EUS-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: Results of a Nationwide Study with Long-Term Follow-Up
Abstract
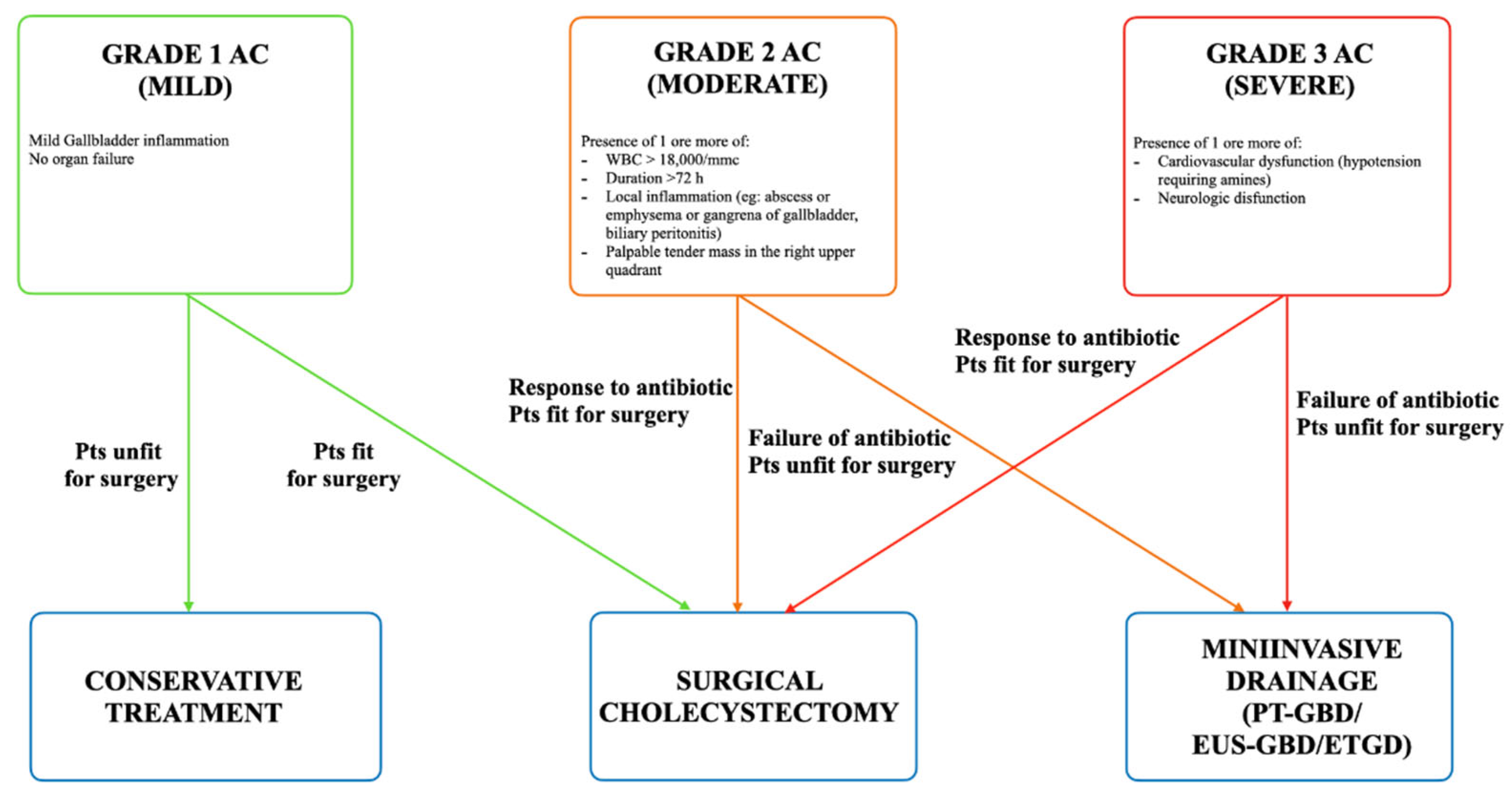
1. Introduction
2. Materials and Methods
2.1. Outcomes
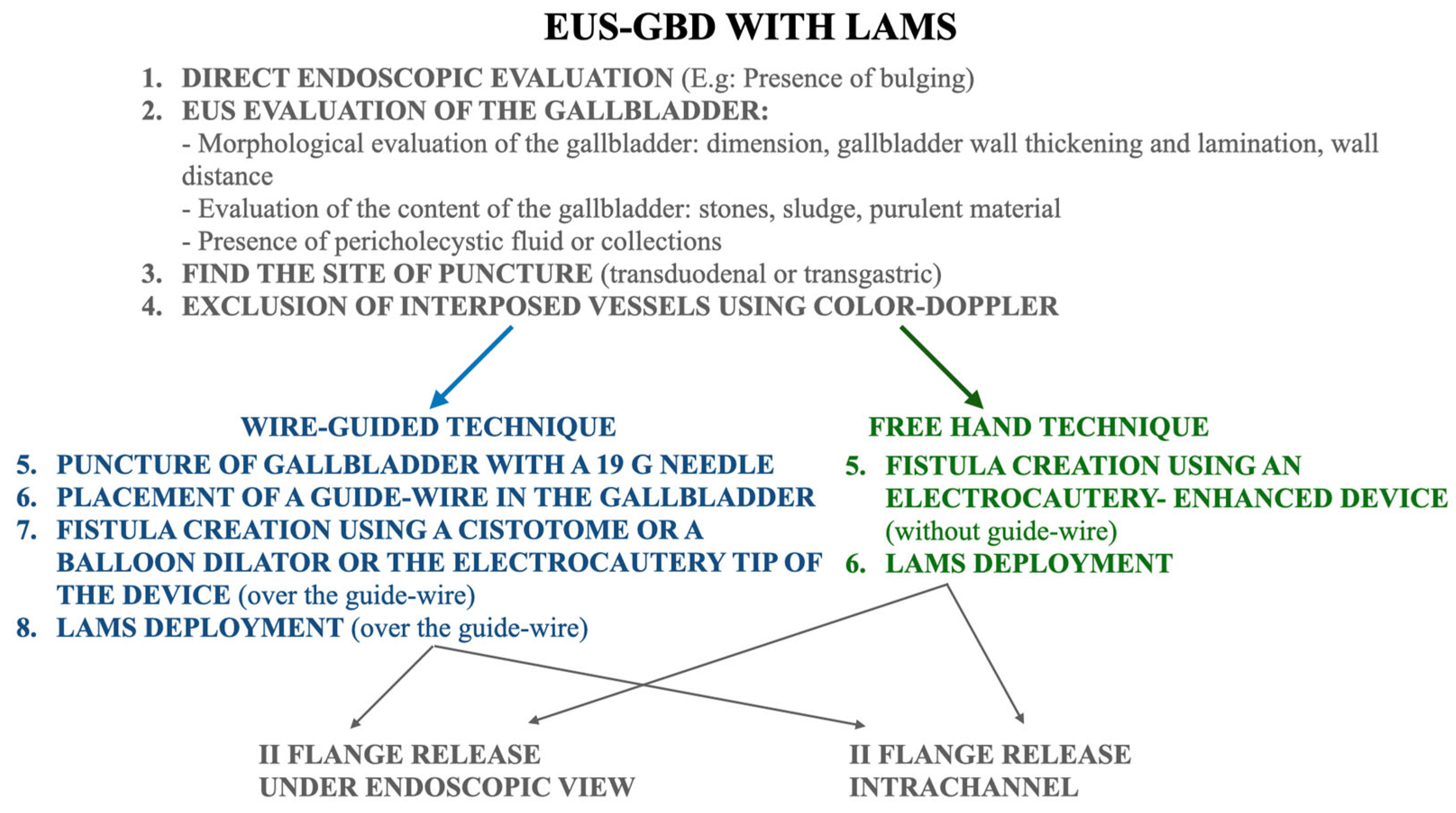
2.2. EUS-Guided Gallbladder Drainage Procedure
2.3. Data
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Gallbladder Drainage Procedure
3.3. Primary Outcomes
3.4. Secondary Outcomes
3.5. Risk Factor Analysis
3.6. Patients with Long-Term Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamoto, K.; Suzuki, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Iwashita, Y.; Hibi, T.; Pitt, H.A.; Umezawa, A.; et al. Tokyo Guidelines 2018: Flowchart for the management of acute cholecystitis. J. Hepatobiliary Pancreat. Sci. 2018, 25, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Kwan, V.; Eisendrath, P.; Antaki, F.; Le Moine, O.; Devière, J. EUS-guided cholecystenterostomy: A new technique (with videos). Gastrointest. Endosc. 2007, 66, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Yamashita, Y.; Ogura, T.; Takenaka, M.; Shimokawa, Y.; Hisa, T.; Itonaga, M.; Kato, H.; Nishikiori, H.; Okuda, A.; et al. Clinical efficacy and safety of endoscopic ultrasound-guided gallbladder drainage replacement of percutaneous drainage: A multicenter retrospective study. Digest. Endosc. 2019, 31, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Saumoy, M.; Tyberg, A.; Brown, E.; Eachempati, S.R.; Lieberman, M.; Afaneh, C.; Kunda, R.; Cosgrove, N.; Siddiqui, A.; Gaidhane, M.; et al. Successful cholecystectomy after endoscopic ultrasound gallbladder drainage compared with percutaneous cholecystostomy, can it be done? J. Clin. Gastroenterol. 2019, 53, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Ngamruengphong, S.; Teoh, A.; Will, U.; Nieto, J.; Abu Dayyeh, B.K.; Gan, S.I.; Larsen, M.; Yip, H.C.; Topazian, M.D.; et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin. Gastroenterol. Hepatol. 2017, 15, 738–745. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Kitano, M.; Itoi, T.; Pérez-Miranda, M.; Ogura, T.; Chan, S.M.; Serna-Higuera, C.; Omoto, S.; Torres-Yuste, R.; Tsuichiya, T.; et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020, 69, 1085–1091. [Google Scholar] [CrossRef]
- Tyberg, A.; Saumoy, M.; Sequeiros, E.V.; Giovannini, M.; Artifon, E.; Teoh, A.; Nieto, J.; Desai, A.P.; Kumta, N.A.; Gaidhane, M.; et al. EUS-guided Versus percutaneous gallbladder drainage: Isn’t it time to convert? J. Clin. Gastroenterol. 2016, 52, 79–84. [Google Scholar] [CrossRef]
- Duncan, C.B.; Riall, T.S. Evidence-based current surgical practice: Calculous gallbladder disease. J. Gastrointest. Surg. 2012, 16, 2011–2025. [Google Scholar] [CrossRef]
- Anderloni, A.; Buda, A.; Vieceli, F.; Khashab, M.A.; Hassan, C.; Repici, A. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: A systematic review and pooled analysis. Surg. Endosc. 2016, 30, 5200–5208. [Google Scholar] [CrossRef]
- Akhan, O.; Akinci, D.; Ozmen, M.N. Percutaneous cholecystostomy. Eur. J. Radiol. 2002, 43, 229–236. [Google Scholar] [CrossRef]
- Súbtil, J.C. Gallbladder drainage guided by endoscopic ultrasound. World J. Gastrointest. Endosc. 2010, 2, 203–209. [Google Scholar] [CrossRef]
- Hatzidakis, A.A.; Prassopoulos, P.; Petinarakis, I.; Sanidas, E.; Chrysos, E.; Chalkiadakis, G.; Tsiftsis, D.; Gourtsoyiannis, N.C. Acute cholecystitis in high-risk patients: Percutaneous cholecystostomy vs conservative treatment. Europ. Radiol. 2002, 12, 1778–1784. [Google Scholar] [CrossRef]
- Adler, D.G. Avoiding the tube: ERCP and EUS approaches to gallbladder drainage as alternatives to percutaneous cholecystostomy in patients with cholecystitis. Gastrointest. Endosc. 2019, 89, 299–300. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, S.S.; Song, T.J.; Hyun, Y.S.; Park, D.Y.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Yun, S.C. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology 2012, 142, 805–811. [Google Scholar] [CrossRef]
- Kedia, P.; Sharaiha, R.Z.; Kumta, N.A.; Widmer, J.; Jamal-Kabani, A.; Weaver, K.; Benvenuto, A.; Millman, J.; Barve, R.; Gaidhane, M.; et al. Endoscopic gallbladder drainage compared with percutaneous drainage. Gastrointest. Endosc. 2015, 82, 1031–1036. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Serna, C.; Penas, I.; Chong, C.C.N.; Perez-Miranda, M.; Ng, E.K.W.; Lau, J.Y.W. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy 2017, 49, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.; Perez-Miranda, M.; Kunda, R.; Lee, S.S.; Irani, S.; Yeaton, P.; Sun, S.; Baron, T.H.; Moon, J.H.; Holt, B.; et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc. Int. Open. 2019, 7, E964–E973. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Coluccio, C.; Binda, C.; Fugazza, A.; Anderloni, A.; Tarantino, I.; i-EUS Group. Lumen-apposing metal stents: How far are we from standardization? An Italian survey. Endosc. Ultrasound. 2022, 11, 59–67. [Google Scholar]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.J.; Han, H.S.; Min, S.K. The safety of a laparoscopic cholecystectomy in acute cholecystitis in high-risk patients older than sixty with stratification based on ASA score. Minim. Invasive Ther. Allied Technol. 2006, 15, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Yates, J.W.; Chalmer, B.; McKegney, F.P. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980, 45, 2220–2224. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest. Endosc. 2011, 74, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Anderloni, A.; Attili, F.; Carrara, S.; Galasso, D.; Di Leo, M.; Costamagna, G.; Repici, A.; Kunda, R.; Larghi, A. Intra-channel stent release technique for fluoroless endoscopic ultrasound-guided lumen-apposing metal stent placement: Changing the paradigm. Endosc. Int. Open 2017, 5, E25–E29. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Fabbri, C.; Di Mitri, R.; Petrone, M.C.; Colombo, M.; Cugia, L.; Amato, A.; Forti, E.; Binda, C.; Maida, M.; et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after failed ERCP: A retrospective nationwide analysis. Gastrointest. Endosc. 2022, 95, 896–904.e1. [Google Scholar] [CrossRef] [PubMed]
- van Wanrooij, R.L.J.; Bronswijk, M.; Kunda, R.; Everett, S.M.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Badaoui, A.; Law, R.; Arcidiacono, P.G.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022, 54, 310–332. [Google Scholar] [CrossRef]
- Tyberg, A.; Jha, K.; Shah, S.; Kedia, P.; Gaidhane, M.; Kahaleh, M. EUS-guided gallbladder drainage: A learning curve modified by technical progress. Endosc. Int. Open 2020, 8, E92–E96. [Google Scholar] [CrossRef]
- Fabbri, C.; Binda, C.; Sbrancia, M.; Dajti, E.; Coluccio, C.; Ercolani, G.; Anderloni, A.; Cucchetti, A. Determinants of outcomes of transmural EUS-guided gallbladder drainage: Systematic review with proportion meta-analysis and meta-regression. Surg. Endosc. 2022, 36, 7974–7985. [Google Scholar] [CrossRef]
- Mohan, B.P.; Asokkumar, R.; Shakhatreh, M.; Garg, R.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Adverse events with lumenapposing metal stents in endoscopic gallbladder drainage: A systematic review and meta-analysis. Endosc. Ultrasound 2019, 8, 241–248. [Google Scholar]
- Lisotti, A.; Linguerri, R.; Bacchilega, I.; Cominardi, A.; Marocchi, G.; Fusaroli, P. EUS-guided gallbladder drainage in high-risk surgical patients with acute cholecystitis-procedure outcomes and evaluation of mortality predictors. Surg. Endosc. 2021, 36, 569–578. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Lowen, C.C.; Martindale, R.G. The 2016 ESPEN Arvid Wretlind lecture: The gut in stress. Clin. Nutr. 2018, 37, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Krezalek, M.A.; DeFazio, J.; Zaborina, O.; Zaborin, A.; Alverdy, J.C. The Shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock 2016, 45, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Coopersmith, C.M. Redefining the gut as the motor of critical illness. Trends Mol. Med. 2014, 20, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Sathiaraj, E.; Murthy, S.; Mansard, M.J.; Rao, G.V.; Mahukar, S.; Reddy, D.N. Clinical trial: Oral feeding with a soft diet compared to clear liquid diet as initial meal in mild acute pancreatitis. Aliment Pharmacol. Ther. 2008, 28, 777–781. [Google Scholar] [CrossRef]
- Sun, J.K.; Mu, X.W.; Li, W.Q.; Tong, Z.H.; Li, J.; Zheng, S.Y. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J. Gastroenterol. 2013, 19, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Storm, A.C.; Vargas, E.J.; Chin, J.Y.; Chandrasekhara, V.; Abu Dayyeh, B.K.; Levy, M.J.; Martin, J.A.; Topazian, M.D.; Andrews, J.C.; Schiller, H.J.; et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest. Endosc. 2021, 94, 742–748.e1. [Google Scholar] [CrossRef]
- Mohan, B.P.; Khan, S.R.; Trakroo, S.; Ponnada, S.; Jayaraj, M.; Asokkumar, R.; Adler, D.G. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: A systematic review and comparative meta-analysis. Endoscopy 2020, 52, 96–106. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Jayaraj, M.; Chandrasekar, V.T.; Singh, D.; Law, J.; Larsen, M.; Ross, A.; Kozarek, R.; Irani, S. EUS-guided versus endoscopic transpapillary gallbladder drainage in high-risk surgical patients with acute cholecystitis: A systematic review and meta-analysis. Surg. Endosc. 2020, 34, 1904–1913. [Google Scholar] [CrossRef]
- Podboy, A.; Yuan, J.; Stave, C.D.; Chan, S.M.; Hwang, J.H.; Teoh, A.Y.B. Comparison of EUS-guided endoscopic transpapillary and percutaneous gallbladder drainage for acute cholecystitis: A systematic review with network meta-analysis. Gastrointest. Endosc. 2021, 93, 797–804.e1. [Google Scholar] [CrossRef] [PubMed]
- Sedarat, A.; Muthusamy, V.R. Endoscopic treatment of acute cholecystitis: Can transpapillary stent placement silence the LAMS? Gastrointest. Endosc. 2021, 94, 749–751. [Google Scholar] [CrossRef] [PubMed]
| Variables | n (%) or Mean ± SD |
|---|---|
| female | 56/116 (48.3%) |
| age, years | 82.7 ± 11 |
| diagnosis | |
| 101/116 (87.1%) |
| 10/116 (8.6%) |
| 3/116 (2.5%) |
| 1/116 (0.8%) |
| 1/116 (0.8%) |
| warfarin or DOAC intake | 14/116 (12%) |
| previous ERCP indication | |
| 28/116 (24.3%) |
| 3/116 (2.6%) |
| concomitant percutaneous drainage | 3/116 (2.6%) |
| gallbladder features | |
| 63/116 (54.3) |
| 14/116 (12.1) |
| 39/116 (33.6) |
| gallbladder length, mm | 71.2 ± 35.8 |
| gallbladder width, mm | 59.4 ± 34 |
| WBC Count (×103) * | 16.13 ± 6.06 |
| Total Bilirubin Levels (mg/dL) * | 5.15 ± 6.32 |
| Direct Bilirubin Levels (mg/dL) * | 4.14 ± 5.15 |
| CRP (mg/dL) * | 21.21 ± 17.5 |
| N° | Type | Severity | Timing | Management | Other |
|---|---|---|---|---|---|
| 1 | bleeding | mild | intraprocedural | conservative | |
| 2 | bleeding | mild | intraprocedural | endoscopy | hemostasis |
| 3 | AC recurrence | moderate | early | conservative | |
| 4 | dislodgement | moderate | intraprocedural | endoscopy | placement of OTSC |
| 5 | dislodgement | moderate | intraprocedural | endoscopy | placement of second LAMS |
| 6 | dislodgement | severe | intraprocedural | surgery | urgent cholecystectomy |
| 7 | migration | severe | delayed | surgery | open cholecystectomy +suturing |
| 8 | malpositioning | severe | intraprocedural | endoscopy | LAMS positioned in renal cyst. Removal of LAMS |
| 9 | perforation | severe | delayed | surgery | open cholecystectomy + suturing |
| 10 | perforation | severe | intraprocedural | surgery | VLS cholecystectomy + suturing |
| 11 | buried stent | severe | delayed | endoscopy | SEMS insertion |
| 12 | cardiac arrest | severe | intraprocedural | conservative |
| Technical Success | |||||
|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | ||||
| Variable | Technical Success (n = 109) | Technical Failure (n = 7) | p Value | Odds Ratios (CI-95%) | p Value |
| WBC (mean ± SD) | 18,207 ± 15,076 | 11,500 ± 3316 | 0.020 | - | |
| stent size (width) | 0.002 | - | |||
| 3 (3.1) | 2 (28.6) | |||
| 95 (96.9) | 5 (71.4) | |||
| Clinical Success | |||||
| Univariable Analysis | Multivariable Analysis | ||||
| Variable | Clinical Success (n = 101) | Clinical Failure (n = 15) | p Value | Odds Ratios (CI-95%) | p Value |
| diagnosis | 0.019 | 10.156 (2.118–48.698) | 0.004 | ||
| 90 (89.1) | 10 (66.7) | |||
| 11 (10.9) | 5 (33.3) | |||
| gallbladder width, mm (mean ± SD) | 60.9 ± 36.5 | 42.1 ± 25.4 | 0.036 | - | |
| stent size (width) | 0.053 | - | |||
| 3 (3.2) | 2 (15.4) | |||
| 90 (96.8) | 11 (84.6) | |||
| procedure time, minutes (mean ± SD) | 22.9 ± 16.5 | 34.5 ± 24.4 | 0.020 | - | |
| beginning of post-procedural enteral diet | 13.691 (3.254–57.601) | <0.001 | |||
| 86 (86.0) | 7 (46.7) | <0.001 | ||
| 14 (14.0) | 8 (63.3) | 0.003 | ||
| adverse events | 6 (5.9) | 6 (60.0) | <0.001 | - | |
| Adverse Events | |||||
| Univariable Analysis | Multivariable Analysis | ||||
| Variable | Adverse Events (n = 12) | no Adverse Events (n = 104) | p Value | Odds Ratios (CI-95%) | p Value |
| release of the 2nd flange | 0.046 | - | |||
| 5 (41.7) | 73 (70.2) | |||
| 7 (58.3) | 31 (29.8) | |||
| beginning of post-procedural enteral diet | 0.036 | - | |||
| 7 (58.3) | 86 (83.5) | |||
| 5 (41.7) | 17 (16.5) | |||
| 30-Days Mortality | |||||
|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | ||||
| Variable | 30-Day Death (n = 21) | 30-Day Survival (n = 85) | p Value | Odds Ratios (CI-95%) | p Value |
| age, years (mean ± SD) | 78.1 ± 14.5 | 83.7 ± 9.9 | 0.038 | 1.031 (0.946–1.122) | 0.488 |
| total bilirubin, mg/dL (mean ± SD) | 10.9 ± 7.6 | 4.4 ± 5.8 | 0.047 | 1.012 (0.872–1.173) | 0.879 |
| diagnosis | <0.001 | 41.7 (1.979–879.0) | 0.016 | ||
| 13 (61.9) | 77 (90.6) | |||
| 8 (38.1) | 8 (9.4) | |||
| beginning of post-procedural enteral diet | 0.005 | 8.578 (0.545–134.197) | 0.126 | ||
| 14 (66.7) | 77 (90.6) | |||
| 7 (33.3) | 8 (9.4) | |||
| Overall Mortality | |||||
| Univariable analysis | Multivariable analysis | ||||
| Variable | Overall Mortality (n = 39) | Overall Survival (n = 67) | p Value | Odds Ratios (CI-95%) | p Value |
| CRP, mg/dL (mean ± SD) | 12.2 ± 8.7 | 22.9 ± 18.4 | 0.005 | 0.918 (0.855–0.987) | 0.020 |
| total bilirubin, mg/dL (mean ± SD) | 9.1 ± 8.7 | 2.9 ± 2.7 | <0.001 | 1.058 (0.960–1.166) | 0.259 |
| diagnosis | 0.001 | 7.542 (0.972–58.510) | 0.053 | ||
| 27 (69.2) | 63 (94.0) | |||
| 12 (30.8) | 4 (6.0) | |||
| beginning of post-procedural enteral diet | 0.002 | 8.582 (1.698–43.381) | 0.009 | ||
| 28 (71.8) | 63 (94.0) | |||
| 11 (28.2) | 4 (6.0) | |||
| length of hospitalization post procedure, days (mean ± SD) | 13.9 ± 11.7 | 9.6 ± 7.4 | 0.021 | 0.954 (0.899–1.013) | 0.125 |
| gallbladder disease | 0.007 | ||||
| 14 (36.8) | 40 (59.7) | |||
| 10 (26.3) | 4 (6.0) | 0.306 (0.026–3.541) | 0.343 | |
| 14 (36.8) | 23 (34.3) | 5.273 (1.229–22.613) | 0.025 | |
| Variable | n (%) or Median + Range |
|---|---|
| patient number | 40 /116 (34.5) |
| follow-up, days | 692 (360–2090) |
| technical success | 38/40 (95) |
| clinical success | 37/40 (92.5) |
| adverse events | 5/40 (12.5) |
| 3/5 (60) |
| 2/5 (40) |
| acute cholecystitis recurrence | 1/40 (2.5) |
| surgical cholecystectomy | 0/40 (0) |
| mortality | 5/40 (12.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binda, C.; Anderloni, A.; Forti, E.; Fusaroli, P.; Macchiarelli, R.; Manno, M.; Fugazza, A.; Redaelli, A.; Aragona, G.; Lovera, M.; et al. EUS-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: Results of a Nationwide Study with Long-Term Follow-Up. Diagnostics 2024, 14, 413. https://doi.org/10.3390/diagnostics14040413
Binda C, Anderloni A, Forti E, Fusaroli P, Macchiarelli R, Manno M, Fugazza A, Redaelli A, Aragona G, Lovera M, et al. EUS-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: Results of a Nationwide Study with Long-Term Follow-Up. Diagnostics. 2024; 14(4):413. https://doi.org/10.3390/diagnostics14040413
Chicago/Turabian StyleBinda, Cecilia, Andrea Anderloni, Edoardo Forti, Pietro Fusaroli, Raffaele Macchiarelli, Mauro Manno, Alessandro Fugazza, Alessandro Redaelli, Giovanni Aragona, Mauro Lovera, and et al. 2024. "EUS-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: Results of a Nationwide Study with Long-Term Follow-Up" Diagnostics 14, no. 4: 413. https://doi.org/10.3390/diagnostics14040413
APA StyleBinda, C., Anderloni, A., Forti, E., Fusaroli, P., Macchiarelli, R., Manno, M., Fugazza, A., Redaelli, A., Aragona, G., Lovera, M., Togliani, T., Armellini, E., Amato, A., Brancaccio, M. L., Badas, R., Leone, N., de Nucci, G., Mangiavillano, B., Sbrancia, M., ... Tarantino, I. (2024). EUS-Guided Gallbladder Drainage Using a Lumen-Apposing Metal Stent for Acute Cholecystitis: Results of a Nationwide Study with Long-Term Follow-Up. Diagnostics, 14(4), 413. https://doi.org/10.3390/diagnostics14040413










