Urine Flow Cytometry and Dipstick Analysis in Diagnosing Bacteriuria and Urinary Tract Infections among Adults in the Emergency Department—A Diagnostic Accuracy Trial
Abstract
1. Introduction
1.1. Background
1.2. Aim and Objectives
2. Materials and Methods
2.1. Study Design
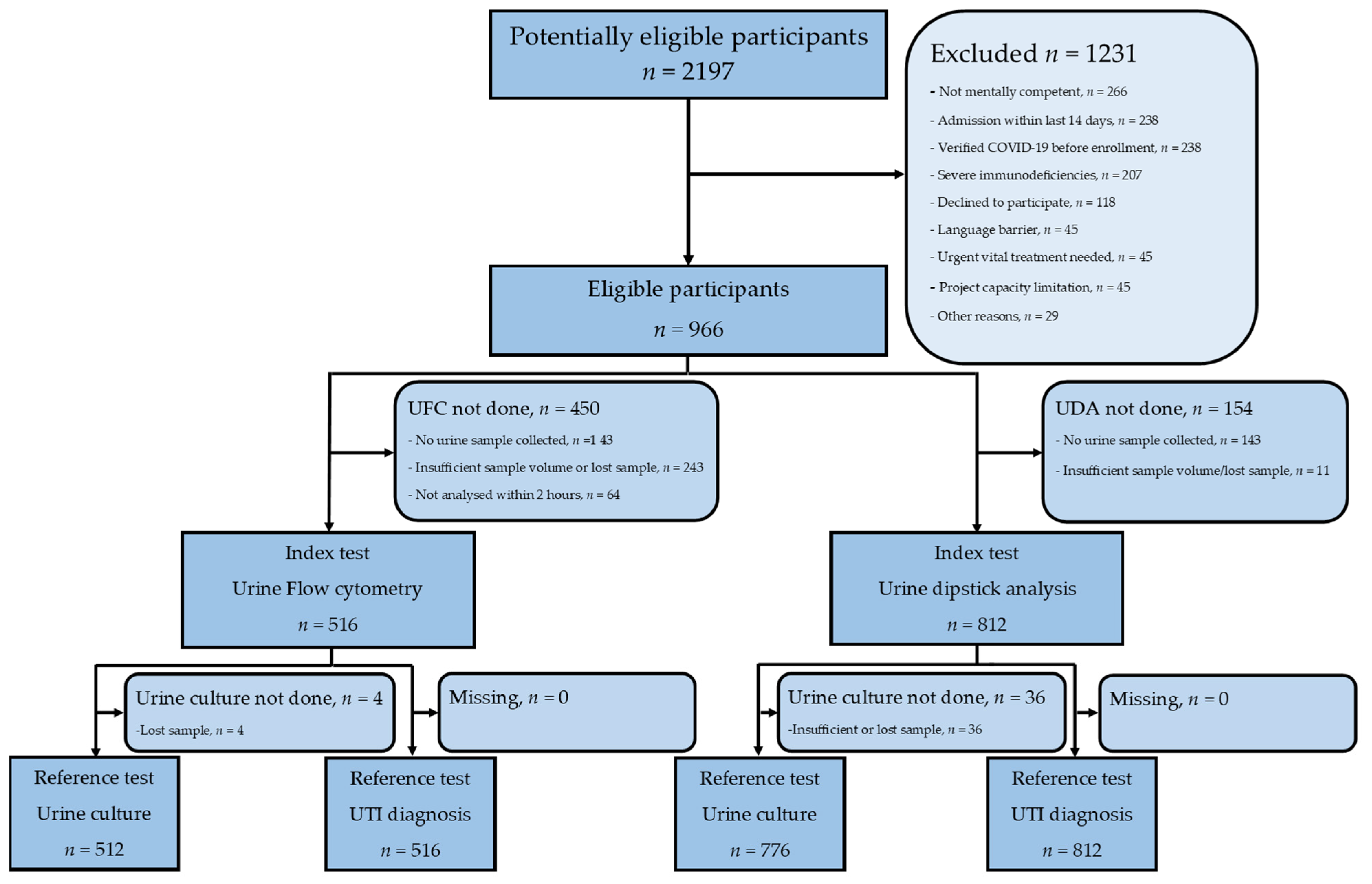
2.2. Participants
2.3. Tests and Variables
2.3.1. Urine Sample Logistics
2.3.2. Urine Flow Cytometry—Index Test
2.3.3. Urine Dipstick Analysis—Index Test
2.3.4. Urine Cultures—Reference Test
2.3.5. Urinary Tract Infection Diagnosis—Reference Test
2.3.6. Other Variables
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Diagnostic Accuracy of UFC and UDA for Bacteriuria
3.3. Diagnostic Accuracy of UFC and UDA for Urinary Tract Infection
3.4. Additional Analyses
3.5. Adverse Events from Performing the Tests
4. Discussion
4.1. Key Results
4.2. Study Limitations
4.3. Implications for Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Hospital | Sygehus Lillebælt | Odense University Hospital | Sygehus Sønderjylland |
|---|---|---|---|
| Sample container | Greiner Bio-One vacuette with no additives (Kremsmünster, Austria) | Sarstedt/Hounisen urine monovette with boric acid (Nümbrecht, Germany) | Sarstedt/Hounisen urine monovette with boric acid (Nümbrecht, Germany) |
| Agar plates | CHROMID biplates CPSE/CNA (BioMerieux, Marcy l’Etoile, France) | CHROMID biplates CPSE/CNA (BioMerieux Marcy l’Etoile, France) | BD CHROMagar Orientation Medium/Columbia CNA Agar Biplate (Becton Dickinson Heidelberg, Germany) |
| Specimen processing | Copan WASP® (Murrieta, CA, USA) | Copan WASP® (Murrieta, CA, USA) | Copan WASP® (Murrieta, CA, USA) |
| Reading | Manual | Manual | Manual |
| Inoculum size | 1 µL | 10 µL | 1 µL |
| Incubation time | 18–48 h | 18–48 h | Negative max 24 h, positive max 48. |
| Final identification | MALDI-TOF (Bruker Daltonic MALDI Biotyper (Bremen, Germany) for all except E. coli which is identified by red colonies on CPS plates and spot indol positive. | MALDI-TOF (Bruker Daltonic MALDI Biotyper (Bremen, Germany) or BioMerieux VITEK MS Marcy l’Etoile, France) | MALDI-TOF (Bruker Daltonics MALDI Biotyper Bremen, Germany) for all except E. coli which is identified by pink colonies on CHROMagar Orientation Medium plates, complete inhibition on Columbia CNA Agar, and DMACA indol positive. |
| Cut-off | 1 × 104 CFU/mL EBC or S. aureus, S. lugdunensis, P. aeruginosa, hemolytic streptococci (grp. A, B, C or D), enterococci, and aerococci. 1 × 105 CFU/mL others. | 1 × 103 CFU/mL EBC, 1 × 104 CFU/mL others | See Table A2 |
| Multiple species | >1 × 104 CFU/mL 3 EBC or S. aureus, S. lugdunensis, P. aeruginosa, hemolytic streptococci (grp. A, B, C or D), enterococci, and aerococci or >1 × 104 CFU/mL, 2 or more others considered contamination. | Three or more different bacterial species > 1 × 104 CFU/mL or 2 or more EBC not exceeding 1 × 103–1 × 104 CFU/mL considered contamination | See Table S1 |
| Urinary Tract Symptoms | Cut-Off | |
|---|---|---|
| Permanent Catheter Urine | Other | |
| No symptoms | 1 × 105 CFU/mL for EBC, S. saprophyticus, P. aeruginosa, S. aureus, enterococci, A. urinae, and ß-hemolytic streptococci in mono-culture | |
| Symptoms | 1 × 103 CFU/mL E. coli or S. saprophyticus in both sexes and P. aeruginosa, S. aureus, Enterococci, A. urinae, and ß-hemolytic streptococci in mono-culture in samples from men. 1 × 104 CFU/mL P. aeruginosa, S. aureus, enterococci, A. urinae, and ß-hemolytic streptococci in mono-culture in samples from women. 1 × 105 CFU/mL P. aeruginosa, A. aureus, enterococci, A. urinae, and ß-hemolytic streptococci if <3 species. Others reported as “mixed flora” | 1 × 104 CFU/mL for E. coli, S. saprophyticus, P. aeruginosa, S. aureus, enterococci, A. urinae, and ß-hemolytic streptococci in mono-culture. 1 × 105 CFU/mlfor two for P. aeruginosa, S. aureus, enterococci, A. urinae, and ß-hemolytic streptococci. A total of three or more 1 × 105 CFU/mL reported as “mixed flora” |
References
- Wolfertz, N.; Bohm, L.; Keitel, V.; Hannappel, O.; Kumpers, P.; Bernhard, M.; Michael, M. Epidemiology, management, and outcome of infection, sepsis, and septic shock in a German emergency department (EpiSEP study). Front. Med. 2022, 9, 997992. [Google Scholar] [CrossRef]
- Schappert, S.M.; Rechtsteiner, E.A. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 2011, 13, 1–38. [Google Scholar]
- Danmarks_Statistik. Danmarks Statistik, Statistikbanken.dk. Available online: https://statistikbanken.dk/ind04 (accessed on 24 May 2023).
- Laan, B.J.; van Horrik, T.; Nanayakkara, P.W.B.; Geerlings, S.E. How many urinalysis and urine cultures are necessary? Eur. J. Intern. Med. 2021, 83, 58–61. [Google Scholar] [CrossRef]
- Shallcross, L.; Gaskell, K.; Fox-Lewis, A.; Bergstrom, M.; Noursadeghi, M. Mismatch between suspected pyelonephritis and microbiological diagnosis: A cohort study from a UK teaching hospital. J. Hosp. Infect. 2018, 98, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gupta, A.; Petty, L.A.; Malani, A.N.; Osterholzer, D.; Patel, P.K.; Younas, M.; Bernstein, S.J.; Burdick, S.; Ratz, D.; et al. A Statewide Quality Initiative to Reduce Unnecessary Antibiotic Treatment of Asymptomatic Bacteriuria. JAMA Intern. Med. 2023, 183, 933–941. [Google Scholar] [CrossRef]
- Richards, D.; Toop, L.; Chambers, S.; Fletcher, L. Response to antibiotics of women with symptoms of urinary tract infection but negative dipstick urine test results: Double blind randomised controlled trial. BMJ 2005, 331, 143. [Google Scholar] [CrossRef]
- Chun, T.T.S.; Ruan, X.; Ng, S.L.; Wong, H.L.; Ho, B.S.H.; Tsang, C.F.; Lai, T.C.T.; Ng, A.T.L.; Ma, W.K.; Lam, W.P.; et al. The diagnostic value of rapid urine test platform UF-5000 for suspected urinary tract infection at the emergency department. Front. Cell Infect. Microbiol. 2022, 12, 936854. [Google Scholar] [CrossRef]
- De Rosa, R.; Grosso, S.; Lorenzi, G.; Bruschetta, G.; Camporese, A. Evaluation of the new Sysmex UF-5000 fluorescence flow cytometry analyser for ruling out bacterial urinary tract infection and for prediction of Gram negative bacteria in urine cultures. Clin. Chim. Acta 2018, 484, 171–178. [Google Scholar] [CrossRef]
- Long, B.; Koyfman, A. The Emergency Department Diagnosis and Management of Urinary Tract Infection. Emerg. Med. Clin. N. Am. 2018, 36, 685–710. [Google Scholar] [CrossRef]
- Skjot-Arkil, H.; Heltborg, A.; Lorentzen, M.H.; Cartuliares, M.B.; Hertz, M.A.; Graumann, O.; Rosenvinge, F.S.; Petersen, E.R.B.; Ostergaard, C.; Laursen, C.B.; et al. Improved diagnostics of infectious diseases in emergency departments: A protocol of a multifaceted multicentre diagnostic study. BMJ Open 2021, 11, e049606. [Google Scholar] [CrossRef]
- Sysmex Europe Fluorescence Flow Cytometry (FFC). Available online: https://www.sysmex-europe.com/academy/knowledge-centre/technologies/fluorescence-flow-cytometry.html (accessed on 29 September 2023).
- Moshaver, B.; de Boer, F.; van Egmond-Kreileman, H.; Kramer, E.; Stegeman, C.; Groeneveld, P. Fast and accurate prediction of positive and negative urine cultures by flow cytometry. BMC Infect. Dis. 2016, 16, 211. [Google Scholar] [CrossRef]
- Davey, H.M.; Kell, D.B.; Weichart, D.H.; Kaprelyants, A.S. Estimation of microbial viability using flow cytometry. Curr. Protoc. Cytom. 2004, 29, 11.3.1–11.3.21. [Google Scholar] [CrossRef]
- Muller, M.; Sagesser, N.; Keller, P.M.; Arampatzis, S.; Steffens, B.; Ehrhard, S.; Leichtle, A.B. Urine Flow Cytometry Parameter Cannot Safely Predict Contamination of Urine-A Cohort Study of a Swiss Emergency Department Using Machine Learning Techniques. Diagnostics 2022, 12, 1008. [Google Scholar] [CrossRef]
- Andersen, E.S.; Ostergaard, C.; Rottger, R.; Christensen, A.F.; Brandslund, I.; Brasen, C.L. POCT urine dipstick versus central laboratory analyses: Diagnostic performance and logistics in the medical emergency department. Clin. Biochem. 2023, 111, 17–25. [Google Scholar] [CrossRef]
- Broeren, M.A.; Bahceci, S.; Vader, H.L.; Arents, N.L. Screening for urinary tract infection with the Sysmex UF-1000i urine flow cytometer. J. Clin. Microbiol. 2011, 49, 1025–1029. [Google Scholar] [CrossRef]
- Conkar, S.; Mir, S. Urine Flow Cytometry in the Diagnosis of Urinary Tract Infection. Indian. J. Pediatr. 2018, 85, 995–999. [Google Scholar] [CrossRef]
- Brilha, S.; Proenca, H.; Cristino, J.M.; Hanscheid, T. Use of flow cytometry (Sysmex) UF-100) to screen for positive urine cultures: In search for the ideal cut-off. Clin. Chem. Lab. Med. 2010, 48, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, S.J.; van Pelt, L.J.; Kampinga, G.A.; Ter Maaten, J.C.; Stegeman, C.A. Routine tests and automated urinalysis in patients with suspected urinary tract infection at the ED. Am. J. Emerg. Med. 2016, 34, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.S.; Brasen, C.L.; Christensen, A.F.; Ostergaard, C.; Brandslund, I. Carryover issues with UF-5000 urine flow cytometry—How did we miss it? Clin. Chem. Lab. Med. 2020, 58, e120–e122. [Google Scholar] [CrossRef] [PubMed]
- Chernaya, A.; Soborg, C.; Midttun, M. Validity of the urinary dipstick test in the diagnosis of urinary tract infections in adults. Dan. Med. J. 2021, 69, A07210607. [Google Scholar]
- Middelkoop, S.J.M.; van Pelt, L.J.; Kampinga, G.A.; Ter Maaten, J.C.; Stegeman, C.A. Influence of gender on the performance of urine dipstick and automated urinalysis in the diagnosis of urinary tract infections at the emergency department. Eur. J. Intern. Med. 2021, 87, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Haugum, K.; Haugan, M.S.; Skage, J.; Tetik, M.; Jakovljev, A.; Nilsen, H.S.; Afset, J.E. Use of Sysmex UF-5000 flow cytometry in rapid diagnosis of urinary tract infection and the importance of validating carryover rates against bacterial count cut-off. J. Med. Microbiol. 2021, 70, 001472. [Google Scholar] [CrossRef] [PubMed]
- El Kettani, A.; Housbane, S.; Wakit, F.; Mikou, K.A.; Belabbes, H.; Zerouali, K. Evaluation of the Sysmex UF-4000i urine analyzer as a screening test to rule out urinary tract infection and reduce urine cultures. Ann. Biol. Clin. 2021, 81, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Alenkaer, L.K.; Pedersen, L.; Szecsi, P.B.; Bjerrum, P.J. Evaluation of the sysmex UF-5000 fluorescence flow cytometer as a screening platform for ruling out urinary tract infections in elderly patients presenting at the Emergency Department. Scand. J. Clin. Lab. Investig. 2021, 81, 379–384. [Google Scholar] [CrossRef]
- Tavenier, A.H.; de Boer, F.J.; Moshaver, B.; van der Leur, S.; Stegeman, C.A.; Groeneveld, P.H.P. Flow cytometric analysis of viable bacteria in urine samples of febrile patients at the emergency department. Cytom. B Clin. Cytom. 2018, 94, 689–695. [Google Scholar] [CrossRef]
- St John, A.; Boyd, J.C.; Lowes, A.J.; Price, C.P. The use of urinary dipstick tests to exclude urinary tract infection: A systematic review of the literature. Am. J. Clin. Pathol. 2006, 126, 428–436. [Google Scholar] [CrossRef]
| Patient Characteristics | Urine Culture | Expert Panel Diagnosis | |||
|---|---|---|---|---|---|
| n = 966 unless stated otherwise. | n = 786 | n = 966 | |||
| Bacteriuria | No Bacteriuria | Urinary tract infection | No urinary tract infection | ||
| no. (%) | 337 (42.9%) | 449 (57.1) | 200 (20.7) | 766 (79.3) | |
| Age, years, median (IQR) | 76 (17) | 69 (27) | 76 (17) | 72 (23) | |
| Sex, no. (%) | Male | 180 (53.4) | 240 (53.4) | 116 (58) | 405 (52.9) |
| Urine sample method, n = 786 (UC)/822 (EPD), no. (%) | Midstream | 181 (53.7) | 349 (77.7) | 105 (55.6) | 444 (70.8) |
| Catheter | 53 (15.7) | 23 (5.1) | 34 (18.0) | 47 (7.5) | |
| Sterile intermittent catheterization | 36 (10.7) | 17 (3.8) | 23 (12.2) | 34 (5.4) | |
| Bedpan or urine bottle | 60 (17.8) | 55 (12.3) | 23 (12.2) | 99 (15.8) | |
| Other/unknown | 7 (2.1) | 5 (1.1) | 7 (3.7) | 6 (1.0) | |
| Catheter type before admission no. (%) | None | 275 (81.6) | 434 (96.7) | 153 (76.5) | 727 (94.9) |
| (more than one catheter type possible) | Catheter a demeure | 34 (10.1) | 5 (1.1) | 24 (12) | 20 (2.6) |
| Clean intermittent catheterization | 7 (2.1) | 7 (1.6) | 9 (4.5) | 6 (0.8) | |
| Sterile intermittent catheterization | 3 (0.9) | 1 (0.2) | 2 (1) | 2 (0.3) | |
| JJ catheter, nephrostomy catheter, suprapubic, or urostomy catheter | 20 (5.9) | 2 (0.4) | 14 (7) | 11 (1.4) | |
| Antibiotic treatment before urine culture, n = 786, no. (%) | Yes | 118 (35.0) | 175 (39.0) | 82 (44.3) | 211 (35.1) |
| Urine culture, n = 786, no. (%) | Positive | 337 (100) | 0 (0) | 147 (79.5) | 190 (31.6) |
| Expert panel diagnosis of UTI, no. (%) | Yes | 147 (43.6) | 38 (8.5) | 200 (100) | 0 (0) |
| Urine dipstick analysis | |||||
| Urine Leukocytes, n = 776 (UC)/812 (EPD), no. (%) | Negative | 95 (28.8) | 326 (73.1) | 29 (15.3) | 326 (67.1) |
| + | 57 (17.3) | 54 (12.1) | 32 (16.9) | 81 (13.0) | |
| ++ | 86 (26.1) | 46 (10.3) | 62 (32.8) | 75 (12.0) | |
| +++ | 44 (13.3) | 12 (2.7) | 30 (15.9) | 29 (4.9) | |
| ++++ | 48 (14.6) | 8 (1.8) | 36 (19.0) | 20 (3.2) | |
| Urine nitrite, n = 776 (UC)/812 (EPD), no. (%) | Positive | 100 (30.3) | 11 (2.5) | 61 (32.3) | 56 (9.0) |
| Urine flow cytometry | |||||
| White blood cells/µL, n = 512 (UC)/516 (EPD), median (IQR) | 205 (1078) | 11.8 (44.4) | 485 (2321) | 17 (70) | |
| Bacterial cells/µL, n = 512 (UC)/516 (EPD), median (IQR) | 1438 (15,483) | 24.9 (190) | 1801 (22,736) | 50 (535) | |
| Squamous epithelial cells/µL, n = 512 (UC)/516 (EPD), median (IQR) | 4.2 (10.2) | 3.4 (15.2) | 4.1 (8.1) | 3.9 (14.4) | |
| Reference Test | Index Test | Variables and Cut-Off | Model AUROC (95%Ci) | Sensitivity | Specificity | PPV | NPV | DA | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteriuria | UFC | BACT/µL–7/µL | 0.809 (0.772–0.847) | 95.3% | 29.1% | 48.9% | 89.7% | 56.6% | 203 | 212 | 10 | 87 |
| n = 786 | n = 512 | WBC/µL–3.2/µL | 0.800 (0.761–0.839) | 95.8% | 25.4% | 47.8% | 89.4% | 54.7% | 204 | 223 | 9 | 76 |
| BACT/µL–7/µL or WBC/µL–3.2/µL | 0.835 (0.800–0.870) | 98.6% | 17.7% | 46.1% | 94.6% | 51.4% | 210 | 246 | 3 | 53 | ||
| BACT/µL–7/µL and WBC/µL–3.2/µL | - | 92.5% | 36.8% | 51.0% | 87.3% | 60.0% | 197 | 189 | 16 | 110 | ||
| UDA | Leucocytes +1 | 0.751 (0.718–0.783) | 71.2% | 73.1% | 66.2% | 77.4% | 72.3% | 235 | 120 | 95 | 326 | |
| n = 776 | Leucocytes +2 | - | 53.9% | 85.2% | 73.0% | 71.4% | 71.9% | 178 | 66 | 152 | 380 | |
| Leucocytes +3 | - | 27.9% | 95.5% | 82.1% | 64.2% | 66.8% | 92 | 20 | 238 | 426 | ||
| Leucocytes +4 | - | 14.5% | 98.2% | 85.7% | 60.8% | 62.6% | 48 | 8 | 282 | 438 | ||
| Nitrite pos | 0.639 (0.613–0.664) | 30.3% | 97.5% | 90.1% | 65.4% | 68.9% | 100 | 11 | 230 | 435 | ||
| Leucocytes +1 or nitrite pos | 0.782 (0.757–0.823) | 74.5% | 71.7% | 66.1% | 79.2% | 72.9% | 246 | 126 | 84 | 320 | ||
| Leucocytes +1 and nitrite pos | - | 27.0% | 98.9% | 94.7% | 64.7% | 68.3% | 89 | 5 | 241 | 441 | ||
| Leucocytes +2 and nitrite pos | - | 22% | 100% | 97% | 63% | 66% | 72 | 2 | 258 | 444 | ||
| Leucocytes +3 and nitrite pos | - | 13% | 100% | 98% | 61% | 63% | 43 | 1 | 287 | 445 | ||
| Leucocytes +4 and nitrite pos | - | 6.4% | 99.8% | 95.5% | 59.0% | 60.1% | 21 | 1 | 309 | 445 | ||
| UTI | UFC | BACT/µL–7.9/µL | 0.743 (0.693–0.793) | 95.8% | 23.9% | 27.2% | 95.0% | 40.3% | 113 | 303 | 5 | 95 |
| n = 966 | n = 512 | WBC/µL–15/µL | 0.856 (0.819–0.894) | 94.9% | 48.7% | 35.4% | 97.0% | 59.3% | 112 | 204 | 6 | 194 |
| WBC/µL–448/µL | - | 50.8% | 91.5% | 63.8% | 86.3% | 82.2% | 60 | 34 | 58 | 364 | ||
| WBC/µL–1125/µL | - | 33.9% | 95.0% | 66.7% | 82.9% | 81.0% | 40 | 20 | 78 | 378 | ||
| BACT/µL–7.9/µL or WBC/µL–15/µl | 0.832 (0.793–0.871) | 98.3% | 21.4% | 27.0% | 97.7% | 39.0% | 116 | 313 | 2 | 85 | ||
| BACT/µL–7.9/µL and WBC/µL–15/µL | - | 92.4% | 51.3% | 36.0% | 95.8% | 60.7% | 109 | 194 | 9 | 204 | ||
| UDA | Leucocytes +1 | 0.796 (0.0761–0.831) | 84.7% | 67.1% | 43.8% | 93.5% | 71.2% | 160 | 205 | 29 | 418 | |
| n = 812 | Leucocytes +2 | - | 67.7% | 80.1% | 50.8% | 89.1% | 77.2% | 128 | 124 | 61 | 499 | |
| Leucocytes +3 | - | 34.9% | 92.1% | 57.4% | 82.4% | 78.8% | 66 | 49 | 123 | 574 | ||
| Leucocytes +4 | - | 19.0% | 96.8% | 64.3% | 79.8% | 78.7% | 36 | 20 | 153 | 603 | ||
| Nitrite pos | 0.616 (0.581–0.652) | 32.3% | 91.0% | 52.1% | 81.6% | 77.3% | 61 | 56 | 128 | 567 | ||
| Leucocytes +1 or nitrite pos | 0.805 (0.771–0.840) | 86.2% | 64.7% | 42.6% | 93.9% | 69.7% | 163 | 220 | 26 | 403 | ||
| Leucocytes +1 and nitrite pos | - | 30.7% | 93.4% | 58.6% | 81.6% | 78.8% | 58 | 41 | 131 | 582 | ||
| Leucocytes +2 and nitrite pos | - | 25% | 95% | 62% | 81% | 79% | 48 | 30 | 141 | 593 | ||
| Leucocytes +3 and nitrite pos | - | 17% | 98% | 71% | 80% | 79% | 32 | 13 | 157 | 610 | ||
| Leucocytes +4 and nitrite pos | - | 7.9% | 98.9% | 68.2% | 78.0% | 77.7% | 15 | 7 | 174 | 616 |
| Reference Test | Index Test | Subgroup | Variables and Cut-Off | Model AUROC (95%CI) | Sensitivity | Specificity | PPV | NPV | DA | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteriuria | UFC | Men | BACT/µL–7/µL or WBC/µL–3.2/µL | 0.900 (0.862–0.938) | 98.2% | 30.0% | 48.9% | 96.0% | 57.6% | 107 | 112 | 2 | 48 |
| n = 269 | BACT/µL–7/µL and WBC/µL–3.2/µL | - | 91.7% | 53.1% | 57.1% | 90.4% | 68.8% | 100 | 75 | 9 | 85 | ||
| Women | BACT/µL–7/µL or WBC/µL–3.2/µL | 0.742 (0.677–0.806) | 99.0% | 3.6% | 43.5% | 83.3% | 44.4% | 103 | 134 | 1 | 5 | ||
| n = 243 | BACT/µL–7/µL and WBC/µL–3.2/µL | - | 93.3% | 18.0% | 46.0% | 78.1% | 50.2% | 97 | 114 | 7 | 25 | ||
| UDA | Men | Leucocytes +1 or nitrite pos | 0.828 (0.789–0.867) | 77.7% | 79.8% | 74.3% | 82.6% | 78.9% | 139 | 48 | 40 | 190 | |
| n = 417 | |||||||||||||
| Women | Leucocytes +1 or nitrite pos | 0.731 (0.679–0.782) | 70.9% | 62.5% | 57.8% | 74.7% | 66.0% | 107 | 78 | 44 | 130 | ||
| n = 359 | |||||||||||||
| UTI | UFC | Men | WBC/µL–15/µl | 0.891 (0.849–0.933) | 95.7% | 61.5% | 46.5% | 97.6% | 70.4% | 67 | 77 | 3 | 123 |
| n = 270 | WBC/µL–448/µL | - | 60.0% | 91.5% | 71.2% | 86.7% | 83.3% | 42 | 17 | 28 | 183 | ||
| WBC/µL–1125/µL | - | 42.9% | 94.0% | 71.4% | 82.5% | 80.7% | 30 | 12 | 40 | 188 | |||
| Women | WBC/µL–15/µL | 0.798 (0.730–0.866) | 93.8% | 35.9% | 26.2% | 95.9% | 47.2% | 45 | 127 | 3 | 71 | ||
| n = 246 | WBC/µL–448/µL | - | 37.5% | 91.4% | 51.4% | 85.8% | 80.9% | 18 | 17 | 30 | 181 | ||
| WBC/µL–1125/µL | - | 20.8% | 96.0% | 55.6% | 83.3% | 81.3% | 10 | 8 | 38 | 190 | |||
| UDA | Men | Leucocytes +1 or nitrite pos | 0.841 (0.799–0.882) | 89.2% | 71.8% | 52.1% | 95.1% | 76.3% | 99 | 91 | 12 | 232 | |
| n = 434 | Leucocytes +2 and nitrite pos | - | 22.5% | 95.4% | 62.5% | 78.2% | 76.7% | 25 | 15 | 86 | 308 | ||
| Leucocytes +3 and nitrite pos | - | 14.4% | 97.2% | 64.0% | 76.8% | 76.0% | 16 | 9 | 95 | 314 | |||
| Women | Leucocytes +1 or nitrite pos | 0.758 (0.697–0.818) | 82.1% | 57.0% | 33.2% | 92.4% | 62.2% | 64 | 129 | 14 | 171 | ||
| n = 378 | Leucocytes +2 and nitrite pos | - | 29.5% | 95.0% | 60.5% | 83.8% | 81.5% | 23 | 15 | 55 | 285 | ||
| Leucocytes +4 and nitrite pos | - | 20.5% | 98.7% | 80.0% | 82.7% | 82.5% | 16 | 4 | 62 | 296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertz, M.A.; Johansen, I.S.; Rosenvinge, F.S.; Brasen, C.L.; Andersen, E.S.; Østergaard, C.; Skovsted, T.A.; Petersen, E.R.B.; Nielsen, S.L.; Mogensen, C.B.; et al. Urine Flow Cytometry and Dipstick Analysis in Diagnosing Bacteriuria and Urinary Tract Infections among Adults in the Emergency Department—A Diagnostic Accuracy Trial. Diagnostics 2024, 14, 412. https://doi.org/10.3390/diagnostics14040412
Hertz MA, Johansen IS, Rosenvinge FS, Brasen CL, Andersen ES, Østergaard C, Skovsted TA, Petersen ERB, Nielsen SL, Mogensen CB, et al. Urine Flow Cytometry and Dipstick Analysis in Diagnosing Bacteriuria and Urinary Tract Infections among Adults in the Emergency Department—A Diagnostic Accuracy Trial. Diagnostics. 2024; 14(4):412. https://doi.org/10.3390/diagnostics14040412
Chicago/Turabian StyleHertz, Mathias Amdi, Isik Somuncu Johansen, Flemming S. Rosenvinge, Claus Lohman Brasen, Eline Sandvig Andersen, Claus Østergaard, Thor Aage Skovsted, Eva Rabing Brix Petersen, Stig Lønberg Nielsen, Christian Backer Mogensen, and et al. 2024. "Urine Flow Cytometry and Dipstick Analysis in Diagnosing Bacteriuria and Urinary Tract Infections among Adults in the Emergency Department—A Diagnostic Accuracy Trial" Diagnostics 14, no. 4: 412. https://doi.org/10.3390/diagnostics14040412
APA StyleHertz, M. A., Johansen, I. S., Rosenvinge, F. S., Brasen, C. L., Andersen, E. S., Østergaard, C., Skovsted, T. A., Petersen, E. R. B., Nielsen, S. L., Mogensen, C. B., & Skjøt-Arkil, H. (2024). Urine Flow Cytometry and Dipstick Analysis in Diagnosing Bacteriuria and Urinary Tract Infections among Adults in the Emergency Department—A Diagnostic Accuracy Trial. Diagnostics, 14(4), 412. https://doi.org/10.3390/diagnostics14040412





