Vertebro-Vertebral Arteriovenous Fistulae: A Case Series of Endovascular Management at a Single Center
Abstract
1. Introduction
2. Materials and Methods
3. Results
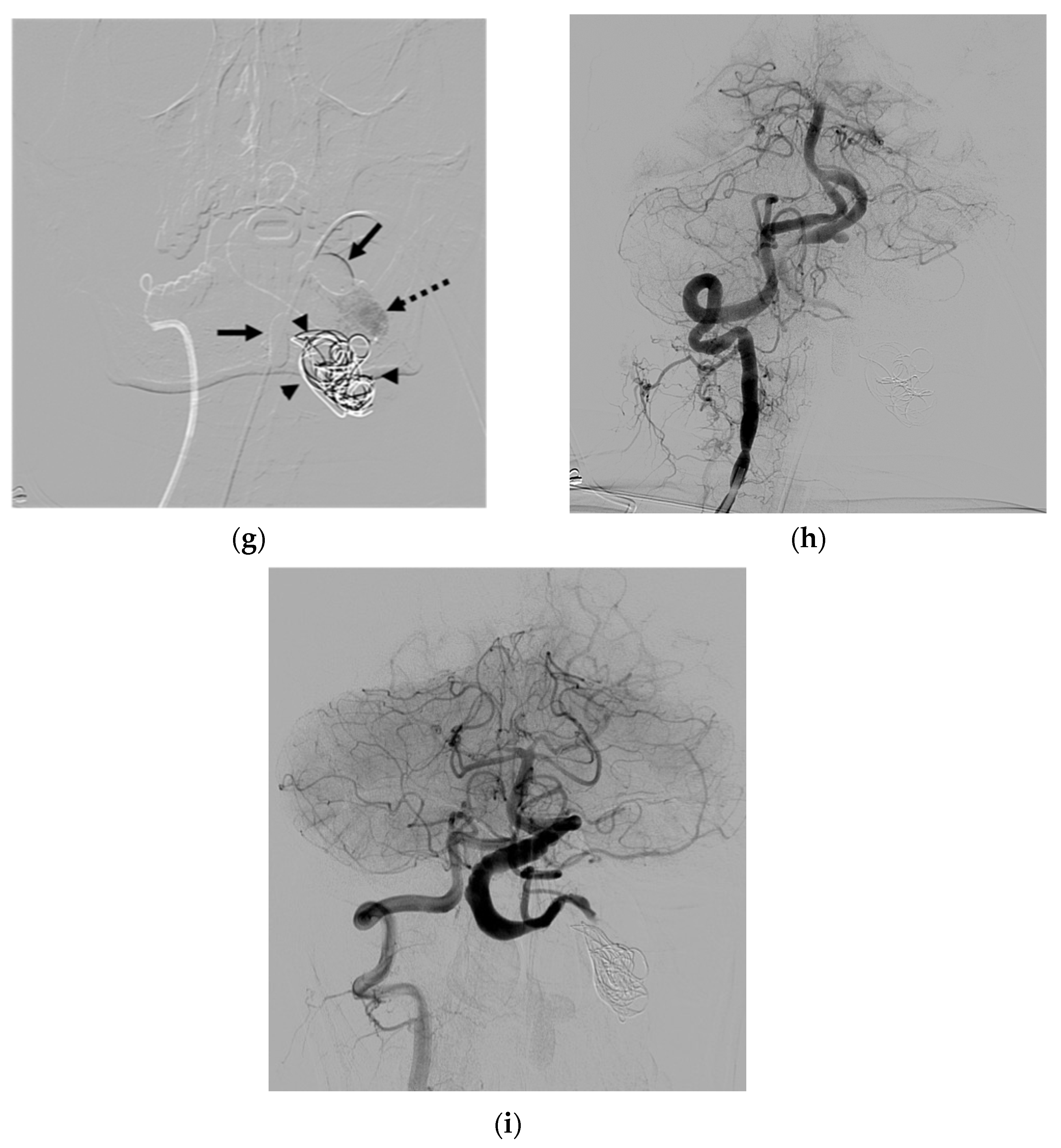
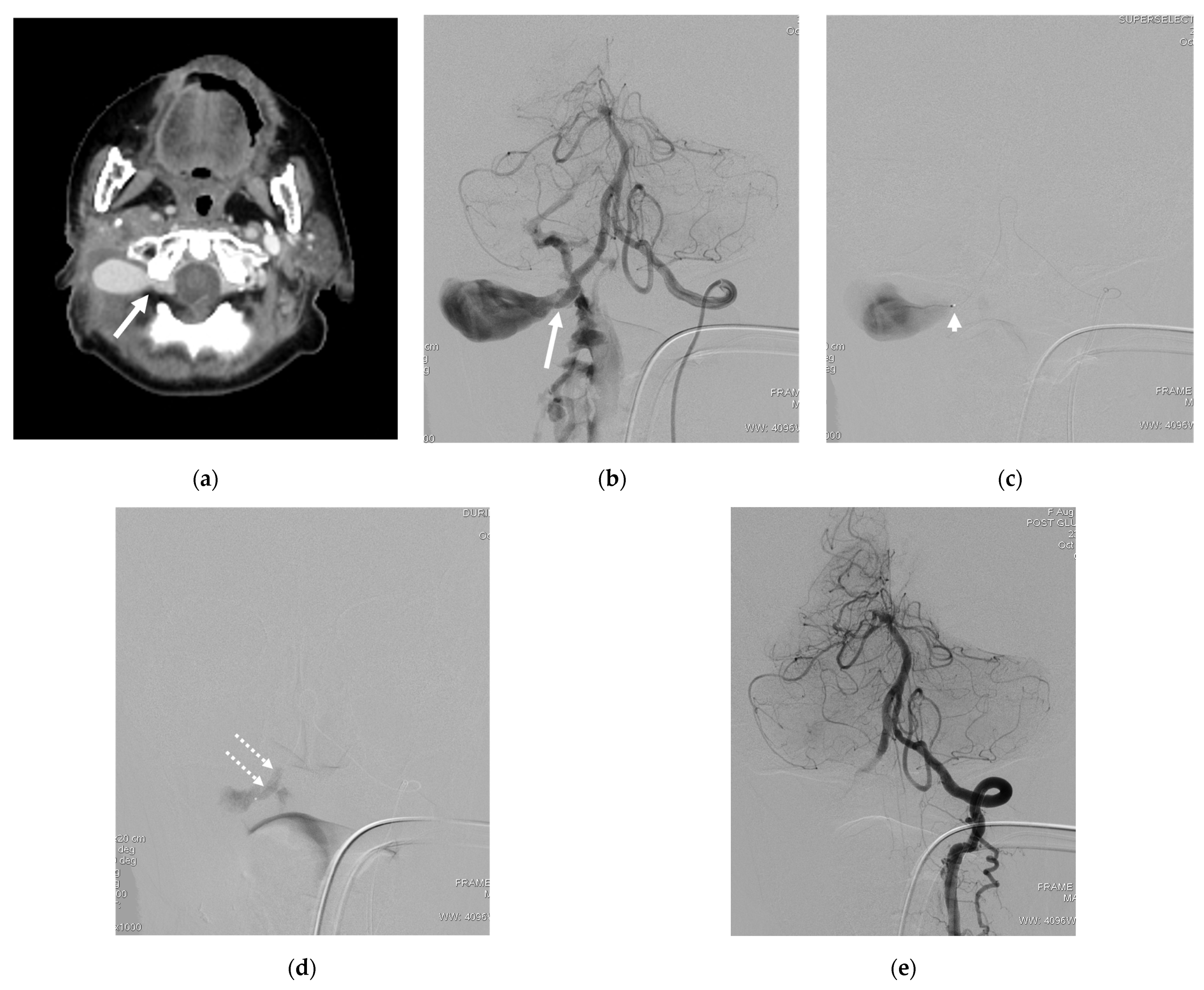
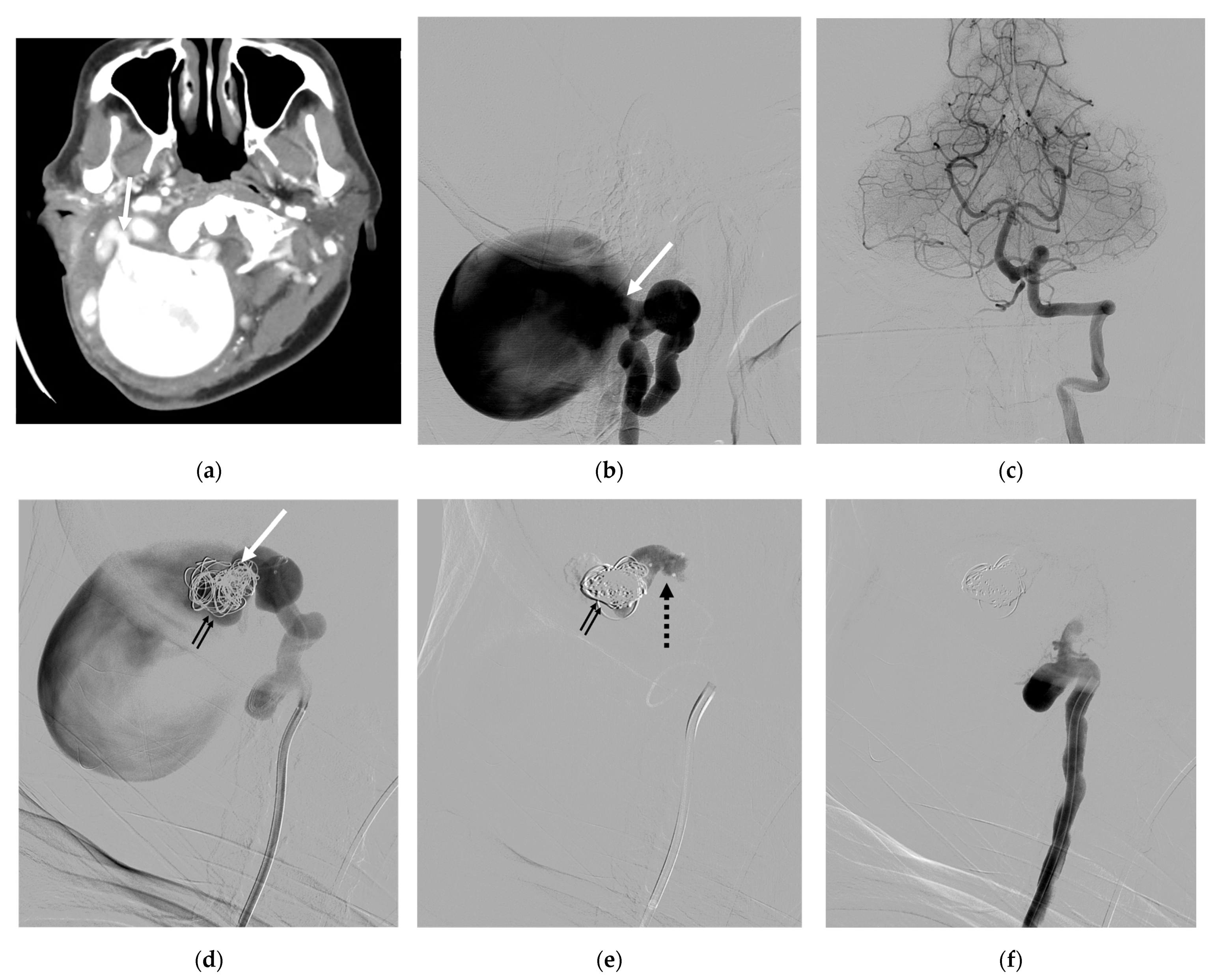
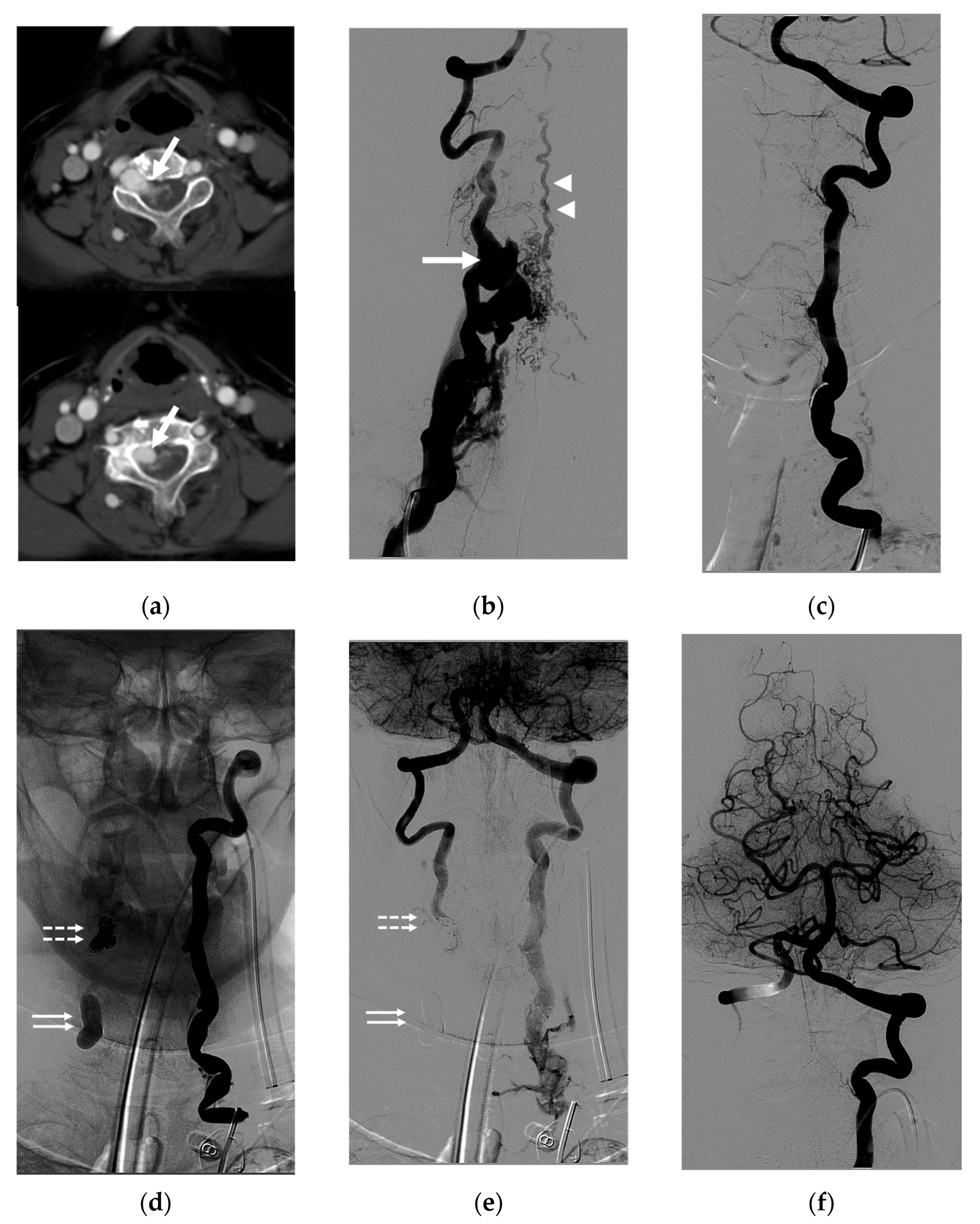
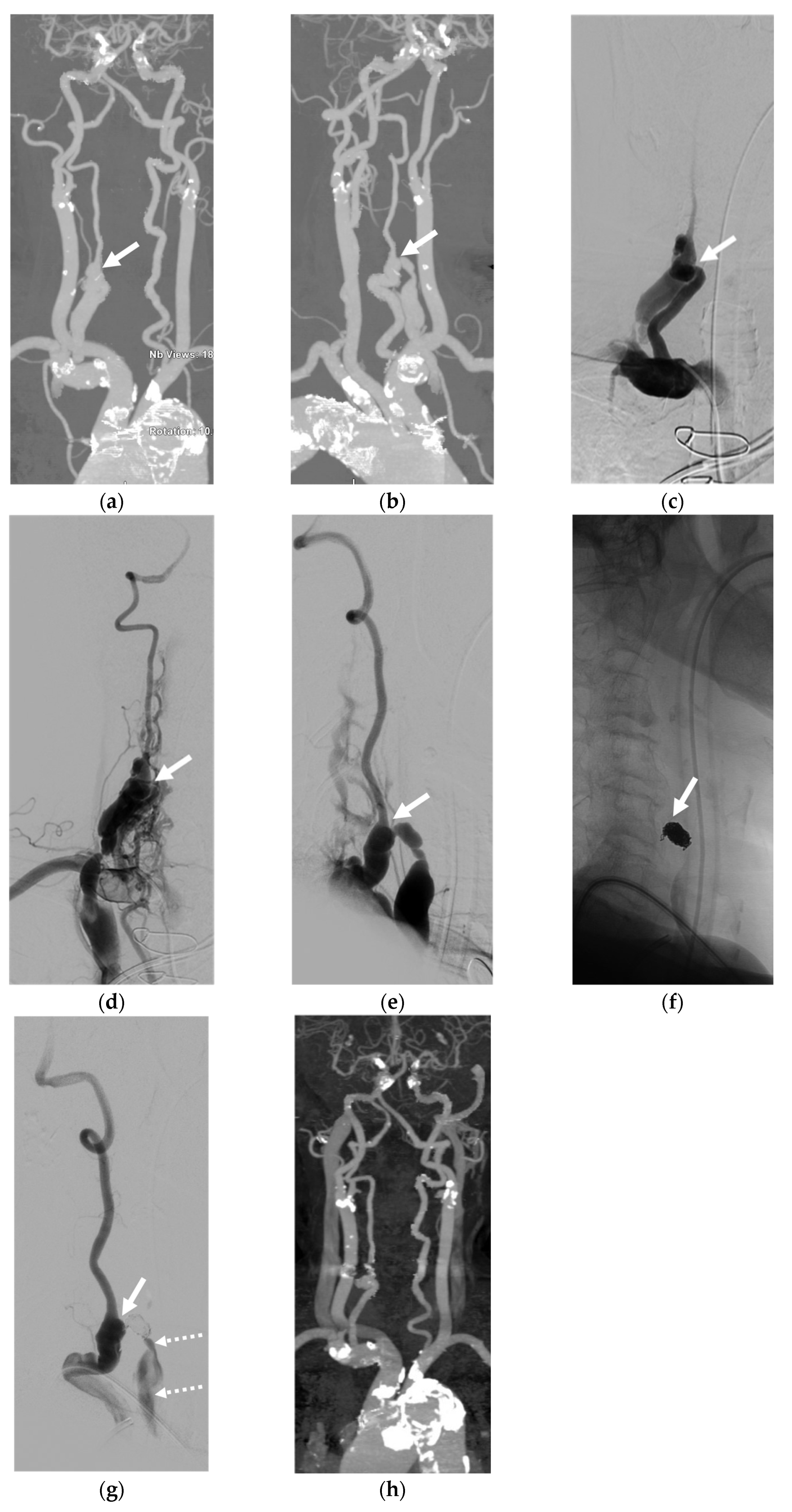
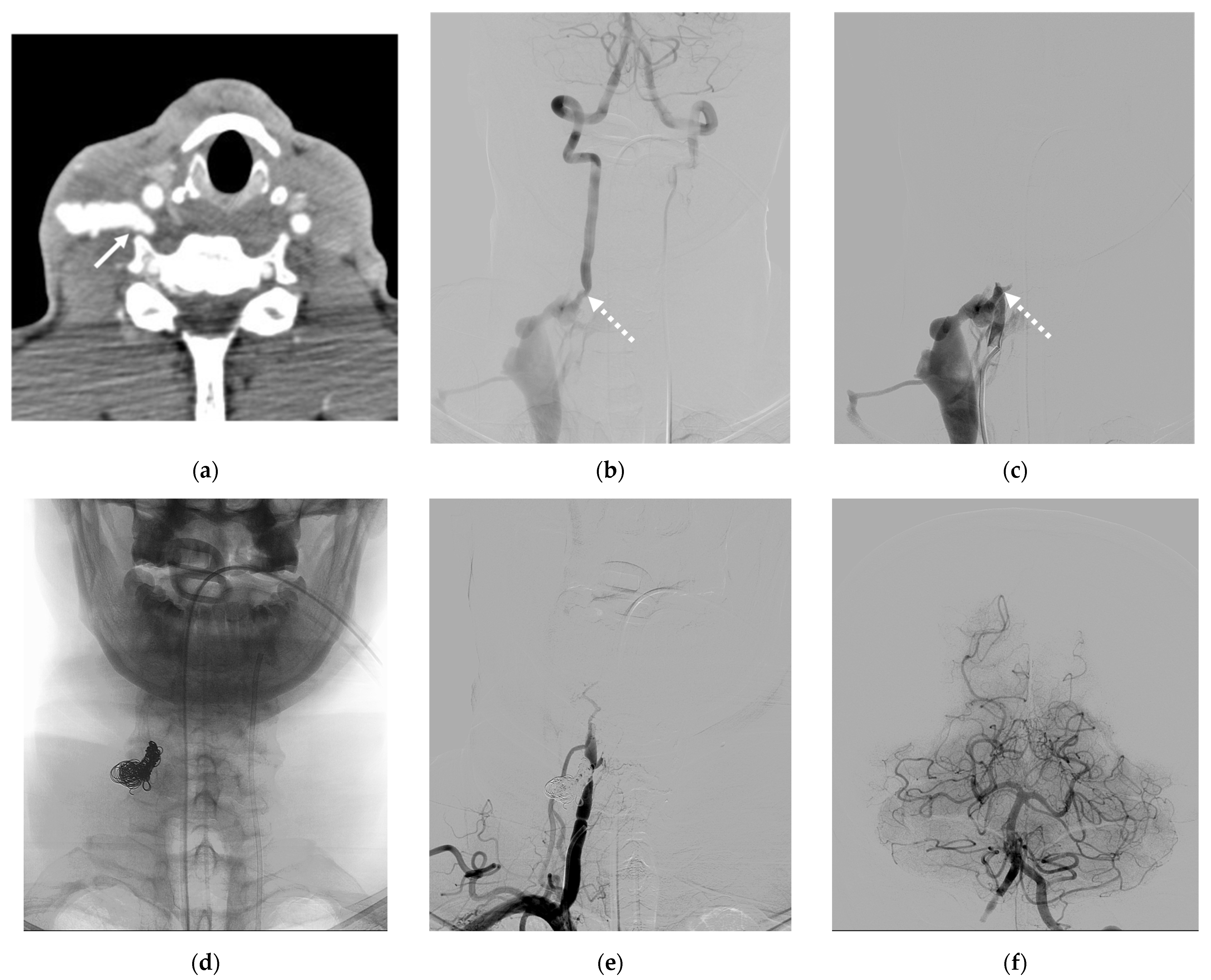
4. Illustrative Cases
5. Discussion
- The affected VA held dominance in the vascular supply of the posterior circulation;
- The shunt presented as being small without the presence of a sizable venous pouch.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aljobeh, A.; Sorenson, T.J.; Bortolotti, C.; Cloft, H.; Lanzino, G. Vertebral Arteriovenous Fistula: A Review Article. World Neurosurg. 2019, 122, e1388–e1397. [Google Scholar] [CrossRef] [PubMed]
- Beaujeux, R.L.; Reizine, D.C.; Casasco, A.; Aymard, A.; Rüfenacht, D.; Khayata, M.H.; Riché, M.C.; Merland, J.J. Endovascular treatment of vertebral arteriovenous fistula. Radiology 1992, 183, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Willinsky, R.; Montanera, W.; Terbrugge, K. Spontaneous vertebrovertebral arteriovenous fistulae clinical features, angioarchitecture and management of twelve patients. Interv. Neuroradiol. 1999, 5, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.N.; Jewkes, D.A.; Kendall, B. Vertebrovertebral arteriovenous fistula. A complication of internal jugular catheterisation. Anaesthesia 1984, 39, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Majoie, C.B.; van Rooij, W.J.; Poll-The, B.T. A vertebro-vertebral fistula as a complication of a jugular line. J. Pediatr. 2006, 149, 576. [Google Scholar] [CrossRef] [PubMed]
- Ricolfi, F.; Valiente, E.; Bodson, F.; Poquet, E.; Chiras, J.; Gaston, A. Arteriovenous fistulae complicating central venous catheterization: Value of endovascular treatment based on a series of seven cases. Intensive Care Med. 1995, 21, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Nishioka, T.; Yamashita, K.; Maeda, Y.; Arita, N. Traumatic vertebro-vertebral arteriovenous fistula manifesting as radiculopathy. Case report. Neurol. Med. Chir. 2008, 48, 167–170. [Google Scholar] [CrossRef]
- Jang, H.J.; Oh, S.Y.; Shim, Y.S.; Yoon, S.H. Endovascular treatment of symptomatic high-flow vertebral arteriovenous fistula as a complication after c1 screw insertion. J. Korean Neurosurg. Soc. 2014, 56, 348–352. [Google Scholar] [CrossRef]
- Deans, W.R.; Bloch, S.; Leibrock, L.; Berman, B.M.; Skultety, F.M. Arteriovenous fistula in patients with neurofibromatosis. Radiology 1982, 144, 103–107. [Google Scholar] [CrossRef]
- Benndorf, G.; Assmann, U.; Bender, A.; Lehmann, T.N.; Lanksch, W.R. Vertebral arteriovenous fistula associated with neurofibromatosis type I misdiagnosed as a giant aneurysm. Interv. Neuroradiol. 2000, 6, 67–74. [Google Scholar] [CrossRef]
- Siddhartha, W.; Chavhan, G.B.; Shrivastava, M.; Limaye, U.S. Endovascular treatment for bilateral vertebral arteriovenous fistulas in neurofibromatosis 1. Australas. Radiol. 2003, 47, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Narayana, R.V.; Pati, R.; Dalai, S. Endovascular management of spontaneous vertebrovertebral arteriovenous fistula associated with neurofibromatosis 1. Indian J. Radiol. Imaging 2015, 25, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Uneda, A.; Suzuki, K.; Okubo, S.; Hirashita, K.; Yunoki, M.; Yoshino, K. Neurofibromatosis Type 1-Associated Extracranial Vertebral Artery Aneurysm Complicated by Vertebral Arteriovenous Fistula After Rupture: Case Report and Literature Review. World Neurosurg. 2016, 96, 609.e13–609.e18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Zhao, K.; Duan, G.; Liu, J.; Huang, Q. Endovascular treatment of vertebro-vertebral arteriovenous fistula in neurofibromatosis type I: A report of two cases and literature review with a focus on endovascular treatment. Clin. Neurol. Neurosurg. 2021, 207, 106806. [Google Scholar] [CrossRef]
- Furtado, S.V.; Vasavada, P.; Baid, A.; Perikal, P.J. Endovascular management of V3 segment vertebro-vertebral fistula: Case management and literature review. Br. J. Neurosurg. 2022, 1–5. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, G.; Lu, L.; Li, C.; Yang, R. Vertebral arteriovenous fistulae (AVF) and vertebral artery aneurysms in neurofibromatosis type 1: A case report and a systematic review. Medicine 2022, 101, e30952. [Google Scholar] [CrossRef]
- Bahar, S.; Chiras, J.; Carpena, J.P.; Meder, J.F.; Bories, J. Spontaneous vertebro-vertebral arterio-venous fistula associated with fibro-muscular dysplasia. Report of two cases. Neuroradiology 1984, 26, 45–49. [Google Scholar] [CrossRef]
- Edwards, M.K.; Christenson, E.N.; Corliss, B.M.; Polifka, A.J.; Allen, B.R. Vertebral Arteriovenous Fistula: An Unwelcome Thrill. Case Rep. Emerg. Med. 2017, 2017, 8386459. [Google Scholar] [CrossRef]
- Breda, M.S.; Amorim, J.; Rocha, J.; Dias, L. Vertebro-vertebral fistula presenting as a pulsatile tinnitus. BMJ Case Rep. 2018, 2018, bcr-2017-222815. [Google Scholar] [CrossRef]
- John, S.; Jaffari, N.; Lu, M.; Hussain, M.S.; Hui, F. Spontaneous vertebral arteriovenous fistula causing cervical myelopathy and acute ischemic strokes treated by endovascular balloon-assisted coiling and Onyx embolization. J. Clin. Neurosci. 2014, 21, 167–170. [Google Scholar] [CrossRef]
- Costa, M.; Basamh, M.; Vivanco-Suarez, J.; Casanova, D.; Baldoncini, M.; Alobaid, A.; Loh, Y.; Patel, A.; McDougall, C.G.; Monteith, S.J. Vertebral-Venous fistulas: Single center experience and practical treatment approach. Interv. Neuroradiol. 2023, 15910199231170079. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Tedeschi, E.; Leone, G.; Marseglia, M.; Cicala, D.; Giamundo, M.; Napoli, M.; Caranci, F. Endovascular treatment of vertebro-vertebral arteriovenous fistula. A report of three cases and literature review. Neuroradiol. J. 2013, 26, 339–346. [Google Scholar] [CrossRef]
- Singh, D.K.; Sinha, K.; Kaif, M.; Yadav, K.; Singh, N.; Chand, V.K.; Sharma, P.K.; Singh, N.; Dabbir, V.K. Endovascular Management of Vertebro-Vertebral Arteriovenous Fistula (VVAVF) with Trapping of the Vertebral Artery. Neurol. India 2023, 71, 898–901. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, Y.L.; Wu, Y.M.; Huang, Y.C.; Wong, H.F. Anatomically based approach for endovascular treatment of vertebro-vertebral arteriovenous fistula. Interv. Neuroradiol. 2014, 20, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Nageshwaran, S.K.; Deng, F.; Regenhardt, R.W.; Das, A.S.; Alotaibi, N.M.; Patel, A.B.; Stapleton, C.J. Deconstructive repair of a traumatic vertebrovertebral arteriovenous fistula via a contralateral endovascular approach. J. Cerebrovasc. Endovasc. Neurosurg. 2022, 24, 291–296. [Google Scholar] [CrossRef]
- Madoz, A.; Desal, H.; Auffray-Calvier, E.; Isnard, J.; Liberge, R.; Taverneau, C.; De Kersaint-Gilly, A. Vertebrovertebral arteriovenous fistula diagnosis and treatment: Report of 8 cases and review of the literature. J. Neuroradiol. 2006, 33, 319–327. [Google Scholar] [CrossRef]
- Kobkitsuksakul, C.; Jiarakongmun, P.; Chanthanaphak, E.; Pongpech, S. Noncavernous arteriovenous shunts mimicking carotid cavernous fistulae. Diagn. Interv. Radiol. 2016, 22, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Endo, T.; Sato, K.; Fesli, R.; Ogawa, Y.; Fujimura, M.; Matsumoto, Y.; Tominaga, T. Massive Intramedullary Hemorrhage After Subarachnoid Hemorrhage in Patient with Vertebrovertebral Arteriovenous Fistula. World Neurosurg. 2019, 129, 432–436. [Google Scholar] [CrossRef]
- Walcott, B.P.; Berkhemer, O.A.; Leslie-Mazwi, T.M.; Chandra, R.V.; Ogilvy, C.S.; Yoo, A.J. Multimodal endovascular treatment of a vertebrovertebral fistula presenting with subarachnoid hemorrhage and hydrocephalus. J. Clin. Neurosci. 2013, 20, 1295–1298. [Google Scholar] [CrossRef]
- Choi, K.-D.; Choi, J.-H.; Kim, J.-S.; Kim, H.J.; Kim, M.-J.; Lee, T.-H.; Lee, H.; Moon, I.S.; Oh, H.J.; Kim, J.-I. Rotational Vertebral Artery Occlusion. Stroke 2013, 44, 1817–1824. [Google Scholar] [CrossRef]
- Xue, S.; Shi, H.; Du, X.; Ma, X. Bow Hunter’s syndrome combined with ipsilateral vertebral artery dissection/pseudoaneurysm: Case study and literature review. Br. J. Neurosurg. 2023, 37, 911–915. [Google Scholar] [CrossRef]
- Berenstein, A.L.P.; ter Brugge, K.G. Vertebro-Vertebral Arteriovenous Fistulae; Berenstein, A.L.P., Brugge, K.G., Eds.; Springer: New York, NY, USA, 2004; Volume 2, p. 9. [Google Scholar]
- Hungerford, G.D.; Perot, P.L. Detachable balloon treatment of carotid-cavernous and vertebro-vertebral fistulas. J. S. C. Med. Assoc. 1982, 78, 479–483. [Google Scholar] [PubMed]
- Modi, M.; Bapuraj, J.R.; Lal, A.; Prabhakar, S.; Khandelwal, N. Vertebral Arteriovenous Fistula Presenting as Cervical Myelopathy: A Rapid Recovery with Balloon Embolization. Cardiovasc. Intervent. Radiol. 2010, 33, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Crowley, R.W.; Medel, R.; Dumont, A.S. Traumatic high flow vertebral-venous fistula presenting with delayed ischemic stroke: Endovascular management with detachable coils and Amplatzer Vascular Plugs. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.H.; Ghasemi-Rad, M.; Eskesen, V.; Frevert, S.C.; Sølling, C.; Benndorf, G. Vertebro-Vertebral Fistula Occlusion Using a Woven EndoBridge™-Device. Neurointervention 2023, 18, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Viñuela, F.; Duckwiler, G.; Dion, J.; Stocker, A. High-flow, small-hole arteriovenous fistulas: Treatment with electrodetachable coils. AJNR Am. J. Neuroradiol. 1995, 16, 325–328. [Google Scholar] [PubMed]
- Miralbés, S.; Cattin, F.; Andrea, I.; Bonneville, J.F. Vertebral arteriovenous fistula: Endovascular treatment with electrodetachable coils. Neuroradiology 1998, 40, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Tenjin, H.; Kimura, S.; Sugawa, N. Coil embolization of vertebro-vertebral arteriovenous fistula: A case report. Surg. Neurol. 2005, 63, 80–83; discussion 83. [Google Scholar] [CrossRef]
- Wang, Q.; Song, D.; Chen, G. Endovascular treatment of high-flow cervical direct vertebro-vertebral arteriovenous fistula with detachable coils and Onyx liquid embolic agent. Acta Neurochir. 2011, 153, 347–352. [Google Scholar] [CrossRef]
- Mizuhashi, S.; Kominami, S.; Fukuda, K. Successful balloon-assisted coil embolization for a diagnostically difficult case of spontaneous vertebrovertebral arteriovenous fistula. Surg. Neurol. Int. 2020, 11, 474. [Google Scholar] [CrossRef]
- He, H.; Li, Q.; Du, M.; Chen, K.; Li, X.; Li, J.; Shu, C. Endovascular and surgical approaches of iatrogenic vertebrovertebral arteriovenous fistula. J Vasc. Surg. Cases Innov. Tech. 2021, 7, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, M.; Izumi, T.; Nishihori, M.; Nagashima, Y.; Nishimura, Y.; Tsukuda, T.; Kropp, A.E.; Goto, S.; Otsuka, T.; Kato, N.; et al. Direct Vertebral Artery Puncture During Open Surgery for the Endovascular Treatment of a Recurrent Vertebro-Vertebral Arteriovenous Fistula. World Neurosurg. 2021, 146, 166–170. [Google Scholar] [CrossRef]
- Kai, Y.; Hamada, J.I.; Mizuno, T.; Kochi, M.; Ushio, Y.; Kitano, I. Transvenous embolization for vertebral arteriovenous fistula: Report of two cases and technical notes. Acta Neurochir. 2001, 143, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Vinchon, M.; George, B.; D’Arrigo, G.; Reizine, D.; Aymard, A.; Riché, M.C.; Merland, J.J.; Laurian, C.; Cormier, J.M. Vertebral arteriovenous fistulas: A study of 49 cases and review of the literature. Vascular 1994, 2, 359–369. [Google Scholar] [CrossRef]
- Sultan, A.; Hassan, T.; Metwaly, T.I. Angiographic predictors of spontaneous obliteration of transarterial partially embolized brain arteriovenous malformations. Interv. Neuroradiol. 2022, 29, 371–378. [Google Scholar] [CrossRef]
- Vollherbst, D.F.; Chapot, R.; Bendszus, M.; Möhlenbruch, M.A. Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas. Clin. Neuroradiol. 2022, 32, 25–38. [Google Scholar] [CrossRef]
- Geng, J.; Hu, P.; Ma, Y.; Zhang, P.; Zhang, H. Endovascular treatment of V3 segment vertebro-vertebral arteriovenous fistula with Willis covered stent: Case report and literature review. Interv. Neuroradiol. 2019, 25, 97–101. [Google Scholar] [CrossRef]
- Li, F.; Song, X.; Liu, C.; Liu, B.; Zheng, Y. Endovascular stent-graft treatment for a traumatic vertebrovertebral arteriovenous fistula with pseudoaneurysm. Ann. Vasc. Surg. 2014, 28, 489. e11–489. e14. [Google Scholar] [CrossRef]
- González, A.; Mayol, A.; Gil-Peralta, A.; González-Marcos, J.R. Endovascular stent-graft treatment of an iatrogenic vertebral arteriovenous fistula. Neuroradiology 2001, 43, 784–786. [Google Scholar] [CrossRef] [PubMed]


| Variables | Number of Spontaneous VVFs (%) | Number of Traumatic VVFs (%) | Total Number of VVFs (%) |
|---|---|---|---|
| Number of patients | 9 (64.3%) | 5 (35.7%) | 14 (100%) |
| Sex (male or female) | 1:8 | 2:3 | 3:11 |
| Age (years), median ± SD | 49 ± 17.9 | 57 ± 15.7 | 49 ± 17.2 |
| Symptoms | |||
| Tinnitus | 5 | 0 | 5 (35.7%) |
| Neck mass | 3 | 1 | 4 (28.6%) |
| Arm weakness | 3 | 1 | 4 (28.6%) |
| Radicular pain | 1 | 0 | 1 (7.1%) |
| Cerebellar insufficiency | 1 | 0 | 1 (7.1%) |
| Proptosis | 1 | 0 | 1 (7.1%) |
| Congestive heart failure | 1 | 0 | 1 (7.1%) |
| Bleeding | 0 | 3 | 3 (21.4%) |
| Incidental finding | 1 | 0 | 1 (7.1%) |
| Shunt locations | |||
| V3 segment | 6 | 2 | 8 (57.1%) |
| C1 level | 3 | 1 | 4 (28.6%) |
| C2 level | 3 | 1 | 4 (28.6%) |
| V2 segment | 2 | 2 | 4 (28.6%) |
| C4 level | 2 | 0 | 2 (14.3%) |
| C4–5 levels | 0 | 1 | 1 (7.1%) |
| C5–6 levels | 0 | 1 | 1 (7.1%) |
| V1 segment | 1 | 1 | 2 (14.3%) |
| C6–7 levels | 1 | 0 | 1 (7.1%) |
| T1 level | 0 | 1 | 1 (7.1%) |
| Venous pouch/Venous aneurysm | 4 | 2 | 6 (42.9%) |
| Embolization techniques | |||
| Parent vessel sacrifice | 7 | 5 | 12 (85.7%) |
| Coil alone | 0 | 2 | 2 (14.3%) |
| Balloon alone | 1 | 2 | 3 (21.4%) |
| NBCA alone | 1 | 1 | 2 (14.3%) |
| Coil + balloon | 2 | 0 | 2 (14.3%) |
| Coil + NBCA | 2 | 0 | 2 (14.3%) |
| Coil + balloon + NBCA | 1 | 0 | 1 (7.1%) |
| Parent vessel preservation | 2 | 0 | 2 (14.3%) |
| Coil alone | 2 | 0 | 2 (14.3%) |
| Clinical outcome | |||
| Improved | 8 | 5 | 13 (92.9%) |
| Stable (no symptoms) | 1 | 0 | 1 (7.1%) |
| Complications | 5 | 0 | 5 (35.7%) |
| Transient occipital pain | 3 | 0 | 3 (21.4%) |
| Puncture-site hematoma | 1 | 0 | 1 (7.1%) |
| Transient vertigo | 1 | 0 | 1 (7.1%) |
| Pt. No. | Sex | Age | Symptoms | Etiology | Type | Pattern of Venous Drainage | Venous Pouch | Embolic Materials | Outcome of VA | Clinical Outcome | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 49 | Neck mass; tinnitus; right arm weakness | Unknown | S | Radicular vein; VVP with venous pouch | Y | C and NBCA | O | Improved | Transient occipital pain |
| 2 | F | 33 | Bleeding | Central line injury | T | VVP | N | C | O | Improved | None |
| 3 | F | 79 | Congestive heart failure | Unknown | S | IJV; subclavian vein | N | C | P | Improved | Puncture-site hematoma |
| 4 | F | 62 | Tinnitus | Unknown | S | VVP | N | B | O | Improved | Transient occipital pain |
| 5 | F | 50 | Right arm weakness | Unknown | S | Radicular vein; peri- medullary veins | N | B and C | O | Improved | None |
| 6 | M | 42 | Proptosis of left eye | Unknown | S | Epidural veins; VVP to IPS; CS and left SOV | N | B and C | O | Improved | None |
| 7 | F | 70 | Radicular pain; right arm weakness; tinnitus | Unknown | S | Lateral epidural vein | N | C | P | Stable | None |
| 8 | F | 33 | Neck mass; tinnitus; paresthesia of left arm; ataxia | NF-1 | S | IJV; VVP with venous pouch | Y | B, C, and NBCA | O | Improved | Vertigo |
| 9 | F | 25 | Neck mass | NF-1 | S | VVP with venous pouch | Y | C and NBCA | O | Improved | None |
| 10 | F | 27 | Incidental finding | NF-1 | S | Lateral epidural vein with venous pouch | Y | NBCA | O | Improved | None |
| 11 | F | 64 | Neck mass | Traffic accident | T | VVP with venous pouch | N | B | O | Improved | Transient occipital pain |
| 12 | F | 73 | Bleeding | Post-surgery | T | VVP with venous pouch | Y | NBCA | O | Improved | None |
| 13 | M | 57 | Neck mass; right arm weakness | Traffic accident | T | Radicular vein; IJV with venous pouch | Y | C | O | Improved | None |
| 14 | M | 36 | Bleeding in epidural space after surgery | Traffic accident | T | VVP with ruptured venous pouch in epidural space | N | B | O | Improved | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Withayasuk, P.; Wichianrat, R.; Sangpetngam, B.; Aurboonyawat, T.; Chankaew, E.; Homsud, S.; Churojana, A. Vertebro-Vertebral Arteriovenous Fistulae: A Case Series of Endovascular Management at a Single Center. Diagnostics 2024, 14, 414. https://doi.org/10.3390/diagnostics14040414
Withayasuk P, Wichianrat R, Sangpetngam B, Aurboonyawat T, Chankaew E, Homsud S, Churojana A. Vertebro-Vertebral Arteriovenous Fistulae: A Case Series of Endovascular Management at a Single Center. Diagnostics. 2024; 14(4):414. https://doi.org/10.3390/diagnostics14040414
Chicago/Turabian StyleWithayasuk, Pattarawit, Ritthikrai Wichianrat, Boonrerk Sangpetngam, Thaweesak Aurboonyawat, Ekawut Chankaew, Saowanee Homsud, and Anchalee Churojana. 2024. "Vertebro-Vertebral Arteriovenous Fistulae: A Case Series of Endovascular Management at a Single Center" Diagnostics 14, no. 4: 414. https://doi.org/10.3390/diagnostics14040414
APA StyleWithayasuk, P., Wichianrat, R., Sangpetngam, B., Aurboonyawat, T., Chankaew, E., Homsud, S., & Churojana, A. (2024). Vertebro-Vertebral Arteriovenous Fistulae: A Case Series of Endovascular Management at a Single Center. Diagnostics, 14(4), 414. https://doi.org/10.3390/diagnostics14040414






