Non-Invasive High-Resolution Imaging of In Vivo Human Myelinated Axons
Abstract
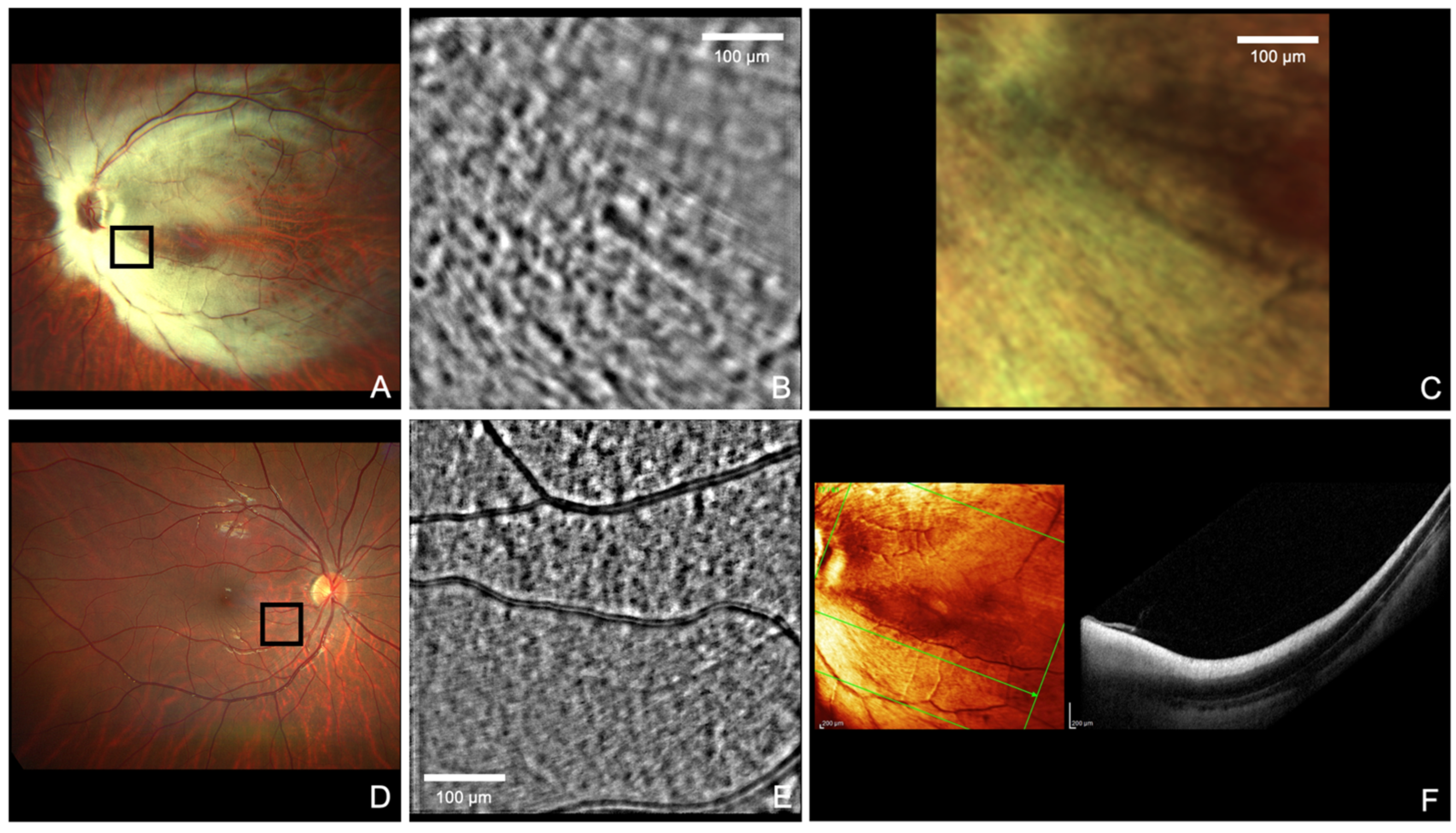
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laforest, T.; Künzi, M.; Kowalczuk, L.; Carpentras, D.; Behar-Cohen, F.; Moser, C. Transscleral Optical Phase Imaging of the Human Retina. Nat. Photonics 2020, 14, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Carpentras, D.; Laforest, T.; Künzi, M.; Moser, C. Effect of Backscattering in Phase Contrast Imaging of the Retina. Opt. Express 2018, 26, 6785. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, H.L.; Verma, R.; Ferreyra, H.A.; Robbins, S.L. Myelinated Retinal Nerve Fiber Layer (RNFL): A Comprehensive Review. Int. Ophthalmol. Clin. 2018, 58, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Straatsma, B.R.; Foos, R.Y.; Heckenlively, J.R.; Taylor, G.N. Myelinated Retinal Nerve Fibers. Am. J. Ophthalmol. 1981, 91, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Sharon, J.M.; Anjanamurthy, R.; Wijesinghe, H.K. Straatsma Syndrome: Unilateral Myelinated Retinal Nerve Fibre Layer, High Myopia, Strabismus and Amblyopia. BMJ Case Rep. 2021, 14, e244362. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.; Cesareo, M.; Falsini, B.; Cusumano, A. Non-Invasive High-Resolution Imaging of In Vivo Human Myelinated Axons. Diagnostics 2024, 14, 253. https://doi.org/10.3390/diagnostics14030253
Lombardo M, Cesareo M, Falsini B, Cusumano A. Non-Invasive High-Resolution Imaging of In Vivo Human Myelinated Axons. Diagnostics. 2024; 14(3):253. https://doi.org/10.3390/diagnostics14030253
Chicago/Turabian StyleLombardo, Marco, Massimo Cesareo, Benedetto Falsini, and Andrea Cusumano. 2024. "Non-Invasive High-Resolution Imaging of In Vivo Human Myelinated Axons" Diagnostics 14, no. 3: 253. https://doi.org/10.3390/diagnostics14030253
APA StyleLombardo, M., Cesareo, M., Falsini, B., & Cusumano, A. (2024). Non-Invasive High-Resolution Imaging of In Vivo Human Myelinated Axons. Diagnostics, 14(3), 253. https://doi.org/10.3390/diagnostics14030253







