Clinical Predictive Modeling of Heart Failure: Domain Description, Models’ Characteristics and Literature Review
Abstract
1. Introduction
1.1. Content and Structure of the Study
1.2. Literature Search Method
2. Medical Domain Description
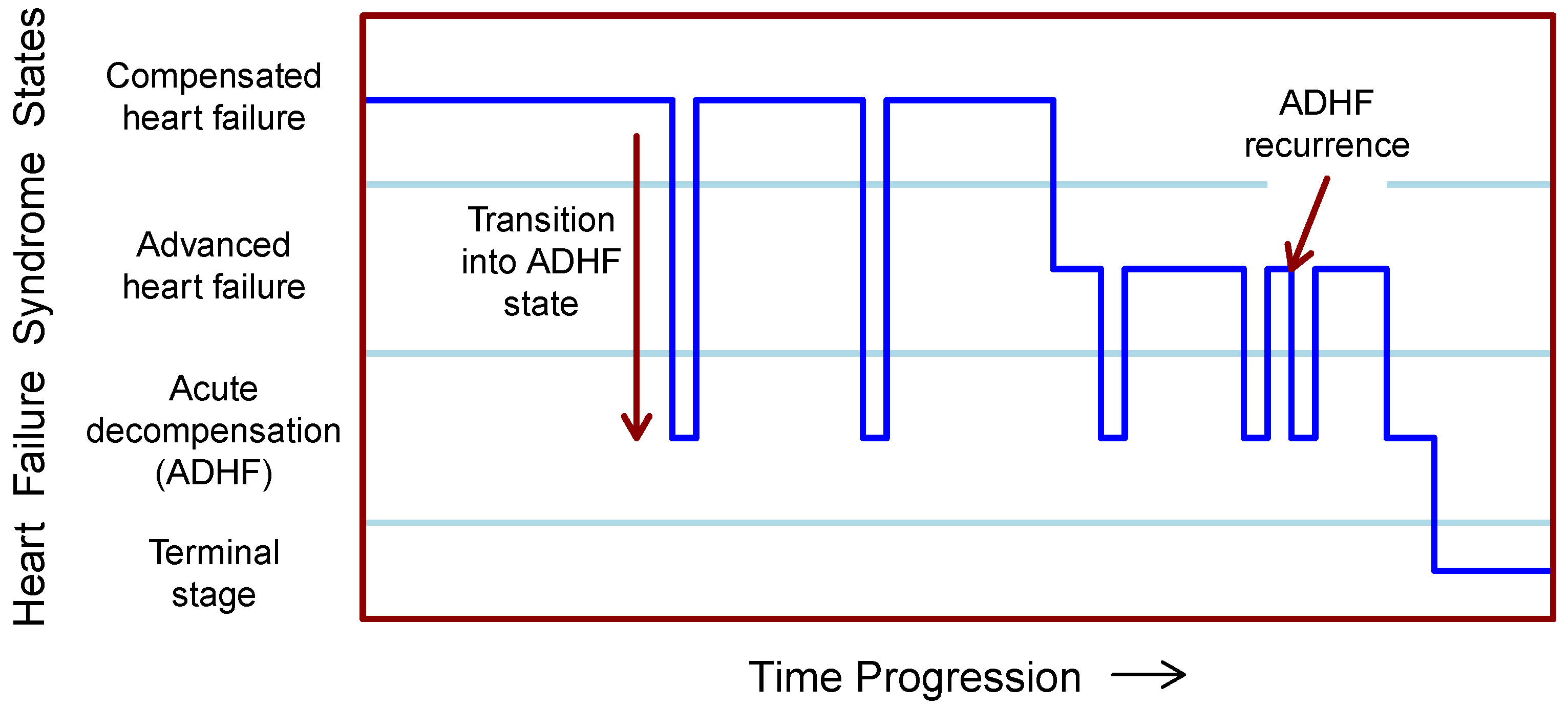
2.1. Chronic Heart Failure Syndrome and Its Decompensation
2.2. Disease-Specific Prognostic and Diagnostic Models
2.3. Telemedical Remote Monitoring of Patients with Heart Failure
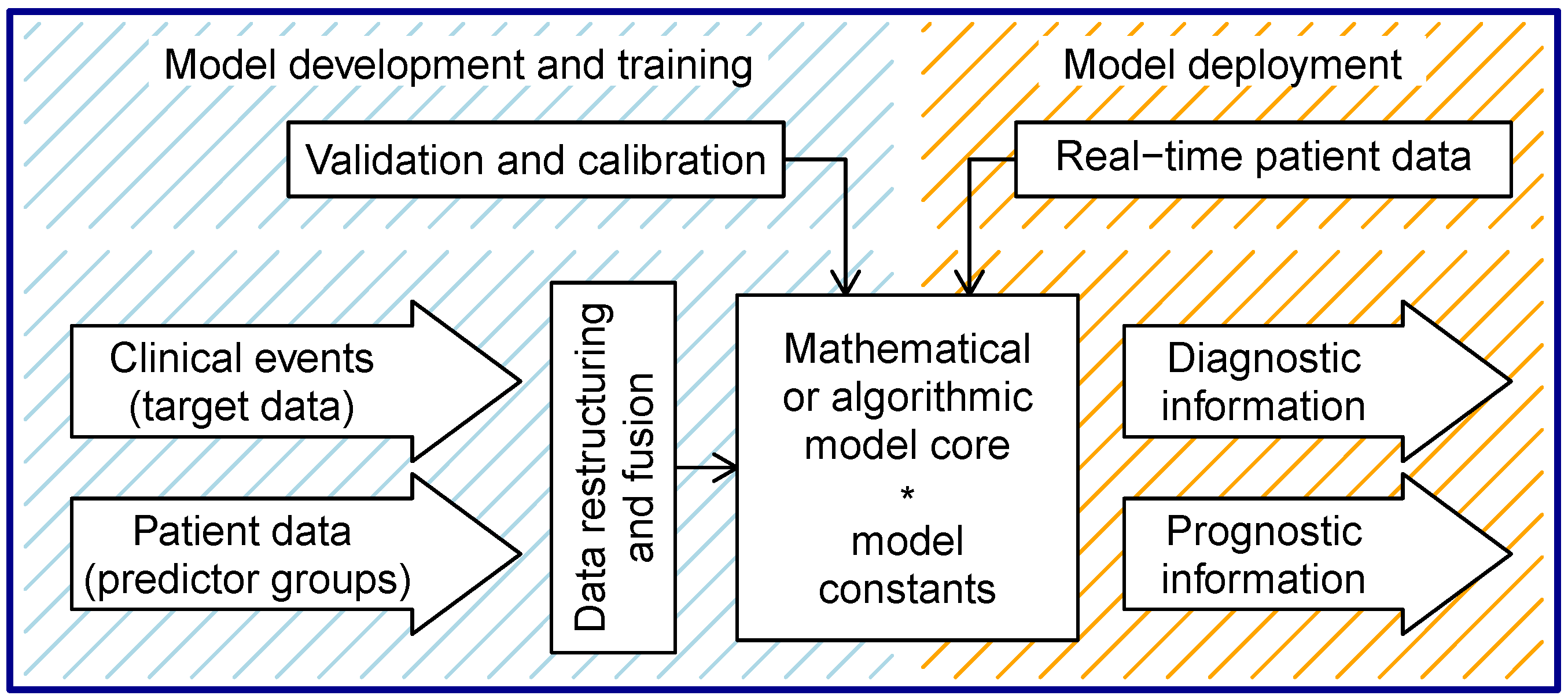
3. Clinical Prediction Models in General
4. Common Characteristics of Quantitative Prediction Models
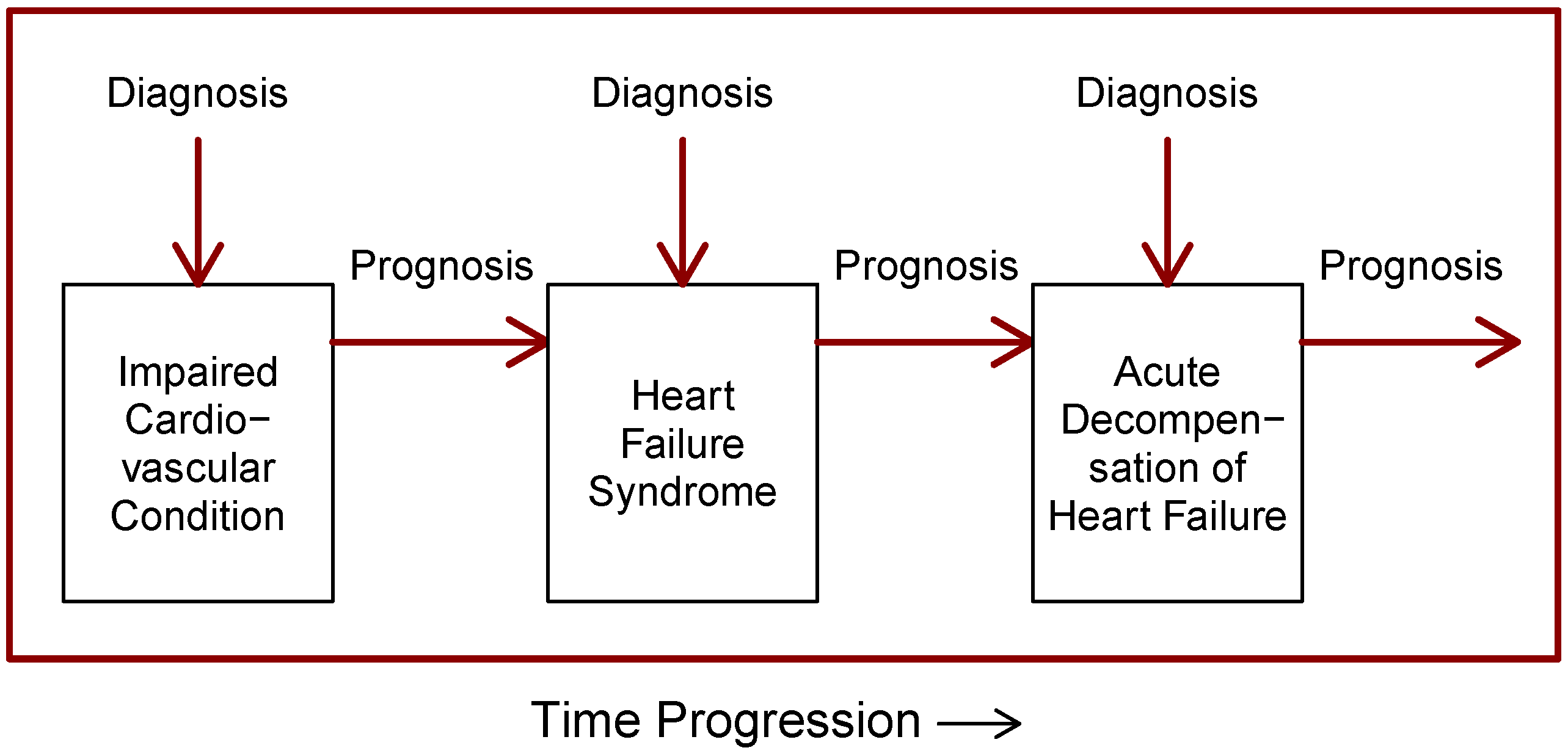
4.1. Characteristic #1—Object of Prediction
4.2. Characteristic #2—Prediction Information Timelines
4.2.1. Diagnostic Information Timelines
4.2.2. Prognostic Information Timelines
4.3. Characteristic #3—Temporal Properties of Target and Predictor Data
4.3.1. Temporal Properties of Target Data
4.3.2. Temporal Properties of Predictor Data
4.3.3. Temporal Properties of Recurrent Diagnostics
4.4. Characteristic #4—Processing of Different Types and Groups of Predictor Data
4.4.1. Increasing Predictive Power by Combining Heterogeneous Groups
4.4.2. Other Methods of Increasing Prediction Ability
4.5. Characteristic #5—Distinction between Prognosis and Diagnosis
4.6. Characteristic #6—Statistical Approach versus Machine Learning
4.7. Mathematics of Quantitative Models: Basic Prognostic and Diagnostic Tasks
4.7.1. Basic Prognostic Model
4.7.2. Basic Diagnostic Model
5. Overview of Heart Failure Prediction Models
5.1. Telemedical and Telemetric Diagnostic Prediction of Acute Heart Failure Decompensation
5.1.1. Published Samples of Telemedical and Telemetric Data
5.1.2. Non-Invasive Prediction Methods
5.1.3. Prediction Models Using Implants
5.1.4. Confirmatory Diagnostics of ADHF
5.2. Prognostic Prediction with Cross-Sectional Predictors
5.3. Advanced Statistical Modeling with Time-Dependent Predictors
- (i)
- a naive approach—simply use the obtained longitudinal data as predictors in models such as the Cox proportional hazards model,
- (ii)
- (iii)
5.4. Other Advanced Statistical Models
5.5. Machine Learning Approaches
5.5.1. Machine Learning for ADHF Detection and Diagnosis
5.5.2. Machine Learning for CHFS Prognosis
6. Future Directions
7. Summary and Conclusions
8. Limitations of this Study
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| EHR | Electronic Health Record |
| TM | Telemedicine |
| UML | Unified Modeling Language |
| HF | Heart Failure |
| CHFS | Chronic Heart Failure Syndrome |
| ADHF | Acute Decompensation of Heart Failure |
| SHFM | Seattle Heart Failure Model |
| ER | Emergency Room |
| ICU | Intensive Care Unit |
| MC | Markov Chain |
| BBN | Bayesian Belief Network |
| AUROC | Area Under the Receiver Operating Characteristics |
| ML | Machine Learning |
| MLP | Multi-Layer Perceptron |
| LSTM | Long Short-Term Memory |
| XGBoost | eXtreme Gradient Boosting |
Appendix A. Determination of Coefficients in Cox Regression Using the Maximum Likelihood Estimation Method
Appendix B. Explanatory Information for the Bayes’ Theorem
| D = 1, = 1 | ||||
|---|---|---|---|---|
| Positive Predictive Value | False Omission Rate * | False Discovery Rate * | Negative Predictive Value | |
| Prevalence | 1 − Prevalence | Prevalence | 1 − Prevalence | |
| 1 − Prevalence | Prevalence | 1 − Prevalence | Prevalence | |
| Positive Predictive Value | False Omission Rate * | False Discovery Rate * | Negative Predictive Value | |
| Sensitivity | 1 − Specificity | 1 − Sensitivity | Specificity | |
| 1 − Sensitivity | Sensitivity | Specificity | 1 − Specificity | |
| Probability of true positive | Probability of false positive | Probability of false negative | Probability of true negative | |
| Probability of false positive | Probability of true positive | Probability of true negative | Probability of false negative |
| Concept Name | Definition |
|---|---|
| Prevalence | Proportion of a defined group in the population having a disease at one point in time |
| Sensitivity | Rate of positive responses in a test from persons with a specific disease, true positive rate |
| Specificity | Rate of negative responses in a test from persons free from a disease, true negative rate |
| True positives | Number of cases in population correctly identified as diseased |
| False positives | Number of cases in population incorrectly identified as diseased, type I error |
| True negatives | Number of cases in population correctly identified as healthy |
| False negatives | Number of cases in population incorrectly identified as healthy, type II error |
References
- Celi, L.A.; Charlton, P.; Ghassemi, M.M.; Johnson, A.; Komorowski, M.; Marshall, D.; Naumann, T.; Paik, K.; Pollard, T.J.; Raffa, J.; et al. Secondary Analysis of Electronic Health Records; MIT Critical Data; Springer Nature: Cham, Switzerland, 2016. [Google Scholar]
- Zhou, Z.R.; Wang, W.W.; Li, Y.; Jin, K.R.; Wang, X.Y.; Wang, Z.W.; Chen, Y.S.; Wang, S.J.; Hu, J.; Zhang, H.N.; et al. In-depth mining of clinical data: The construction of clinical prediction model with R. Ann. Transl. Med. 2019, 7, 796. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Sweeting, M.; Morris, T.; Crowther, M.J. A scoping methodological review of simulation studies comparing statistical and machine learning approaches to risk prediction for time-to-event data. Diagn. Progn. Res. 2022, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association and Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Chin. J. Cardiovasc. Dis. Zhonghua Xin Xue Guan Bing Zhi 2018, 46, 760–789. [Google Scholar]
- Kurmani, S.; Squire, I. Acute heart failure: Definition, classification and epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Straw, S.; Napp, A.; Witte, K.K. ‘Acute Heart Failure’: Should We Abandon the Term Altogether? Curr. Heart Fail. Rep. 2022, 19, 425–434. [Google Scholar] [CrossRef]
- Hummel, A.; Empen, K.; Dörr, M.; Felix, S.B. De novo acute heart failure and acutely decompensated chronic heart failure. Dtsch. Ärzteblatt Int. 2015, 112, 298. [Google Scholar] [CrossRef]
- Mann, D.L.; Bristow, M.R. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation 2005, 111, 2837–2849. [Google Scholar] [CrossRef]
- Schiff, G.D.; Fung, S.; Speroff, T.; McNutt, R.A. Decompensated heart failure: Symptoms, patterns of onset, and contributing factors. Am. J. Med. 2003, 114, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Houwelingen, H.; Putter, H. Dynamic Prediction in Clinical Survival Analysis; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Januzzi, J.L., Jr.; Butler, J. The importance of worsening heart failure: Hiding in plain sight. J. Am. Coll. Cardiol. 2022, 80, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Kahwash, R.; Sarkar, S.; Koehler, J.; Butler, J. Temporal characteristics of device-based individual and integrated risk metrics in patients with chronic heart failure. Heart Fail. 2023, 11, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Lyass, A.; Lee, D.S.; Vasan, R.S.; Kannel, W.B.; Larson, M.G.; Levy, D. Predictors of new-onset heart failure: Differences in preserved versus reduced ejection fraction. Circ. Heart Fail. 2013, 6, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Dworzynski, K.; Roberts, E.; Ludman, A.; Mant, J. Diagnosing and managing acute heart failure in adults: Summary of NICE guidance. BMJ 2014, 349, g5695. [Google Scholar] [CrossRef] [PubMed]
- Ramani, G.V.; Uber, P.A.; Mehra, M.R. Chronic heart failure: Contemporary diagnosis and management. Mayo Clin. Proc. 2010, 85, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Wakai, A.; Collins, S.P.; Levy, P.D.; Diercks, D.; Hiestand, B.C.; Fermann, G.J.; Desouza, I.; Sinert, R. Diagnosing acute heart failure in the emergency department: A systematic review and meta-analysis. Acad. Emerg. Med. 2016, 23, 223–242. [Google Scholar] [CrossRef]
- Kellett, J.; Sebat, F. Make vital signs great again–A call for action. Eur. J. Intern. Med. 2017, 45, 13–19. [Google Scholar] [CrossRef]
- Drews, T.E.; Laukkanen, J.; Nieminen, T. Non-invasive home telemonitoring in patients with decompensated heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2021, 8, 3696–3708. [Google Scholar] [CrossRef]
- Kropf, M.; Modre-Osprian, R.; Hayn, D.; Fruhwald, F.; Schreier, G. Telemonitoring in heart failure patients with clinical decision support to optimize medication doses based on guidelines. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3168–3171. [Google Scholar]
- Louis, A.A.; Turner, T.; Gretton, M.; Baksh, A.; Cleland, J.G. A systematic review of telemonitoring for the management of heart failure. Eur. J. Heart Fail. 2003, 5, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Zito, A.; Princi, G.; Romiti, G.F.; Galli, M.; Basili, S.; Liuzzo, G.; Sanna, T.; Restivo, A.; Ciliberti, G.; Trani, C.; et al. Device-based remote monitoring strategies for congestion-guided management of patients with heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2022, 24, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Curtain, J.P.; Lee, M.M.; McMurray, J.J.; Gardner, R.S.; Petrie, M.C.; Jhund, P.S. Efficacy of implantable haemodynamic monitoring in heart failure across ranges of ejection fraction: A systematic review and meta-analysis. Heart 2023, 109, 823–831. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.J.; Ray, M.; Brienesse, S.C.; Sritharan, S.; Boyle, A.J.; Jackson, N.; Leitch, J.W.; Sverdlov, A.L. Remote monitoring in patients with heart failure with cardiac implantable electronic devices: A systematic review and meta-analysis. Open Heart 2022, 9, e002096. [Google Scholar] [CrossRef] [PubMed]
- Straw, S.; Witte, K.K. Remote monitoring in heart failure: It’s the data you collect and what you do with them. Heart 2023, 109, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous wearable monitoring analytics predict heart failure hospitalization: The LINK-HF multicenter study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Yuan, W.L.; Huang, T.C.; Zhang, H.F.; Mai, J.T.; Wang, J.F. Clinical effectiveness of telemedicine for chronic heart failure: A systematic review and meta-analysis. J. Investig. Med. 2017, 65, 899–911. [Google Scholar] [CrossRef]
- Kuan, P.X.; Chan, W.K.; Ying, D.K.F.; Rahman, M.A.A.; Peariasamy, K.M.; Lai, N.M.; Mills, N.L.; Anand, A. Efficacy of telemedicine for the management of cardiovascular disease: A systematic review and meta-analysis. Lancet Digit. Health 2022, 4, e676–e691. [Google Scholar] [CrossRef]
- Alvarez, P.; Sianis, A.; Brown, J.; Ali, A.; Briasoulis, A. Chronic disease management in heart failure: Focus on telemedicine and remote monitoring. Rev. Cardiovasc. Med. 2021, 22, 403–413. [Google Scholar] [CrossRef]
- Umeh, C.A.; Torbela, A.; Saigal, S.; Kaur, H.; Kazourra, S.; Gupta, R.; Shah, S. Telemonitoring in heart failure patients: Systematic review and meta-analysis of randomized controlled trials. World J. Cardiol. 2022, 14, 640. [Google Scholar] [CrossRef]
- Rebolledo Del Toro, M.; Herrera Leano, N.M.; Barahona-Correa, J.E.; Munoz Velandia, O.M.; Fernández Ávila, D.G.; García Peña, Á.A. Effectiveness of mobile telemonitoring applications in heart failure patients: Systematic review of literature and meta-analysis. Heart Fail. Rev. 2023, 28, 431–452. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in patients with heart failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Galinier, M.; Roubille, F.; Berdague, P.; Brierre, G.; Cantie, P.; Dary, P.; Ferradou, J.M.; Fondard, O.; Labarre, J.P.; Mansourati, J.; et al. Telemonitoring versus standard care in heart failure: A randomised multicentre trial. Eur. J. Heart Fail. 2020, 22, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: The better effectiveness after transition–heart failure (BEAT-HF) randomized clinical trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Black, J.T.; Romano, P.S.; Sadeghi, B.; Auerbach, A.D.; Ganiats, T.G.; Greenfield, S.; Kaplan, S.H.; Ong, M.K.; BEAT-HF Research Group. A remote monitoring and telephone nurse coaching intervention to reduce readmissions among patients with heart failure: Study protocol for the Better Effectiveness After Transition-Heart Failure (BEAT-HF) randomized controlled trial. Trials 2014, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Binuya, M.; Engelhardt, E.; Schats, W.; Schmidt, M.; Steyerberg, E. Methodological guidance for the evaluation and updating of clinical prediction models: A systematic review. BMC Med. Res. Methodol. 2022, 22, 316. [Google Scholar] [CrossRef]
- Kappen, T.H.; van Klei, W.A.; van Wolfswinkel, L.; Kalkman, C.J.; Vergouwe, Y.; Moons, K.G. Evaluating the impact of prediction models: Lessons learned, challenges, and recommendations. Diagn. Progn. Res. 2018, 2, 11. [Google Scholar] [CrossRef]
- Duran, A.; De Anda-Duran, I.; Ventura, H.O. The era of heart failure risk prediction models, is it time to test their utility? Int. J. Cardiol. 2022, 352, 98–99. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) the TRIPOD statement. Circulation 2015, 131, 211–219. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.F.; Moons, K.G.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; PROBAST Group. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Moons, K.G.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A tool to assess risk of bias and applicability of prediction model studies: Explanation and elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Harhay, M.O.; Lederer, D.J.; Abramson, M.; Adjei, A.A.; Bakker, J.; Ballas, Z.K.; Barreiro, E.; Bell, S.C.; Bellomo, R.; et al. Development and reporting of prediction models: Guidance for authors from editors of respiratory, sleep, and critical care journals. Crit. Care Med. 2020, 48, 623. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, M.M.; Loftus, T.J.; Small, C.; Li, H.; Ozrazgat-Baslanti, T.; Balch, J.; Holmes, R.; Tighe, P.J.; Upchurch, G.R., Jr.; Efron, P.A.; et al. Predictive Modeling for Readmission to Intensive Care: A Systematic Review. Crit. Care Explor. 2023, 5, e0848. [Google Scholar] [CrossRef] [PubMed]
- Collett, D. Modelling Survival Data in Medical Research, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Therneau, T.; Grambsch, P.M. Modelling Survival Data: Extending the Cox Model, 1st ed.; Springer: New York, NY, USA, 2000. [Google Scholar]
- Liu, X. Survival Analysis: Models and Applications; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Efron, B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J. Am. Stat. Assoc. 1988, 83, 414–425. [Google Scholar] [CrossRef]
- Cook, N.R. Statistical evaluation of prognostic versus diagnostic models: Beyond the ROC curve. Clin. Chem. 2008, 54, 17–23. [Google Scholar] [CrossRef]
- Ahmad, T.; Munir, A.; Bhatti, S.H.; Aftab, M.; Raza, M.A. Survival analysis of heart failure patients: A case study. PLoS ONE 2017, 12, e0181001. [Google Scholar] [CrossRef]
- Javed, F.; Farrugia, S.; Colefax, M.; Schindhelm, K. Early warning of acute decompensation in heart failure patients using a noncontact measure of stability index. IEEE Trans. Biomed. Eng. 2015, 63, 438–448. [Google Scholar] [CrossRef]
- Henriques, J.; Carvalho, P.; Paredes, S.; Rocha, T.; Habetha, J.; Antunes, M.; Morais, J. Prediction of heart failure decompensation events by trend analysis of telemonitoring data. IEEE J. Biomed. Health Inform. 2014, 19, 1757–1769. [Google Scholar] [CrossRef]
- Zhang, J.; Goode, K.M.; Cuddihy, P.E.; Cleland, J.G.; TEN-HMS Investigators. Predicting hospitalization due to worsening heart failure using daily weight measurement: Analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur. J. Heart Fail. 2009, 11, 420–427. [Google Scholar] [CrossRef]
- Gardner, R.S.; Thakur, P.; Hammill, E.F.; Nair, D.G.; Eldadah, Z.; Stančák, B.; Ferrick, K.; Sriratanasathavorn, C.; Duray, G.Z.; Wariar, R.; et al. Multiparameter diagnostic sensor measurements during clinically stable periods and worsening heart failure in ambulatory patients. ESC Heart Fail. 2021, 8, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, L.; Astor, B.; Yang, W.; Greene, T. Predicting the risk of a clinical event using longitudinal data: The generalized landmark analysis. BMC Med. Res. Methodol. 2023, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39,372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.A.; Clark, C.A.; Zhang, S.; Halm, E.A.; Shannon, J.J.; Girod, C.E.; Cooper, L.; Amarasingham, R. Predicting out of intensive care unit cardiopulmonary arrest or death using electronic medical record data. BMC Med. Inform. Decis. Mak. 2013, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Robicsek, A.A.; Meltzer, D.O.; Gibbons, R.D.; Edelson, D.P. Multicenter development and validation of a risk stratification tool for ward patients. Am. J. Respir. Crit. Care Med. 2014, 190, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Warman, E.N.; Cho, Y.K.; Sarkar, S. Using Biomarker Information for Heart Failure Risk Computation. U.S. Patent 16/708,572, 18 June 2020. [Google Scholar]
- Fahimi, F.; Guo, Y.; Tong, S.C.; Ng, A.; Bing, S.O.Y.; Choo, B.; Weiliang, H.; Lee, S.; Ramasamy, S.; Chow, W.L.; et al. A Vital Signs Telemonitoring Programme Improves the Dynamic Prediction of Readmission Risk in Patients with Heart Failure. Amia Annu. Symp. Proc. 2020, 2020, 432. [Google Scholar] [PubMed]
- Valko, M.; Hauskrecht, M. Feature importance analysis for patient management decisions. Stud. Health Technol. Inform. 2010, 160, 861. [Google Scholar]
- Sarkar, S.; Koehler, J. A dynamic risk score to identify increased risk for heart failure decompensation. IEEE Trans. Biomed. Eng. 2012, 60, 147–150. [Google Scholar] [CrossRef]
- Joshi, R.; Gyllensten, I.C. Changes in daily measures of blood pressure and heart rate improve weight-based detection of heart failure deterioration in patients on telemonitoring. IEEE J. Biomed. Health Inform. 2018, 23, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Ledwidge, M.; Mcdonald, K. Evaluation of a Subject’s Weight. U.S. Patent 20120330683-A1, 31 August 2011. [Google Scholar]
- Larburu, N.; Artetxe, A.; Escolar, V.; Lozano, A.; Kerexeta, J. Artificial intelligence to prevent mobile heart failure patients decompensation in real time: Monitoring-based predictive model. Mob. Inf. Syst. 2018, 2018, 1546210. [Google Scholar] [CrossRef]
- Gyllensten, I.G.L.C.; Joshi, R.; ter Horst, H.J. System and Method for Predicting Heart Failure Decompensation. U.S. Patent 10,638,980, 5 May 2020. [Google Scholar]
- Van Smeden, M.; Reitsma, J.B.; Riley, R.D.; Collins, G.S.; Moons, K.G. Clinical prediction models: Diagnosis versus prognosis. J. Clin. Epidemiol. 2021, 132, 142–145. [Google Scholar] [CrossRef]
- Fu, L.H.; Schwartz, J.; Moy, A.; Knaplund, C.; Kang, M.J.; Schnock, K.O.; Garcia, J.P.; Jia, H.; Dykes, P.C.; Cato, K.; et al. Development and validation of early warning score system: A systematic literature review. J. Biomed. Inform. 2020, 105, 103410. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Park, S.Y.; Gibbons, R.; Edelson, D.P. Using electronic health record data to develop and validate a prediction model for adverse outcomes on the wards. Crit. Care Med. 2014, 42, 841. [Google Scholar] [CrossRef] [PubMed]
- Assa, S.; Vernooy, K.; van Stipdonk, A.M. Cardiovascular Implantable Electronic Devices Enabled Remote Heart Failure Monitoring; What We Have Learned and Where to Go Next. J. Cardiovasc. Dev. Dis. 2023, 10, 152. [Google Scholar] [CrossRef]
- Breiman, L. Statistical modeling: The two cultures (with comments and a rejoinder by the author). Stat. Sci. 2001, 16, 199–231. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, W.; Shi, H.; Ma, H.; Cheng, L.; Huang, Z. Comparing machine learning models and statistical models for predicting heart failure events: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 812276. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Austin, P.C.; Harrell, F.E., Jr.; Steyerberg, E.W. Predictive performance of machine and statistical learning methods: Impact of data-generating processes on external validity in the “large N, small p” setting. Stat. Methods Med. Res. 2021, 30, 1465–1483. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Mauff, K.; Tomer, A.; Rizopoulos, D. An overview of joint modeling of time-to-event and longitudinal outcomes. Annu. Rev. Stat. Appl. 2019, 6, 223–240. [Google Scholar] [CrossRef]
- Bours, M.J. Bayes’ rule in diagnosis. J. Clin. Epidemiol. 2021, 131, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics Notes: Diagnostic tests 2: Predictive values. BMJ 1994, 309, 102. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.J. The predictive value of simple rules for combining two diagnostic tests. Biometrics 1989, 45, 1213–1222. [Google Scholar] [CrossRef]
- Raffaello, W.M.; Henrina, J.; Huang, I.; Lim, M.A.; Suciadi, L.P.; Siswanto, B.B.; Pranata, R. Clinical characteristics of de novo heart failure and acute decompensated chronic heart failure: Are they distinctive phenotypes that contribute to different outcomes? Card. Fail. Rev. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Sahle, B.W.; Owen, A.J.; Chin, K.L.; Reid, C.M. Risk prediction models for incident heart failure: A systematic review of methodology and model performance. J. Card. Fail. 2017, 23, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.I.; Wang, Y.; Concato, J.; Gill, T.M.; Krumholz, H.M. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007, 116, 1549–1554. [Google Scholar] [CrossRef]
- Ledwidge, M.T.; O’Hanlon, R.; Lalor, L.; Travers, B.; Edwards, N.; Kelly, D.; Voon, V.; McDonald, K.M. Can individualized weight monitoring using the HeartPhone algorithm improve sensitivity for clinical deterioration of heart failure? Eur. J. Heart Fail. 2013, 15, 447–455. [Google Scholar] [CrossRef]
- Darling, C.E.; Dovancescu, S.; Saczynski, J.S.; Riistama, J.; Kuniyoshi, F.S.; Rock, J.; Meyer, T.E.; McManus, D.D. Bioimpedance-based heart failure deterioration prediction using a prototype fluid accumulation vest-mobile phone dyad: An observational study. JMIR Cardio 2017, 1, e6057. [Google Scholar] [CrossRef]
- Yu, C.M.; Wang, L.; Chau, E.; Chan, R.H.W.; Kong, S.L.; Tang, M.O.; Christensen, J.; Stadler, R.W.; Lau, C.P. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005, 112, 841–848. [Google Scholar] [CrossRef]
- Lewin, J.; Ledwidge, M.; O’Loughlin, C.; McNally, C.; McDonald, K. Clinical deterioration in established heart failure: What is the value of BNP and weight gain in aiding diagnosis? Eur. J. Heart Fail. 2005, 7, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Hettrick, D.A.; Stadler, R.W. Using Multiple Diagnostic Parameters for Predicting Heart Failure Events. U.S. Patent 9,713,701, 25 July 2017. [Google Scholar]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Koehler, J.; Sarkar, S.; Butler, J. Prediction of worsening heart failure events and all-cause mortality using an individualized risk stratification strategy. ESC Heart Fail. 2020, 7, 4277–4289. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: Results from the MultiSENSE study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Sriratanasathavorn, C.; Fisher, J.; Bransford, P.; Chan, R.; Sweeney, R.J.; Ahmed, R.; Zhang, Y.; Averina, V.; An, Q.; et al. Heart failure diagnostics sensor measurements change prior to heart failure decompensation events. J. Card. Fail. 2017, 23, S65. [Google Scholar] [CrossRef][Green Version]
- López-Azor, J.C.; de la Torre, N.; Carmena, M.D.G.C.; Pérez, P.C.; Munera, C.; MarcoClement, I.; León, R.C.; Álvarez-García, J.; Pachón, M.; Ynsaurriaga, F.A.; et al. Clinical Utility of HeartLogic, a Multiparametric Telemonitoring System, in Heart Failure. Card. Fail. Rev. 2022, 8, e13. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Mulvey, G.K.; Stauffer, B.; Patlolla, V.; Bernheim, S.M.; Keenan, P.S.; Krumholz, H.M. Statistical models and patient predictors of readmission for heart failure: A systematic review. Arch. Intern. Med. 2008, 168, 1371–1386. [Google Scholar] [CrossRef]
- Artetxe, A.; Beristain, A.; Grana, M. Predictive models for hospital readmission risk: A systematic review of methods. Comput. Methods Programs Biomed. 2018, 164, 49–64. [Google Scholar] [CrossRef]
- Liu, J.; Liu, P.; Lei, M.R.; Zhang, H.W.; You, A.L.; Luan, X.R. Readmission Risk Prediction Model for Patients with Chronic Heart Failure: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2022, 51, 1481. [Google Scholar] [CrossRef]
- Rich, J.D.; Burns, J.; Freed, B.H.; Maurer, M.S.; Burkhoff, D.; Shah, S.J. Meta-Analysis Global Group in Chronic (MAGGIC) heart failure risk score: Validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2018, 7, e009594. [Google Scholar] [CrossRef]
- Canepa, M.; Fonseca, C.; Chioncel, O.; Laroche, C.; Crespo-Leiro, M.G.; Coats, A.J.; Mebazaa, A.; Piepoli, M.F.; Tavazzi, L.; Maggioni, A.P.; et al. Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail. 2018, 6, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Codina, P.; Lupón, J.; Borrellas, A.; Spitaleri, G.; Cediel, G.; Domingo, M.; Simpson, J.; Levy, W.C.; Santiago-Vacas, E.; Zamora, E.; et al. Head-to-head comparison of contemporary heart failure risk scores. Eur. J. Heart Fail. 2021, 23, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.A.; Boye, M.E.; Crowther, M.J.; Ibrahim, J.G.; Quartey, G.; Micallef, S.; Bois, F.Y. Joint modeling of survival and longitudinal non-survival data: Current methods and issues. Report of the DIA Bayesian joint modeling working group. Stat. Med. 2015, 34, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Luo, J.; Liu, J.; Wan, F.; Wang, G.; Gordon, M.; Xiong, C. Comparing statistical methods in assessing the prognostic effect of biomarker variability on time-to-event clinical outcomes. BMC Med. Res. Methodol. 2022, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, D. Joint Models for Longitudinal and Time-to-Event Data: With Applications in R; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Suresh, K.; Taylor, J.M.; Spratt, D.E.; Daignault, S.; Tsodikov, A. Comparison of joint modeling and landmarking for dynamic prediction under an illness-death model. Biom. J. 2017, 59, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, L.; Astor, B.C. A comparison of two approaches to dynamic prediction: Joint modeling and landmark modeling. Stat. Med. 2023, 42, 2101–2115. [Google Scholar] [CrossRef] [PubMed]
- van Vark, L.C.; Lesman-Leegte, I.; Baart, S.J.; Postmus, D.; Pinto, Y.M.; Orsel, J.G.; Westenbrink, B.D.; Brunner-la Rocca, H.P.; van Miltenburg, A.J.; Boersma, E.; et al. Prognostic value of serial ST2 measurements in patients with acute heart failure. J. Am. Coll. Cardiol. 2017, 70, 2378–2388. [Google Scholar] [CrossRef]
- Canepa, M.; Siri, G.; Puntoni, M.; Latini, R.; Tavazzi, L.; Maggioni, A.P. Testing longitudinal data for prognostication in ambulatory heart failure patients with reduced ejection fraction. A proof of principle from the GISSI-HF database. Int. J. Cardiol. 2020, 313, 89–96. [Google Scholar] [CrossRef]
- Jenkins, D.A.; Sperrin, M.; Martin, G.P.; Peek, N. Dynamic models to predict health outcomes: Current status and methodological challenges. Diagn. Progn. Res. 2018, 2, 23. [Google Scholar] [CrossRef]
- Van Calster, B.; McLernon, D.J.; Van Smeden, M.; Wynants, L.; Steyerberg, E.W. Calibration: The Achilles heel of predictive analytics. BMC Med. 2019, 17, 230. [Google Scholar] [CrossRef]
- Therneau, T.; Crowson, C.; Atkinson, E. Using time dependent covariates and time dependent coefficients in the cox model. Surviv. Vignettes 2017, 2, 1–25. [Google Scholar]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G. Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Croon, P.; Selder, J.; Allaart, C.; Bleijendaal, H.; Chamuleau, S.; Hofstra, L.; Išgum, I.; Ziesemer, K.; Winter, M. Current state of artificial intelligence-based algorithms for hospital admission prediction in patients with heart failure: A scoping review. Eur. Heart J. Digit. Health 2022, 3, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Ghanta, S.N.; Mueller, J.; Mansour, M.; Chen, Z.; Puente, C.; Ha, Y.M.; Tarun, T.; Dhar, G.; Sivakumar, K.; et al. Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications. Diagnostics 2022, 12, 2964. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B.J.; Downing, N.S.; Bucholz, E.M.; Dharmarajan, K.; Manhapra, A.; Li, S.X.; Negahban, S.N.; Krumholz, H.M. Analysis of machine learning techniques for heart failure readmissions. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Kerexeta, J.; Larburu, N.; Escolar, V.; Lozano-Bahamonde, A.; Macía, I.; Beristain Iraola, A.; Graña, M. Prediction and analysis of heart failure decompensation events based on telemonitored data and artificial intelligence methods. J. Cardiovasc. Dev. Dis. 2023, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Austin, P.C.; Ross, H.J.; Abdel-Qadir, H.; Freitas, C.; Tomlinson, G.; Chicco, D.; Mahendiran, M.; Lawler, P.R.; Billia, F.; et al. Machine learning vs. conventional statistical models for predicting heart failure readmission and mortality. ESC Heart Fail. 2021, 8, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Tan, X.; Liu, Y.; Kannapur, K.; Ramanan, D.; Kessler, G.; Lautsch, D.; Fonarow, G. Comparison of machine learning algorithms for predicting hospital readmissions and worsening heart failure events in patients with heart failure with reduced ejection fraction: Modeling study. JMIR Form. Res. 2023, 7, e41775. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Sant’Anna, A.; Lingman, M.; Nowaczyk, S. Readmission prediction using deep learning on electronic health records. J. Biomed. Inform. 2019, 97, 103256. [Google Scholar] [CrossRef]



| Data Group | Temporal Characteristics |
|---|---|
| Demographic and comorbidity data (baseline) | Not changing during the trial |
| Entry examination data (baseline) | Collected at the time of entry examination |
| Signs and symptoms data (telemedicine data) | Time dependent (high repetitive rate) |
| Episodical or regular visit data | Time dependent (low repetitive rate) |
| Final examination data | Collected at the time of final examination |
| Risk-changing clinical milestones * | Episodic or in the patient’s clinical history |
| Drug dosing and other therapeutic actions | Episodic or in the patient’s clinical history |
| Entry Examination (Baseline Data) * | Signs and Symptoms (Highly Repetitive Data) |
|---|---|
| NYHA II–IV | Heart rate |
| LVEF | Systolic blood pressure |
| ECG | Diastolic blood pressure |
| Haemoglobin | Body weight |
| Serum sodium | Oxygen saturation |
| Serum potassium | Symptom intensity level |
| Serum creatine | |
| NT-proBNP | Demographics (baseline data) |
| CRP | Age |
| BUN | Race |
| KCCQ-12 | Gender |
| 6-min walk test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odrobina, I. Clinical Predictive Modeling of Heart Failure: Domain Description, Models’ Characteristics and Literature Review. Diagnostics 2024, 14, 443. https://doi.org/10.3390/diagnostics14040443
Odrobina I. Clinical Predictive Modeling of Heart Failure: Domain Description, Models’ Characteristics and Literature Review. Diagnostics. 2024; 14(4):443. https://doi.org/10.3390/diagnostics14040443
Chicago/Turabian StyleOdrobina, Igor. 2024. "Clinical Predictive Modeling of Heart Failure: Domain Description, Models’ Characteristics and Literature Review" Diagnostics 14, no. 4: 443. https://doi.org/10.3390/diagnostics14040443
APA StyleOdrobina, I. (2024). Clinical Predictive Modeling of Heart Failure: Domain Description, Models’ Characteristics and Literature Review. Diagnostics, 14(4), 443. https://doi.org/10.3390/diagnostics14040443






