoletta, A.; Caposiena, D.; Faricelli, B.; Gabrielli, D.; Caulo, M. Rectal cancer MRI: Protocols, signs and future perspectives radiologists should consider in everyday clinical practice. Insights Imaging 2018, 9, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, A.; Mastrodicasa, D.; Marchioni, M.; Primiceri, G.; Di Fabio, F.; Cianci, R.; Seccia, B.; Sessa, B.; Mincuzzi, E.; Romanelli, M.; et al. Bladder cancer: Do we need contrast injection for MRI assessment of muscle invasion? A prospective multi-reader VI-RADS approach. Eur. Radiol. 2021, 31, 3874–3883. [Google Scholar] [CrossRef]
- Aslan, S.; Cakir, I.M.; Oguz, U.; Bekci, T.; Demirelli, E. Comparison of the diagnostic accuracy and validity of biparametric MRI and multiparametric MRI-based VI-RADS scoring in bladder cancer; is contrast material really necessary in detecting muscle invasion? Abdom. Radiol. 2022, 47, 771–780. [Google Scholar] [CrossRef]
- Watanabe, M.; Taguchi, S.; Machida, H.; Tambo, M.; Takeshita, Y.; Kariyasu, T.; Fukushima, K.; Shimizu, Y.; Okegawa, T.; Fukuhara, H.; et al. Clinical validity of non-contrast-enhanced VI-RADS: Prospective study using 3-T MRI with high-gradient magnetic field. Eur. Radiol. 2022, 32, 7513–7521. [Google Scholar] [CrossRef]
- Ye, L.; Chen, Y.; Xu, H.; Xie, H.; Yao, J.; Liu, J.; Song, B. Biparametric magnetic resonance imaging assessment for detection of muscle-invasive bladder cancer: A systematic review and meta-analysis. Eur. Radiol. 2022, 32, 6480–6492. [Google Scholar] [CrossRef]
- Elshetry, A.S.F.; El-fawakry, R.M.; Hamed, E.M.; Metwally, M.I.; Zaid, N.A. Diagnostic accuracy and discriminative power of biparametric versus multiparametric MRI in predicting muscle-invasive bladder cancer. Eur. J. Radiol. 2022, 151, 110282. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Hu, H.; Wang, Y.; Feng, C.; Hu, D.; Liu, Z.; Kamel, I.R.; Li, Z. Accuracy and Challenges in the VesicalImaging-Reportingand Data System for Staging Bladder Cancer. J. Magn. Reson. Imaging 2022, 56, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Barchetti, G.; Simone, G.; Ceravolo, I.; Salvo, V.; Campa, R.; Del Giudice, F.; De Berardinis, E.; Buccilli, D.; Catalano, C.; Gallucci, M.; et al. Multiparametric MRI of the bladder: Inter-observer agreement and accuracy with the Vesical Imaging-Reporting and Data System (VI-RADS) at a single reference center. Eur. Radiol. 2019, 29, 5498–5506. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.C.; Pecoraro, M.; Pisciotti, M.L.; Dehghanpour, A.; Forookhi, A.; Lucciola, S.; Bicchetti, M.; Messina, E.; Catalano, C.; Panebianco, V. The learning curve in bladder MRI using VI-RADS assessment score during an interactive dedicated training program. Eur. Radiol. 2022, 32, 7494–7503. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.I.; Zeed, N.A.; Hamed, E.M.; Elshetry, A.S.F.; Elfwakhry, R.M.; Alaa Eldin, A.M.; Sakr, A.; Aly, S.A.; Mosallam, W.; Ziada, Y.M.A.; et al. The validity, reliability, and reviewer acceptance of VI-RADS in assessing muscle invasion by bladder cancer: A multicenter prospective study. Eur. Radiol. 2021, 31, 6949–6961. [Google Scholar] [CrossRef] [PubMed]
- Bricio, T.G.M.; Gouvea, G.L.; Barros, R.V.; Chahud, F.; Elias, J.; Reis, R.B.; Muglia, V.F. What is the impact of dynamic contrast-enhancement sequence in the Vesical Imaging, Reporting and Data System (VI-RADS)? A subgroup analysis. Cancer Imaging 2022, 22, 20. [Google Scholar] [CrossRef]
- Jazayeri, S.B.; Dehghanbanadaki, H.; Hosseini, M.; Taghipour, P.; Bazargani, S.; Thomas, D.; Feibus, A.; Sarabchian, E.; Bacchus, M.W.; Di Valerio, E.A.; et al. Inter-reader reliability of the vesical imaging-reporting and data system (VI-RADS) for muscle-invasive bladder cancer: A systematic review and meta-analysis. Abdom. Radiol. 2022, 47, 4173–4185. [Google Scholar] [CrossRef]
- Nicola, R.; Pecoraro, M.; Lucciola, S.; Reis, R.B.D.; Narumi, Y.; Panebianco, V.; Muglia, V.F. VI-RADS score system—A primer for urologists. Int. Braz. J. Urol. 2022, 48, 609–622. [Google Scholar] [CrossRef]
- Li, S.; Liang, P.; Wang, Y.; Feng, C.; Shen, Y.; Hu, X.; Hu, D.; Meng, X.; Li, Z. Combining volumetric apparent diffusion coefficient histogram analysis with vesical imaging reporting and data system to predict the muscle invasion of bladder cancer. Abdom. Radiol. 2021, 46, 4301–4310. [Google Scholar] [CrossRef]
- Akcay, A.; Yagci, A.B.; Celen, S.; Ozlulerden, Y.; Turk, N.S.; Ufuk, F. VI-RADS score and tumor contact length in MRI: A potential method for the detection of muscle invasion in bladder cancer. Clin. Imaging 2021, 77, 25–36. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, F.; Gu, Z.; Yan, Y.; Xu, T.; Liu, S.; Yao, X. Integrating multiparametric MRI radiomics features and the Vesical Imaging-Reporting and Data System (VI-RADS) for bladder cancer grading. Abdom. Radiol. 2021, 46, 4311–4323. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, F.; Gu, Z.; Yan, Y.; Xu, T.; Liu, S.; Yao, X. Combining Multiparametric MRI Radiomics Signature with the Vesical Imaging-Reporting and Data System (VI-RADS) Score to Preoperatively Differentiate Muscle Invasion of Bladder Cancer. Front. Oncol. 2021, 11, 619893. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Altun, E. MR Imaging of the Urinary Bladder. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Calareso, G.; Raggi, D.; Marandino, L.; Colecchia, M.; Gallina, A.; Giannatempo, P.; Pederzoli, F.; Gandaglia, G.; Fossati, N.; et al. The Value of Multiparametric Magnetic Resonance Imaging Sequences to Assist in the Decision Making of Muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2021, 4, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Del Giudice, F.; Magliocca, F.; Simone, G.; Flammia, S.; Leonardo, C.; Messina, E.; De Berardinis, E.; Cortesi, E.; Panebianco, V. Vesical Imaging-Reporting and Data System (VI-RADS) for assessment of response to systemic therapy for bladder cancer: Preliminary report. Abdom. Radiol. 2022, 47, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Leonardo, C.; Simone, G.; Pecoraro, M.; De Berardinis, E.; Cipollari, S.; Flammia, S.; Bicchetti, M.; Busetto, G.M.; Chung, B.I.; et al. Preoperative detection of Vesical Imaging-Reporting and Data System (VI-RADS) score 5 reliably identifies extravesical extension of urothelial carcinoma of the urinary bladder and predicts significant delayed time to cystectomy: Time to reconsider the nee. BJU Int. 2020, 126, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Weatherall, P.T.; McColl, R.W.; Tripathy, D.; Mason, R.P. Blood oxygenation level-dependent (BOLD) contrast magnetic resonance imaging (MRI) for prediction of breast cancer chemotherapy response: A pilot study. J. Magn. Reson. Imaging 2013, 37, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Hu, X.; Li, Z.; Feng, C.; Hu, D.; Kamel, I.R. Application of R2* and Apparent Diffusion Coefficient in Estimating Tumor Grade and T Category of Bladder Cancer. Am. J. Roentgenol. 2020, 214, 383–389. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ahn, S.S.; Lee, S.; Chang, J.H.; Kang, S.; Kim, S.H.; Zhou, J. Amide proton transfer imaging to discriminate between low- and high-grade gliomas: Added value to apparent diffusion coefficient and relative cerebral blood volume. Eur. Radiol. 2017, 27, 3181–3189. [Google Scholar] [CrossRef]
- Zhang, H.; Yong, X.; Ma, X.; Zhao, J.; Shen, Z.; Chen, X.; Tian, F.; Chen, W.; Wu, D.; Zhang, Y. Differentiation of low- and high-grade pediatric gliomas with amide proton transfer imaging: Added value beyond quantitative relaxation times. Eur. Radiol. 2021, 31, 9110–9119. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Y.; Xiang, Y.; Wu, P.; Shen, A.; Wang, P. The feasibility of amide proton transfer imaging at 3 T for bladder cancer: A preliminary study. Clin. Radiol. 2022, 77, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Cai, Q.; Huang, Y.P.; Li, M.Q.; Wen, Z.H.; Lin, Y.Y.; Ouyang, L.Y.; Qian, L.; Guo, Y. Amide Proton Transfer-weighted MRI in Predicting Histologic Grade of Bladder Cancer. Radiology 2022, 305, 127–134. [Google Scholar] [CrossRef]
- Boca, B.; Caraiani, C.; Telecan, T.; Pintican, R.; Lebovici, A.; Andras, I.; Crisan, N.; Pavel, A.; Diosan, L.; Balint, Z.; et al. MRI-Based Radiomics in Bladder Cancer: A Systematic Review and Radiomics Quality Score Assessment. Diagnostics 2023, 13, 2300. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, G.; Liu, H.; Jiang, W.; Mai, S.; Zhang, L.; Zeng, H.; Wu, S.; Chen, C.Y.; Wu, Z. MRI-based automated machine learning model for preoperative identification ofvariant histology in muscle-invasive bladder carcinoma. Eur. Radiol. 2023. [Google Scholar] [CrossRef]
- Chen, G.; Fan, X.; Wang, T.; Zhang, E.; Shao, J.; Chen, S.; Zhang, D.; Zhang, J.; Guo, T.; Yuan, Z.; et al. A machine learning model based on MRI for the preoperative prediction of bladdercancer invasion depth. Eur. Radiol. 2023, 33, 8821–8832. [Google Scholar] [CrossRef]
- Fan, Z.C.; Zhang, L.; Yang, G.Q.; Li, S.; Guo, J.T.; Bai, J.J.; Wang, B.; Li, Y.; Wang, L.; Wang, X.C. MRI radiomics for predicting poor disease-free survival in muscle invasivebladder cancer: The results of the retrospective cohort study. Abdom. Radiol. 2023, 49, 151–162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Meng, X.; Wang, Y.; Feng, C.; Liu, Z.; Li, Z.; Niu, Y. Progress of Multiparameter Magnetic Resonance Imaging in Bladder Cancer: A Comprehensive Literature Review. Diagnostics 2024, 14, 442. https://doi.org/10.3390/diagnostics14040442
He K, Meng X, Wang Y, Feng C, Liu Z, Li Z, Niu Y. Progress of Multiparameter Magnetic Resonance Imaging in Bladder Cancer: A Comprehensive Literature Review. Diagnostics. 2024; 14(4):442. https://doi.org/10.3390/diagnostics14040442
Chicago/Turabian StyleHe, Kangwen, Xiaoyan Meng, Yanchun Wang, Cui Feng, Zheng Liu, Zhen Li, and Yonghua Niu. 2024. "Progress of Multiparameter Magnetic Resonance Imaging in Bladder Cancer: A Comprehensive Literature Review" Diagnostics 14, no. 4: 442. https://doi.org/10.3390/diagnostics14040442
APA StyleHe, K., Meng, X., Wang, Y., Feng, C., Liu, Z., Li, Z., & Niu, Y. (2024). Progress of Multiparameter Magnetic Resonance Imaging in Bladder Cancer: A Comprehensive Literature Review. Diagnostics, 14(4), 442. https://doi.org/10.3390/diagnostics14040442




