MRI-Based Risk Factors for Adverse Maternal Outcomes in Prophylactic Aortic Balloon Occlusion for Placenta Accreta Spectrum and Placenta Previa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
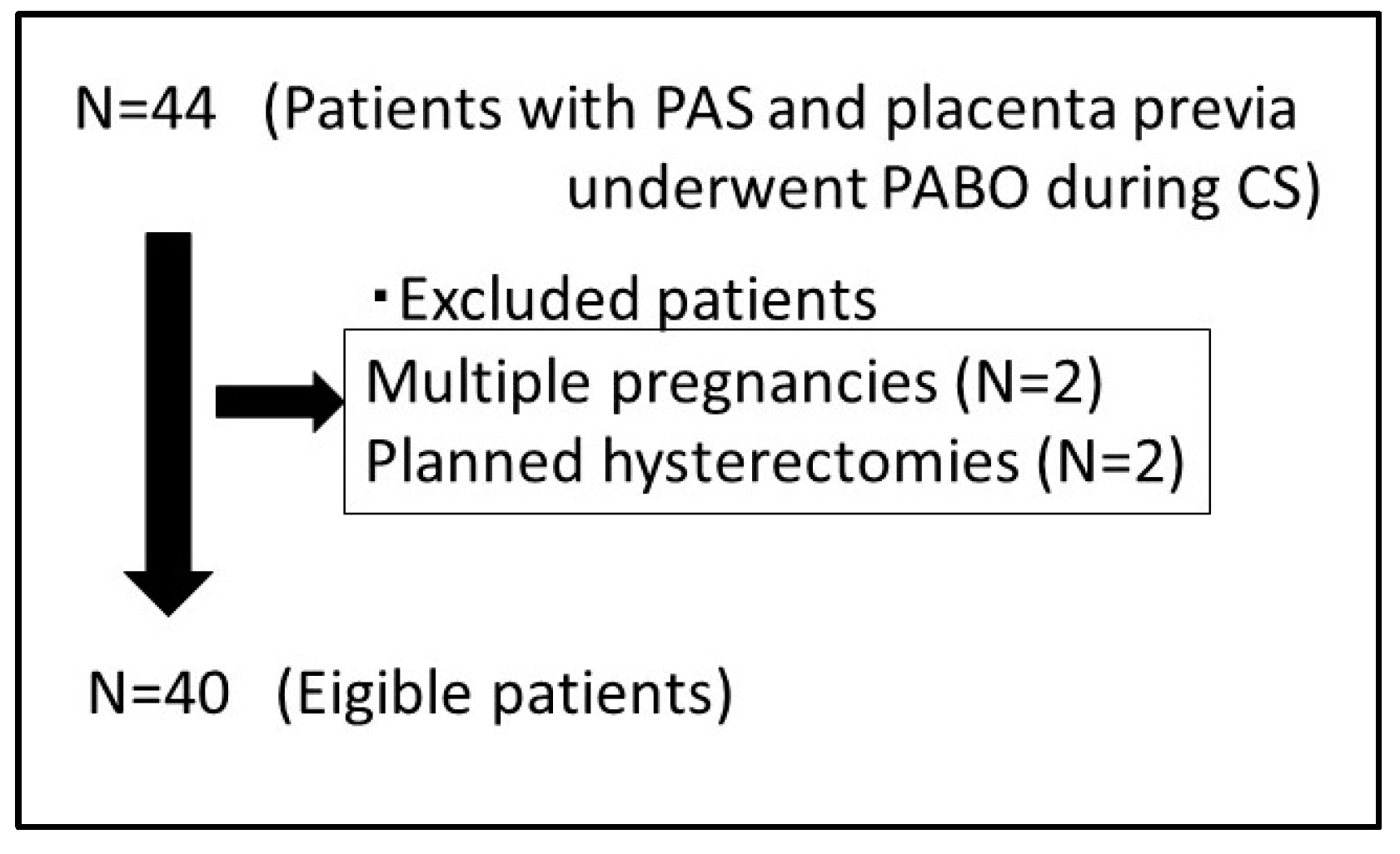
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Data Collection
2.4. MRI Protocols
2.5. Imaging Analysis and Assessment of MRI Features of PAS Disorders
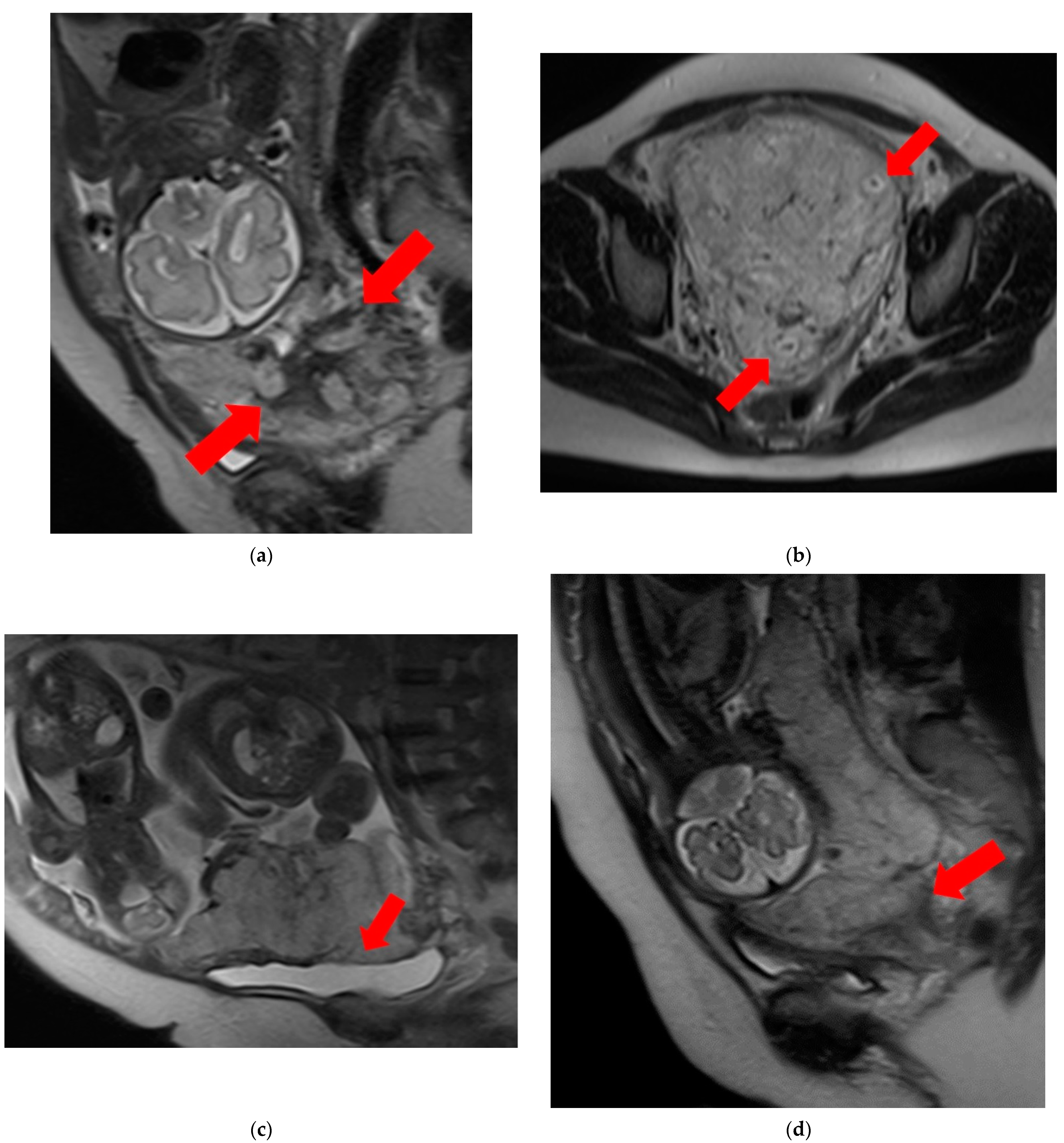
- T2 dark bands: dark lines on T2-weighted images showing nodular or linear patterns from the uterus to the placenta (Figure 1a).
- Placental heterogeneity: uneven signal intensity observed inside the placenta, often due to repeated bleeding (Figure 1b).
- Placental bulge: lower uterine protrusion caused by the placenta, usually toward the bladder (Figure 1c).
- Placental cervical protrusion sign: placental tissue sticking to the cervical canal (Figure 1d).
- Abnormal vascularization of the placental bed: disturbed blood vessels in the placental bed affecting the uteroplacental connection (Figure 1e).
- Focal exophytic mass: placental tissue extending through the uterine wall (Figure 1f).
- Myometrial thinning: Thinning of the uterine muscle over the placenta, sometimes becoming nearly invisible (Figure 1g).
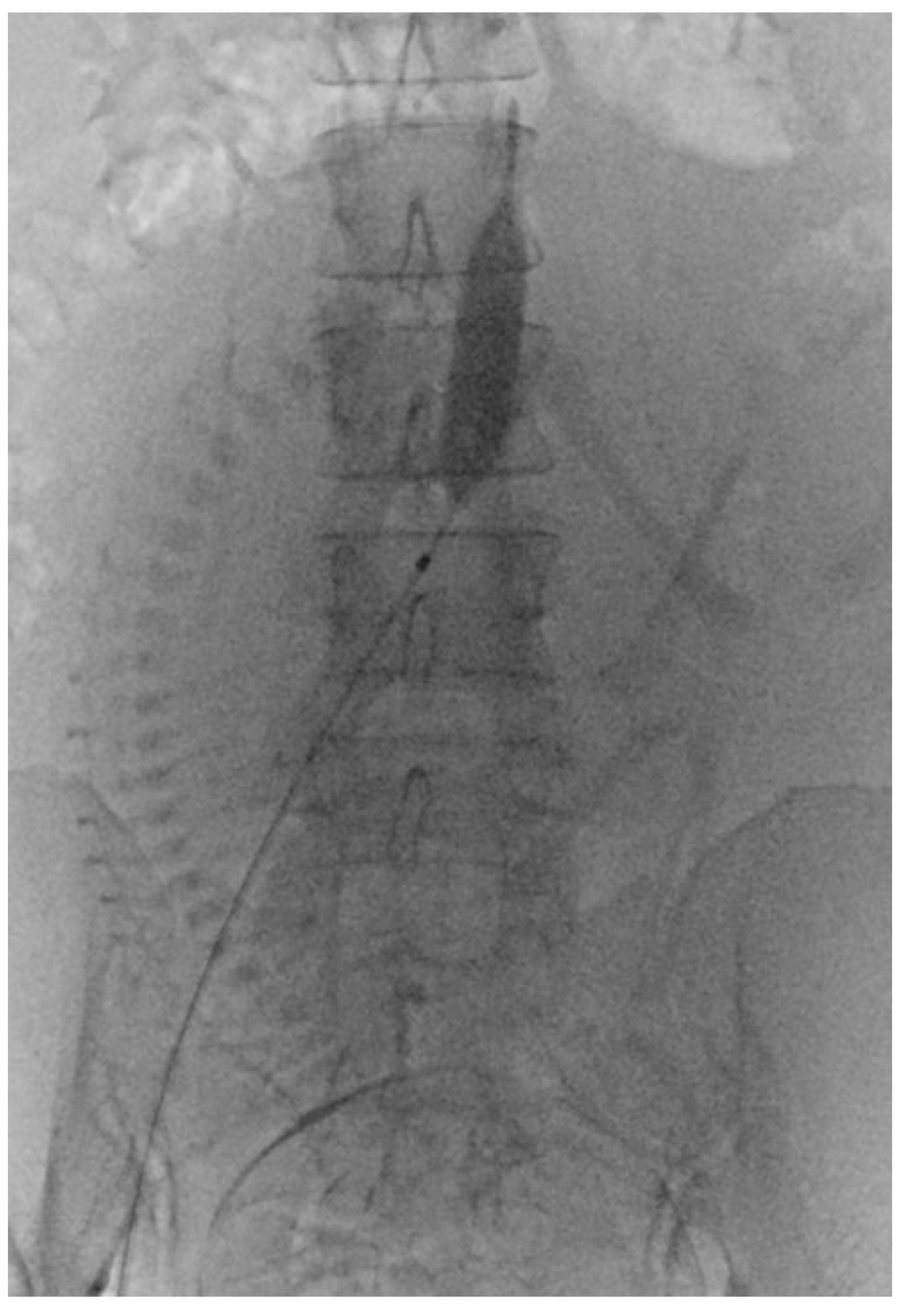
2.6. PABO Procedure
2.7. Statistical Analysis
3. Results
3.1. Background of the Participants
3.2. Comparison of Clinical Characteristics
3.3. MRI Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angolile, C.M.; Max, B.L.; Mushemba, J.; Mashauri, H.L. Global increased cesarean section rates and public health implications: A call to action. Health Sci. Rep. 2023, 6, e1274. [Google Scholar] [CrossRef] [PubMed]
- Oyelese, Y.; Smulian, J.C. Placenta previa, placenta accreta, and vasa previa. Obstet. Gynecol. 2006, 107, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Morlando, M.; Collins, S. Placenta Accreta Spectrum Disorders: Challenges, Risks, and Management Strategies. Int. J. Womens Health. 2020, 12, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.H.; Xiong, Z.; Zhao, B.S.; Zhang, G.B.; Song, W.; Tao, L.X.; Zhang, X.Z. Prophylactic abdominal aortic balloon occlusion: An effective method of controlling hemorrhage in patients with placenta previa or accreta. Exp. Ther. Med. 2019, 17, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, N.; Fichera, A.; Prefumo, F. An update of diagnostic efficacy of ultrasound and magnetic resonance imaging in the diagnosis of clinically significant placenta accreta spectrum disorders. Curr. Opin. Obstet. Gynecol. 2022, 34, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Le, Y.; Lio, K.U.; Zhang, T.; Zhang, Y.; Zhang, N. Performance comparison of ultrasonography and magnetic resonance imaging in their diagnostic accuracy of placenta accreta spectrum disorders: A systematic review and meta-analysis. Insights Imaging 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Tokue, H.; Tokue, A.; Tsushima, Y. Risk factors of MRI findings for predicting patient outcomes of placenta accreta spectrum and placenta previa after prophylactic balloon occlusion of the internal iliac artery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 282, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tokue, H.; Tokue, A.; Tsushima, Y.; Kameda, T. Risk factors for massive bleeding based on angiographic findings in patients with placenta previa and accreta who underwent balloon occlusion of the internal iliac artery during cesarean section. Br. J. Radiol. 2019, 92, 20190127. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Pōder, L.; Bourgioti, C.; Bharwani, N.; Lewis, S.; Kamath, A.; Nougaret, S.; Soyer, P.; Weston, M.; Castillo, R.P.; et al. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur. Radiol. 2020, 30, 2604–2615. [Google Scholar] [CrossRef] [PubMed]
- Bour, L.; Placé, V.; Bendavid, S.; Fargeaudou, Y.; Portal, J.J.; Ricbourg, A.; Sebbag, D.; Dohan, A.; Vicaut, E.; Soyer, P. Suspected invasive placenta: Evaluation with magnetic resonance imaging. Eur. Radiol. 2014, 24, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Kayem, G.; Chandraharan, E.; Palacios-Jaraquemada, J.; Jauniaux, E. FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management. Int. J. Gynaecol. Obstet. 2018, 140, 291–298. [Google Scholar] [PubMed]
- Zhou, X.; Sun, X.; Wang, M.; Huang, L.; Xiong, W. The effectiveness of prophylactic internal iliac artery balloon occlusion in the treatment of patients with pernicious placenta previa coexisting with placenta accreta. J. Matern. Fetal Neonatal Med. 2021, 34, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Murayama, Y.; Itakura, A.; Baba, K.; Seki, H.; Takeda, S. Limitations of internal iliac artery ligation for the reduction of intraoperative hemorrhage during cesarean hysterectomy in cases of placenta previa accreta. J. Obstet. Gynaecol. Res. 2010, 36, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Li, X.; Zhou, Z.; Chen, C.; Xu, M.; Wang, Z.; Ji, J. Intraplacental T2-hypointense bands may help predict placental invasion depth and postpartum hemorrhage in placenta accrete spectrum disorders in high-risk gravid patients. Magn. Reson. Imaging 2022, 94, 73–79. [Google Scholar]
- Derman, A.Y.; Nikac, V.; Haberman, S.; Zelenko, N.; Opsha, O.; Flyer, M. MRI of placenta accreta: A new imaging perspective. AJR Am. J. Roentgenol. 2011, 197, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, H.; Xin, Y.; Zhang, C.; Liu, Z.; Han, X.; Liu, Q.; Li, Y.; Huang, Z. Assessment of the massive hemorrhage in placenta accreta spectrum with magnetic resonance imaging. Exp. Ther. Med. 2020, 19, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Capannolo, G.; D’Amico, A.; Alameddine, S.; Di Girolamo, R.; Khalil, A.; Calì, G.; Trish, I.T.; Coutinho, C.M.; Herrera, M.; Liberati, M.; et al. Placenta accreta spectrum disorders clinical practice guidelines: A systematic review. J. Obstet. Gynaecol. Res. 2023, 49, 1313–1321. [Google Scholar] [CrossRef] [PubMed]

| Nonmassive Bleeding Group | Massive Bleeding Group | p | |
|---|---|---|---|
| N | 18 | 22 | |
| Age (years) | 34.7 ± 4.2 | 36.2 ± 4.8 | 0.39 |
| BMI (kg/m2) | 26.8 ± 2.9 | 25.0 ± 2.7 | 0.72 |
| Parity | 1.5 ± 0.9 | 1.9 ± 0.5 | 0.29 |
| Number of prior caesarean sections | 1.2 ± 0.6 | 1.5 ± 0.6 | 0.48 |
| Degree of placental adhesion | |||
| Accreta | 16 | 13 | 0.073 |
| Increta | 2 | 5 | 0.43 |
| Percreta | 0 | 4 | 0.11 |
| Gestational age at the CS (days) | 248 ± 13 | 251 ± 10 | 0.79 |
| Gestational age at the MRI (days) | 227 ± 10 | 129 ± 12 | 0.89 |
| Foetal weight (g) | 2482.6 ± 431.2 | 2641.2 ± 169.2 | 0.21 |
| Operative duration (min) | 100.4 ± 33.6 | 213 ± 41.2 | <0.001 |
| Estimated blood loss (mL) | 1526.5 ± 842 | 5822.1 ± 4223.4 | <0.001 |
| Hysterectomy | 0 | 13 | <0.001 |
| Number of pRBCs transfused | 3.2 ± 4.1 | 10.7 ± 8.5 | <0.001 |
| Postoperative hospital stay (days) | 9.9 ± 3.2 | 11.2 ± 4.2 | 0.47 |
| Nonmassive Bleeding Group | Massive Bleeding Group | p | |
|---|---|---|---|
| T2 dark bands | 10 (55.6%) | 20 (90.9%) | 0.025 |
| Placental heterogeneity | 8 (44.4%) | 13 (59.1%) | 0.53 |
| Placental bulge | 7 (38.9%) | 13 (59.1%) | 0.34 |
| Placental cervical protrusion sign | 7 (38.9%) | 12 (54.5%) | 0.36 |
| Abnormal vascularization of the placental bed | 3 (16.7%) | 9 (40.9%) | 0.17 |
| Focal exophytic mass | 8 (44.4%) | 11 (50.0%) | 0.76 |
| Myometrial thinning | 8 (44.4%) | 9 (40.9%) | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokue, H.; Ebara, M.; Yokota, T.; Yasui, H.; Tokue, A.; Tsushima, Y. MRI-Based Risk Factors for Adverse Maternal Outcomes in Prophylactic Aortic Balloon Occlusion for Placenta Accreta Spectrum and Placenta Previa. Diagnostics 2024, 14, 333. https://doi.org/10.3390/diagnostics14030333
Tokue H, Ebara M, Yokota T, Yasui H, Tokue A, Tsushima Y. MRI-Based Risk Factors for Adverse Maternal Outcomes in Prophylactic Aortic Balloon Occlusion for Placenta Accreta Spectrum and Placenta Previa. Diagnostics. 2024; 14(3):333. https://doi.org/10.3390/diagnostics14030333
Chicago/Turabian StyleTokue, Hiroyuki, Masashi Ebara, Takayuki Yokota, Hiroyuki Yasui, Azusa Tokue, and Yoshito Tsushima. 2024. "MRI-Based Risk Factors for Adverse Maternal Outcomes in Prophylactic Aortic Balloon Occlusion for Placenta Accreta Spectrum and Placenta Previa" Diagnostics 14, no. 3: 333. https://doi.org/10.3390/diagnostics14030333
APA StyleTokue, H., Ebara, M., Yokota, T., Yasui, H., Tokue, A., & Tsushima, Y. (2024). MRI-Based Risk Factors for Adverse Maternal Outcomes in Prophylactic Aortic Balloon Occlusion for Placenta Accreta Spectrum and Placenta Previa. Diagnostics, 14(3), 333. https://doi.org/10.3390/diagnostics14030333







