Feasibility of Tear Meniscus Height Measurements Obtained with a Smartphone-Attachable Portable Device and Agreement of the Results with Standard Slit Lamp Examination
Abstract
1. Introduction
2. Materials and Methods
2.1. Smart Eye Camera
2.2. Fluorescein Staining Protocol
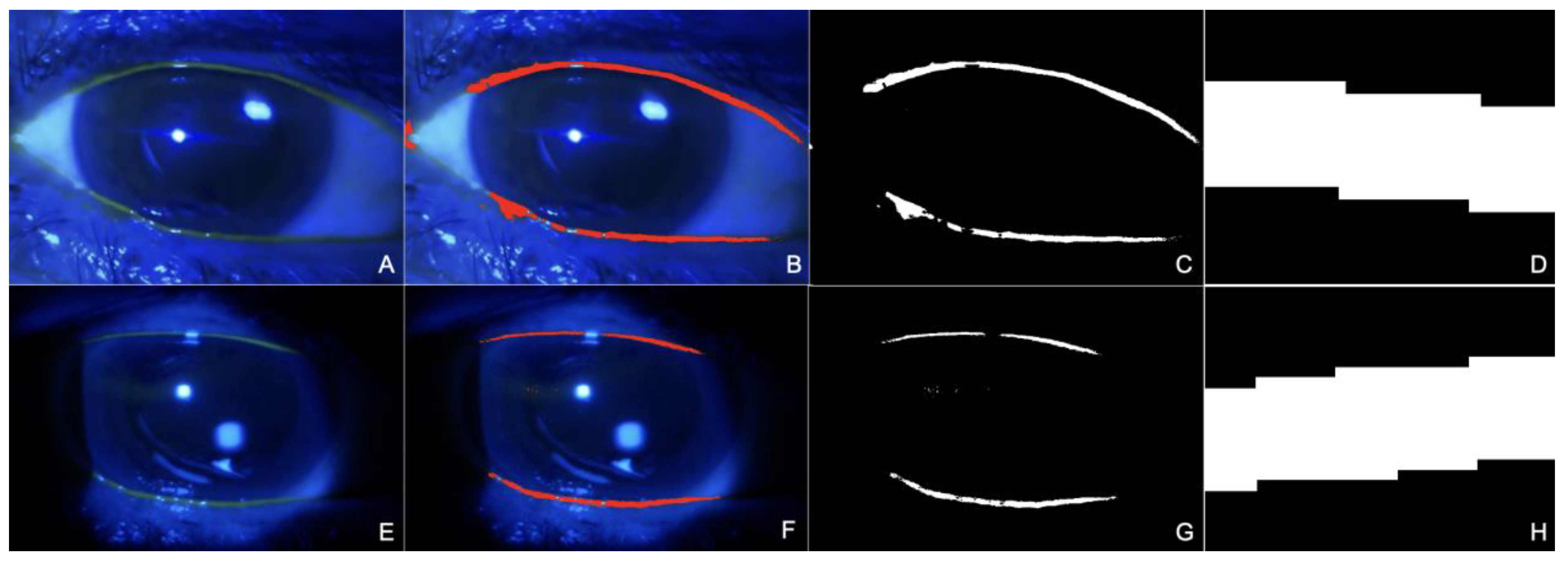
2.3. Image Analysis
2.4. Sample Size
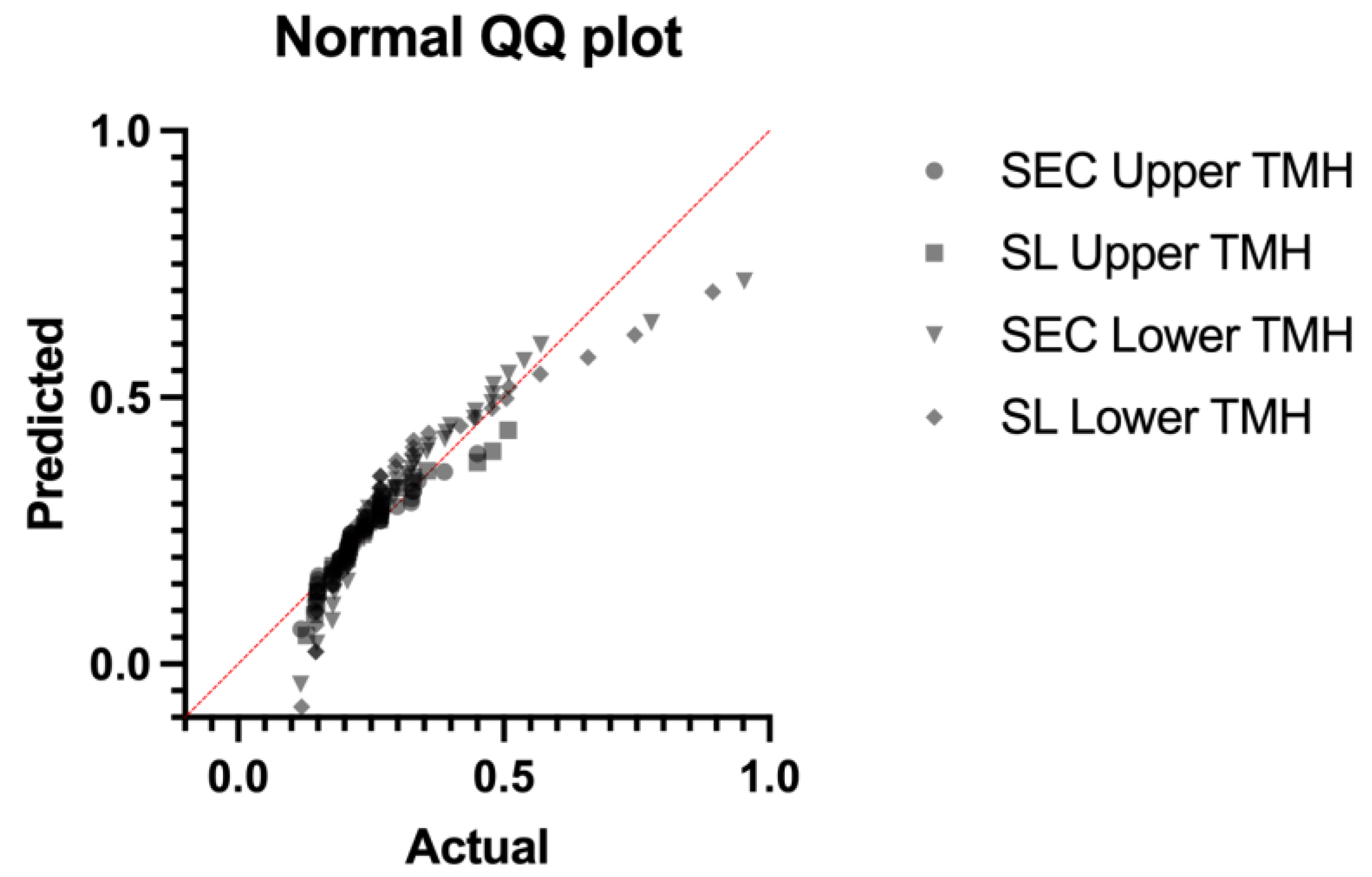
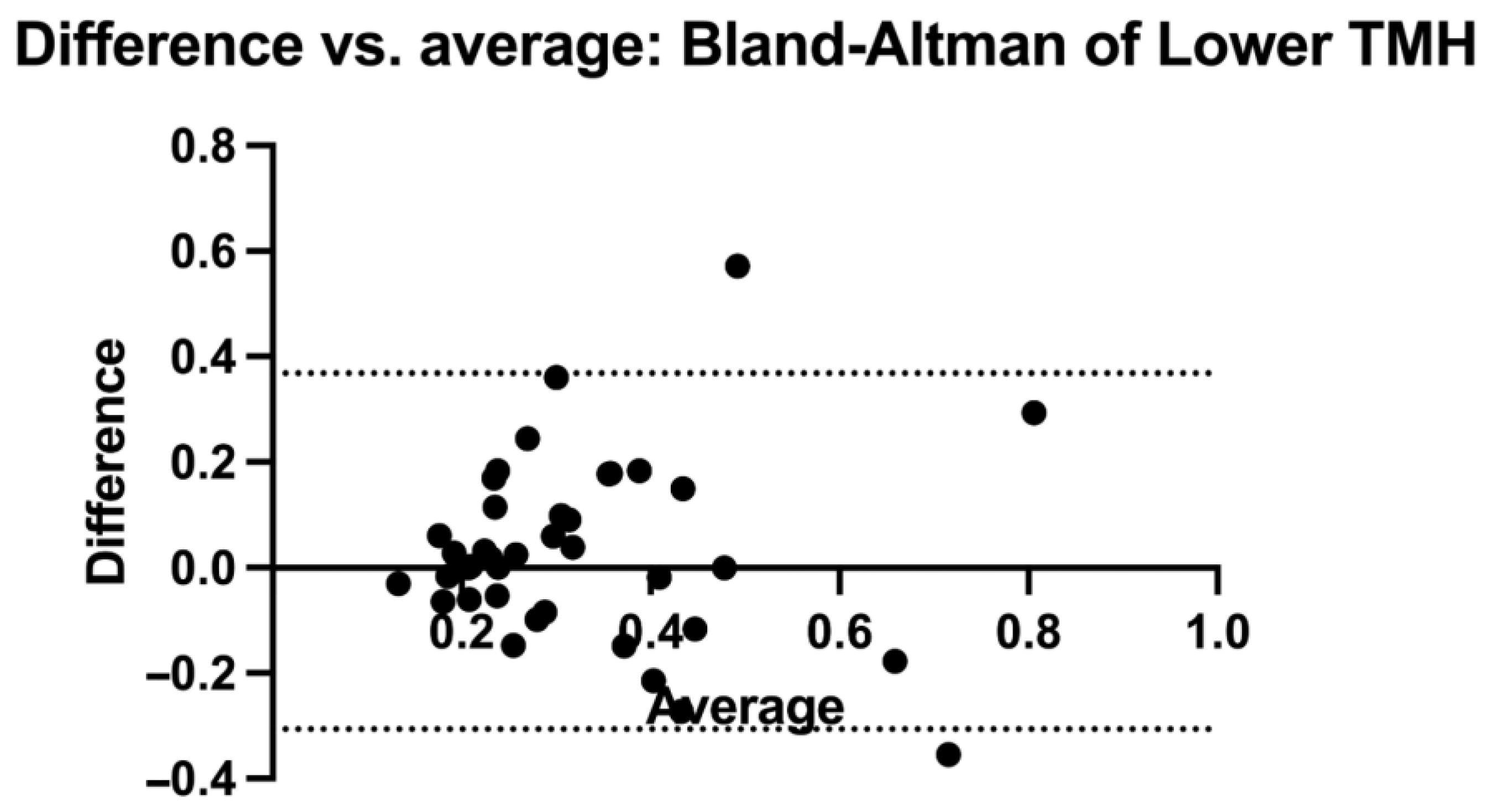
2.5. Statistical Analysis
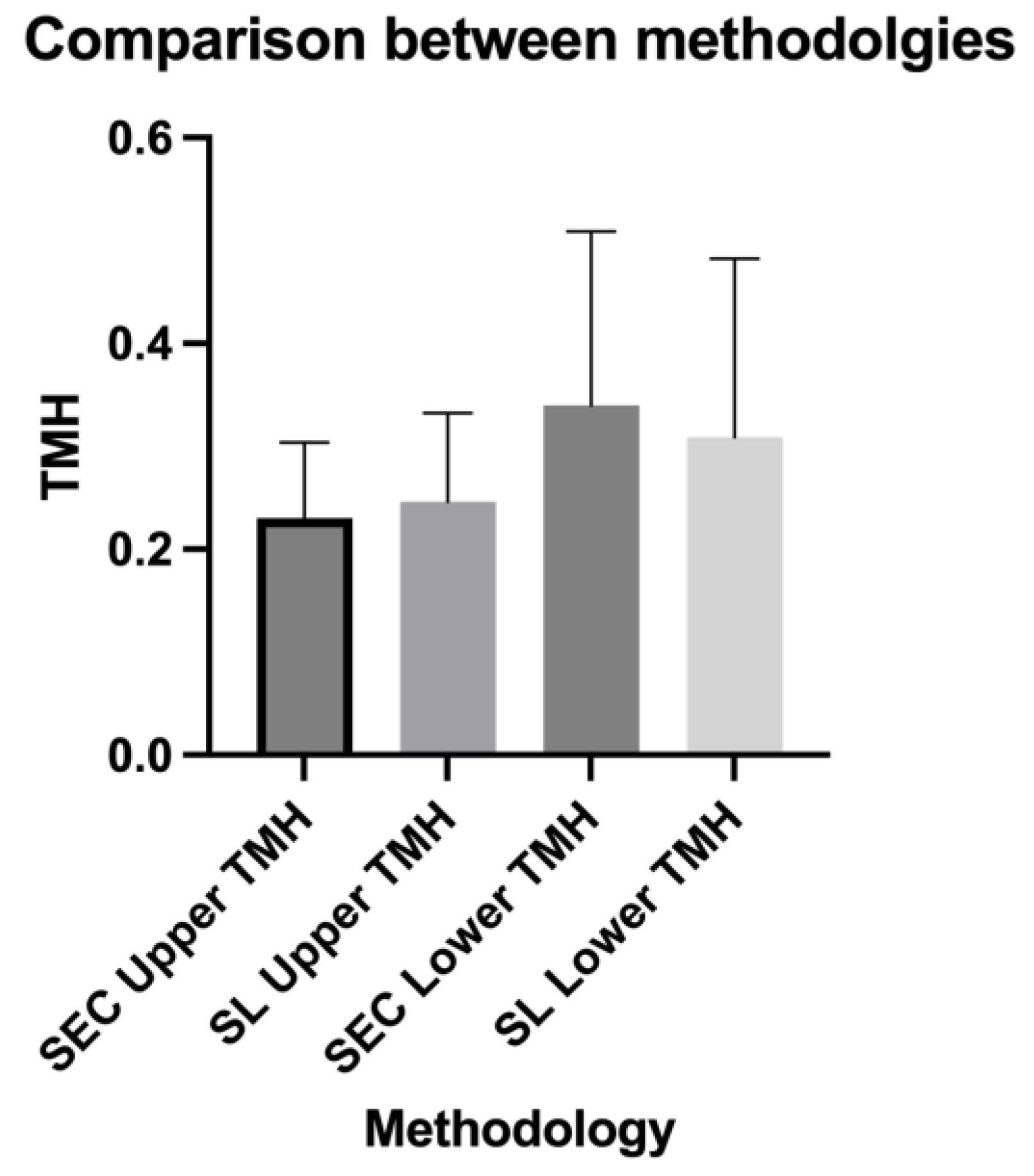
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winebrake, J.P.; Drinkwater, O.J.; Brissette, A.R.; Starr, C.E. The TFOS Dry Eye Workshop II: Key Updates; Eyenet Magazine: San Francisco, CA, USA, 2017; Volume 15, pp. 63–65. [Google Scholar]
- Ayaki, M.; Tachi, N.; Hashimoto, Y.; Kawashima, M.; Tsubota, K.; Negishi, K. Diurnal variation of human tear meniscus volume measured with tear strip meniscometry self-examination. PLoS ONE 2019, 14, e0215922. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, J.; Tao, A.; Chen, Q.; Lin, S.; Qu, J.; Lu, F. Diurnal variation of upper and lower tear menisci. Am. J. Ophthalmol. 2008, 145, 801–806, Erratum in: Am. J. Ophthalmol. 2008, 146, following 479. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510, Erratum in: Ocul. Surf. 2019, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.Q.; Corrales, R.M.; Pflugfelder, S.C. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 2005, 31, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Chen, Z.; Song, X.J.; Luo, L.; Pflugfelder, S.C. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Investig. Opthalmology Vis. Sci. 2004, 45, 4302–4311. [Google Scholar] [CrossRef]
- Rhee, M.K.; Mah, F.S. Inflammation in Dry Eye Disease: How Do We Break the Cycle? Ophthalmology 2017, 124, S14–S19. [Google Scholar] [CrossRef]
- Kim, Y.H.; Graham, A.D.; Li, W.; Radke, C.J.; Lin, M.C. Human Lacrimal Production Rate and Wetted Length of Modified Schirmer's Tear Test Strips. Transl. Vis. Sci. Technol. 2019, 8, 40. [Google Scholar] [CrossRef]
- Doughty, M.J.; Laiquzzaman, M.; Oblak, E.; Button, N. The tear (lacrimal) meniscus height in human eyes: A useful clinical measure or an unusable variable sign? Contact Lens Anterior Eye 2002, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Hua, R.; Guo, P.; Lin, P.; Wang, J.; Yang, W.; Hong, X. Measurement method of tear meniscus height based on deep learning. Front. Med. 2023, 10, 1126754. [Google Scholar] [CrossRef] [PubMed]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Categorization of the Aqueous Deficient Dry Eye by a cut-off Criterion of TMH Measured with Tearscope. Life 2022, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Fodor, E.; Hagyó, K.; Resch, M.; Somodi, D.; Németh, J. Comparison of Tearscope-plus versus slit lamp measurements of inferior tear meniscus height in normal individuals. Eur. J. Ophthalmol. 2010, 20, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Bandlitz, S.; Purslow, C.; Murphy, P.J.; Pult, H. Comparison of a new portable digital meniscometer and optical coherence tomography in tear meniscus radius measurement. Acta Ophthalmol. 2014, 92, e112–e118. [Google Scholar] [CrossRef] [PubMed]
- Niedernolte, B.; Trunk, L.; Wolffsohn, J.S.; Pult, H.; Bandlitz, S. Evaluation of tear meniscus height using different clinical methods. Clin. Exp. Optom. 2021, 104, 583–588. [Google Scholar] [CrossRef] [PubMed]
- LPena-Verdeal, H.; Garcia-Resua, C.; Barreira, N.; Giraldez, M.J.; Yebra-Pimentel, E. Interobserver variability of an open-source software for tear meniscus height measurement. Contact Lens Anterior Eye 2016, 39, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Yamada, M.; Kawashima, M.; Inoue, M.; Goto, E.; Mashima, Y.; Tsubota, K. Quantitative evaluation of tear meniscus height from fluorescein photographs. Cornea 2007, 26, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Di Cello, L.; Pellegrini, M.; Vagge, A.; Borselli, M.; Ferro Desideri, L.; Scorcia, V.; Traverso, C.E.; Giannaccare, G. Advances in the Noninvasive Diagnosis of Dry Eye Disease. Appl. Sci. 2021, 11, 10384. [Google Scholar] [CrossRef]
- Yazu, H.; Shimizu, E.; Okuyama, S.; Katahira, T.; Aketa, N.; Yokoiwa, R.; Sato, Y.; Ogawa, Y.; Fujishima, H. Evaluation of Nuclear Cataract with Smartphone-Attachable Slit-Lamp Device. Diagnostics 2020, 10, 576. [Google Scholar] [CrossRef]
- Shimizu, E.; Ogawa, Y.; Yazu, H.; Aketa, N.; Yang, F.; Yamane, M.; Sato, Y.; Kawakami, Y.; Tsubota, K. “Smart Eye Camera”: An innovative technique to evaluate tear film breakup time in a murine dry eye disease model. PLoS ONE 2019, 14, e0215130. [Google Scholar] [CrossRef]
- Shimizu, E.; Yazu, H.; Aketa, N.; Yokoiwa, R.; Sato, S.; Katayama, T.; Hanyuda, A.; Sato, Y.; Ogawa, Y.; Tsubota, K. Smart Eye Camera: A Validation Study for Evaluating the Tear Film Breakup Time in Human Subjects. Transl. Vis. Sci. Technol. 2021, 10, 28. [Google Scholar] [CrossRef]
- Shimizu, E.; Yazu, H.; Aketa, N.; Yokoiwa, R.; Sato, S.; Yajima, J.; Katayama, T.; Sato, R.; Tanji, M.; Sato, Y.; et al. A Study Validating the Estimation of Anterior Chamber Depth and Iridocorneal Angle with Portable and Non-Portable Slit-Lamp Microscopy. Sensors 2021, 21, 1436. [Google Scholar] [CrossRef] [PubMed]
- GSchiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- HPflugfelder, S.; Nakhleh, L.; Kikukawa, Y.; Tanaka, S.; Kosugi, T. Non-Invasive Tear Break-Up Detection with the Kowa DR-1α and Its Relationship to Dry Eye Clinical Severity. Int. J. Mol. Sci. 2022, 23, 14774. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Tharoo, A.; Lakhlani, N. Optimal time following fluorescein instillation to evaluate rigid gas permeable contact lens fit. Contact Lens Anterior Eye 2015, 38, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, D.; Borselli, M.; Gratteri, S.; Zampogna, G.; Feola, A.; Della Corte, M.; Guarna, F.; Scorcia, V.; Giannaccare, G. Applications and Current Medico-Legal Challenges of Telemedicine in Ophthalmology. Int. J. Environ. Res. Public Health 2022, 19, 5614. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Lee, J.H. Measurement of the tear meniscus height using 0.25% fluorescein sodium. Korean J. Ophthalmol. 1991, 5, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Oguz, H.; Yokoi, N.; Kinoshita, S. The height and radius of the tear meniscus and methods for examining these parameters. Cornea 2000, 19, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.; Flanagan, J.; Simpson, T.; Jones, L. Tear meniscus height determination using the OCT2 and the RTVue-100. Optom. Vis. Sci. 2009, 86, 1154–1159. [Google Scholar] [CrossRef]
- MGiavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Tung, C.I.; Perin, A.F.; Gumus, K.; Pflugfelder, S.C. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am. J. Ophthalmol. 2014, 157, 301–310. [Google Scholar] [CrossRef]
- Czajkowski, G.; Kaluzny, B.J.; Laudencka, A.; Malukiewicz, G.; Kaluzny, J.J. Tear meniscus measurement by spectral optical coherence tomography. Optom. Vis. Sci. 2012, 89, 336–342. [Google Scholar] [CrossRef]
- Baek, J.; Doh, S.H.; Chung, S.K. Comparison of Tear Meniscus Height Measurements Obtained with the Keratograph and Fourier Domain Optical Coherence Tomography in Dry Eye. Cornea 2015, 34, 1209–1213. [Google Scholar] [CrossRef]
- Martin-García, B.; Palomo-Álvarez, C.; Piedrahita-Alonso, E.; Gomez-de-Liaño, R.; Ferrer, M.E.F.; Arriola-Villalobos, P. Lower tear meniscus height measured by optical coherence tomography in children. Ophthalmic Physiol. Opt. 2023, 43, 1059–1064. [Google Scholar] [CrossRef]
- Wei, A.; Le, Q.; Hong, J.; Wang, W.; Wang, F.; Xu, J. Assessment of Lower Tear Meniscus. Optom. Vis. Sci. 2016, 93, 1420–1425. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; He, J.; Li, S.; Chen, Y.; Xu, C.; Lin, X.; Kang, J.; Li, W.; Luo, Z.; et al. A Fully Automatic Estimation of Tear Meniscus Height Using Artificial Intelligence. Investig. Opthalmology Vis. Sci. 2023, 64, 7. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B.H. Impact of conjunctival folds on central tear meniscus height. Investig. Opthalmology Vis. Sci. 2015, 56, 1459–1466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yazu, H.; Shimizu, E.; Sato, S.; Aketa, N.; Katayama, T.; Yokoiwa, R.; Sato, Y.; Fukagawa, K.; Ogawa, Y.; Tsubota, K.; et al. Clinical Observation of Allergic Conjunctival Diseases with Portable and Recordable Slit-Lamp Device. Diagnostics 2021, 11, 535. [Google Scholar] [CrossRef]
- Handayani, A.T.; Valentina, C.; Suryaningrum, I.G.A.R.; Megasafitri, P.D.; Juliari, I.G.A.M.; Pramita, I.A.A.; Nakayama, S.; Shimizu, E.; Triningrat, A.A.M.P. Interobserver Reliability of Tear Break-up Time Examination Using “Smart Eye Camera” in Indonesian Remote Area. Clin. Ophthalmol. 2023, 17, 2097–2107. [Google Scholar] [CrossRef]
- Andhare, P.; Ramasamy, K.; Ramesh, R.; Shimizu, E.; Nakayama, S.; Gandhi, P. A study establishing sensitivity and accuracy of smartphone photography in ophthalmologic community outreach programs: Review of a smart eye camera. Indian J. Ophthalmol. 2023, 71, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Rhee, M.K. COVID-19 and Dry Eye. Eye Contact Lens 2021, 47, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.J.; Ong, H.S.; Tong, L. A practical framework for telemedicine in dry eye disease. Ocul. Surf. 2022, 23, 143–145. [Google Scholar] [CrossRef] [PubMed]

| SEC Upper TMH | SL Upper TMH | SEC Lower TMH | SL Lower TMH | |
|---|---|---|---|---|
| Number of values | 40 | 40 | 40 | 40 |
| Minimum | 0.119 | 0.129 | 0.118 | 0.12 |
| 25% Percentile | 0.1853 | 0.1823 | 0.211 | 0.2063 |
| Median | 0.2095 | 0.2355 | 0.297 | 0.26 |
| 75% Percentile | 0.2688 | 0.2698 | 0.4338 | 0.3518 |
| Maximum | 0.45 | 0.508 | 0.953 | 0.893 |
| Range | 0.331 | 0.379 | 0.835 | 0.773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borselli, M.; Toro, M.D.; Rossi, C.; Taloni, A.; Khemlani, R.; Nakayama, S.; Nishimura, H.; Shimizu, E.; Scorcia, V.; Giannaccare, G. Feasibility of Tear Meniscus Height Measurements Obtained with a Smartphone-Attachable Portable Device and Agreement of the Results with Standard Slit Lamp Examination. Diagnostics 2024, 14, 316. https://doi.org/10.3390/diagnostics14030316
Borselli M, Toro MD, Rossi C, Taloni A, Khemlani R, Nakayama S, Nishimura H, Shimizu E, Scorcia V, Giannaccare G. Feasibility of Tear Meniscus Height Measurements Obtained with a Smartphone-Attachable Portable Device and Agreement of the Results with Standard Slit Lamp Examination. Diagnostics. 2024; 14(3):316. https://doi.org/10.3390/diagnostics14030316
Chicago/Turabian StyleBorselli, Massimiliano, Mario Damiano Toro, Costanza Rossi, Andrea Taloni, Rohan Khemlani, Shintato Nakayama, Hiroki Nishimura, Eisuke Shimizu, Vincenzo Scorcia, and Giuseppe Giannaccare. 2024. "Feasibility of Tear Meniscus Height Measurements Obtained with a Smartphone-Attachable Portable Device and Agreement of the Results with Standard Slit Lamp Examination" Diagnostics 14, no. 3: 316. https://doi.org/10.3390/diagnostics14030316
APA StyleBorselli, M., Toro, M. D., Rossi, C., Taloni, A., Khemlani, R., Nakayama, S., Nishimura, H., Shimizu, E., Scorcia, V., & Giannaccare, G. (2024). Feasibility of Tear Meniscus Height Measurements Obtained with a Smartphone-Attachable Portable Device and Agreement of the Results with Standard Slit Lamp Examination. Diagnostics, 14(3), 316. https://doi.org/10.3390/diagnostics14030316









