Discordance of Biomarker Expression Profile between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis in Preoperative Core Needle Biopsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemistry (IHC) and Fluorescence In Situ Hybridization (FISH)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Breast Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2019. [Google Scholar]
- Hanrahan, E.O.; Gonzalez-Angulo, A.M.; Giordano, S.H.; Rouzier, R.; Broglio, K.R.; Hortobagyi, G.N.; Valero, V. Overall Survival and Cause-Specific Mortality of Patients with Stage T1a, BN0M0 Breast Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4952–4960. [Google Scholar] [CrossRef]
- Beenken, S.W.; Urist, M.M.; Zhang, Y.; Desmond, R.; Krontiras, H.; Medina, H.; Bland, K.I. Axillary Lymph Node Status, but Not Tumor Size, Predicts Locoregional Recurrence and Overall Survival after Mastectomy for Breast Cancer. Ann. Surg. 2003, 237, 732–739. [Google Scholar] [CrossRef]
- Jatoi, I.; Hilsenbeck, S.G.; Clark, G.M.; Osborne, C.K. Significance of Axillary Lymph Node Metastasis in Primary Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 2334–2340. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Falck, A.-K.; Fernö, M.; Bendahl, P.-O.; Rydén, L. Does Analysis of Biomarkers in Tumor Cells in Lymph Node Metastases Give Additional Prognostic Information in Primary Breast Cancer? World J. Surg. 2010, 34, 1434–1441. [Google Scholar] [CrossRef]
- Heim, S.; Teixeira, M.R.; Dietrich, C.U.; Pandis, N. Cytogenetic Polyclonality in Tumors of the Breast. Cancer Genet. Cytogenet. 1997, 95, 16–19. [Google Scholar] [CrossRef]
- Rasbridge, S.A.; Gillett, C.E.; Seymour, A.M.; Patel, K.; Richards, M.A.; Rubens, R.D.; Millis, R.R. The Effects of Chemotherapy on Morphology, Cellular Proliferation, Apoptosis and Oncoprotein Expression in Primary Breast Carcinoma. Br. J. Cancer 1994, 70, 335–341. [Google Scholar] [CrossRef]
- Falck, A.-K.; Bendahl, P.-O.; Chebil, G.; Olsson, H.; Fernö, M.; Rydén, L. Biomarker Expression and St Gallen Molecular Subtype Classification in Primary Tumours, Synchronous Lymph Node Metastases and Asynchronous Relapses in Primary Breast Cancer Patients with 10 Years’ Follow-Up. Breast Cancer Res. Treat. 2013, 140, 93–104. [Google Scholar] [CrossRef]
- Snell, C.E.; Gough, M.; Middleton, K.; Hsieh, M.; Furnas, L.; Seidl, B.; Gibbons, K.; Pyke, C.; Shannon, C.; Woodward, N.; et al. Absent Progesterone Receptor Expression in the Lymph Node Metastases of ER-Positive, HER2-Negative Breast Cancer Is Associated with Relapse on Tamoxifen. J. Clin. Pathol. 2017, 70, 954–960. [Google Scholar] [CrossRef]
- Kinoe, H.; Yamanouchi, K.; Kuba, S.; Morita, M.; Sakimura, C.; Kanetaka, K.; Takatsuki, M.; Abe, K.; Yano, H.; Matsumoto, M.; et al. Discordance of Hormone Receptor, Human Epidermal Growth Factor Receptor-2, and Ki-67 between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis. J. BUON 2018, 23, 60–66. [Google Scholar]
- Kuncman, W.; Orzechowska, M.; Kuncman, Ł.; Kordek, R.; Taran, K. Intertumoral Heterogeneity of Primary Breast Tumors and Synchronous Axillary Lymph Node Metastases Reflected in IHC-Assessed Expression of Routine and Nonstandard Biomarkers. Front. Oncol. 2021, 11, 660318. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-X.; Lu, L.-J.; Wang, R.-J.; Jin, L.-B.; Liu, S.-C.; Li, H.-Y.; Ren, G.-S.; Wu, K.-N.; Wang, D.-L.; Kong, L.-Q. Discordance and Clinical Significance of ER, PR, and HER2 Status between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis. Med. Oncol. 2014, 31, 798. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Basso, M.; Strippoli, A.; Dadduzio, V.; Cerchiaro, E.; Barile, R.; D’Argento, E.; Cassano, A.; Schinzari, G.; Barone, C. Hormone Receptor Status and HER2 Expression in Primary Breast Cancer Compared with Synchronous Axillary Metastases or Recurrent Metastatic Disease. Clin. Breast Cancer 2015, 15, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Pellas, U.; Bauer, A.; Baroš, I.V.; Fattorini, C.; Tot, T. HER2-Low Metastases of HER2-Negative Primary Tumors: A Single Institution Analysis of Intertumoral and Internodal Heterogeneity in Node-Positive Breast Cancer. Front. Oncol. 2023, 13, 1167567. [Google Scholar] [CrossRef] [PubMed]
- Baroš, I.V.; Tanasković, N.; Pellas, U.; Eri, Ž.; Tadić Latinović, L.; Tot, T. Internodal HER2 Heterogeneity of Axillary Lymph Node Metastases in Breast Cancer Patients. Bosn. J. Basic Med. Sci. 2019, 19, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Rizzo, A.; Costarelli, L.; Santinelli, A.; Cerbelli, B.; Scatena, C.; Macrì, E.; Pietribiasi, F.; d’Amati, G.; Sapino, A.; et al. Pathological Examination of Breast Cancer Samples before and after Neoadjuvant Therapy: Recommendations from the Italian Group for the Study of Breast Pathology-Italian Society of Pathology (GIPaM-SIAPeC). Pathologica 2022, 114, 104–110. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J. Strategies for Subtypes--Dealing with the Diversity of Breast Cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Lombardi, A.; Lazzeroni, R.; Bersigotti, L.; Vitale, V.; Amanti, C. The Proper Ki-67 Cut-Off in Hormone Responsive Breast Cancer: A Monoinstitutional Analysis with Long-Term Follow-Up. Breast Cancer 2021, 13, 213–217. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology–College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2023, 147, 993–1000. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, J.; Cho, M.-S.; Park, S.; Sung, S.H. Evaluation of Ki-67 Index in Core Needle Biopsies and Matched Breast Cancer Surgical Specimens. Arch. Pathol. Lab. Med. 2018, 142, 364–368. [Google Scholar] [CrossRef]
- Tamaki, K.; Sasano, H.; Ishida, T.; Miyashita, M.; Takeda, M.; Amari, M.; Tamaki, N.; Ohuchi, N. Comparison of Core Needle Biopsy (CNB) and Surgical Specimens for Accurate Preoperative Evaluation of ER, PgR and HER2 Status of Breast Cancer Patients. Cancer Sci. 2010, 101, 2074–2079. [Google Scholar] [CrossRef]
- Skibinski, A.; Kuperwasser, C. The Origin of Breast Tumor Heterogeneity. Oncogene 2015, 34, 5309–5316. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2023, 21, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Gaglia, A.; Arapantoni-Dadioti, P.; Bobota, A.; Valavanis, C.; Kostopoulou, V.; Mylonakis, N.; Karabelis, A.; Pectasides, M.; Economopoulos, T. HER-2/Neu Status of Primary Breast Cancer and Corresponding Metastatic Sites in Patients with Advanced Breast Cancer Treated with Trastuzumab-Based Therapy. Anticancer Res. 2006, 26, 647–653. [Google Scholar] [PubMed]
- Xi, X.; Huang, X.-W.; Yuan, H.-Z.; He, C.; Ni, J.; Yang, F.-L. Biomarker Heterogeneity between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastases. Oncol. Lett. 2020, 20, 273. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, L.; Liu, W.; Lv, C.; Zhang, K.; Gao, H.; Wang, J.; Ma, R. Comparison of the Expression of Prognostic Biomarkers between Primary Tumor and Axillary Lymph Node Metastases in Breast Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 5744–5748. [Google Scholar] [PubMed]
- Aitken, S.J.; Thomas, J.S.; Langdon, S.P.; Harrison, D.J.; Faratian, D. Quantitative Analysis of Changes in ER, PR and HER2 Expression in Primary Breast Cancer and Paired Nodal Metastases. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Liu, J.; Hayashi, N.; Mittendorf, E.A.; Gong, Y.; Palla, S.L.; Tokuda, Y.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Ueno, N.T. Loss of Human Epidermal Growth Factor Receptor 2 (HER2) Expression in Metastatic Sites of HER2-Overexpressing Primary Breast Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 593–599. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Park, D.; Kåresen, R.; Noren, T.; Sauer, T. Ki-67 Expression in Primary Breast Carcinomas and Their Axillary Lymph Node Metastases: Clinical Implications. Virchows Arch. 2007, 451, 11–18. [Google Scholar] [CrossRef]
- Tökés, A.-M.; Szász, A.M.; Geszti, F.; Lukács, L.V.; Kenessey, I.; Turányi, E.; Meggyesházi, N.; Molnár, I.A.; Fillinger, J.; Soltész, I.; et al. Expression of Proliferation Markers Ki67, Cyclin A, Geminin and Aurora-Kinase A in Primary Breast Carcinomas and Corresponding Distant Metastases. J. Clin. Pathol. 2015, 68, 274–282. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E.J.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef]
- Najjar, M.K.; Manore, S.G.; Regua, A.T.; Lo, H.-W. Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer. Genes 2022, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. Evolution of HER2-Low Expression from Primary to Recurrent Breast Cancer. NPJ Breast Cancer 2021, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Janeva, S.; Parris, T.Z.; Krabbe, E.; Sundquist, M.; Karlsson, P.; Audisio, R.A.; Olofsson Bagge, R.; Kovács, A. Clinical Relevance of Biomarker Discordance between Primary Breast Cancers and Synchronous Axillary Lymph Node Metastases. Clin. Exp. Metastasis 2023, 40, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Kimbung, S.; Kovács, A.; Danielsson, A.; Bendahl, P.-O.; Lövgren, K.; Frostvik Stolt, M.; Tobin, N.P.; Lindström, L.; Bergh, J.; Einbeigi, Z.; et al. Contrasting Breast Cancer Molecular Subtypes across Serial Tumor Progression Stages: Biological and Prognostic Implications. Oncotarget 2015, 6, 33306–33318. [Google Scholar] [CrossRef] [PubMed]
- Marletta, S.; Treanor, D.; Eccher, A.; Pantanowitz, L. Whole-Slide Imaging in Cytopathology: State of the Art and Future Directions. Diagn. Histopathol. 2021, 27, 425–430. [Google Scholar] [CrossRef]
- Sallout, L.; Tashkandi, M.; Moqnas, A.; AlMajed, H.; Al-Naeem, A.; Alwelaie, Y. Fine-Needle Aspiration Biopsy of Axillary Lymph Nodes: A Reliable Diagnostic Tool for Breast Cancer Staging. Cancer Cytopathol. 2023. [Google Scholar] [CrossRef] [PubMed]
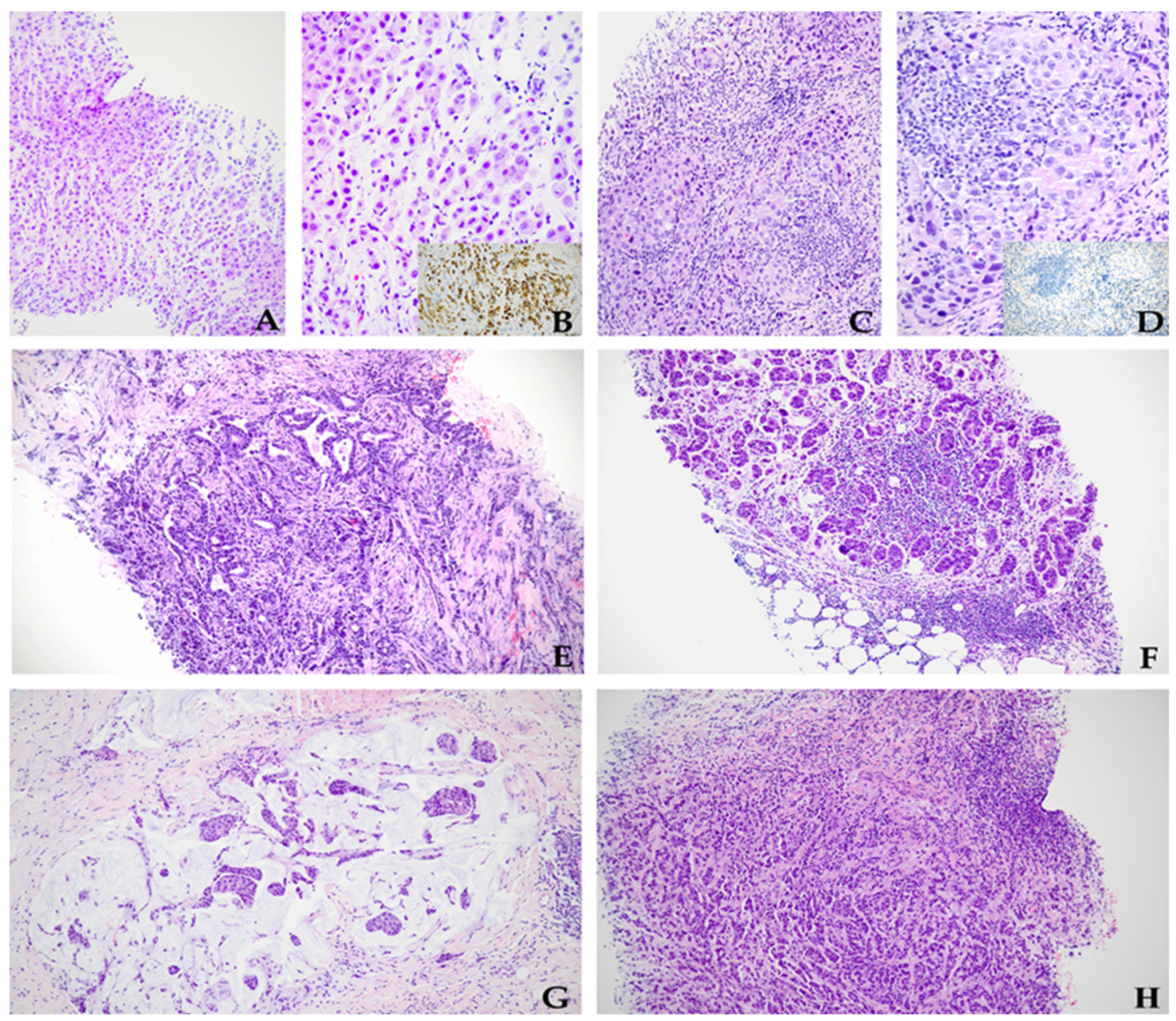
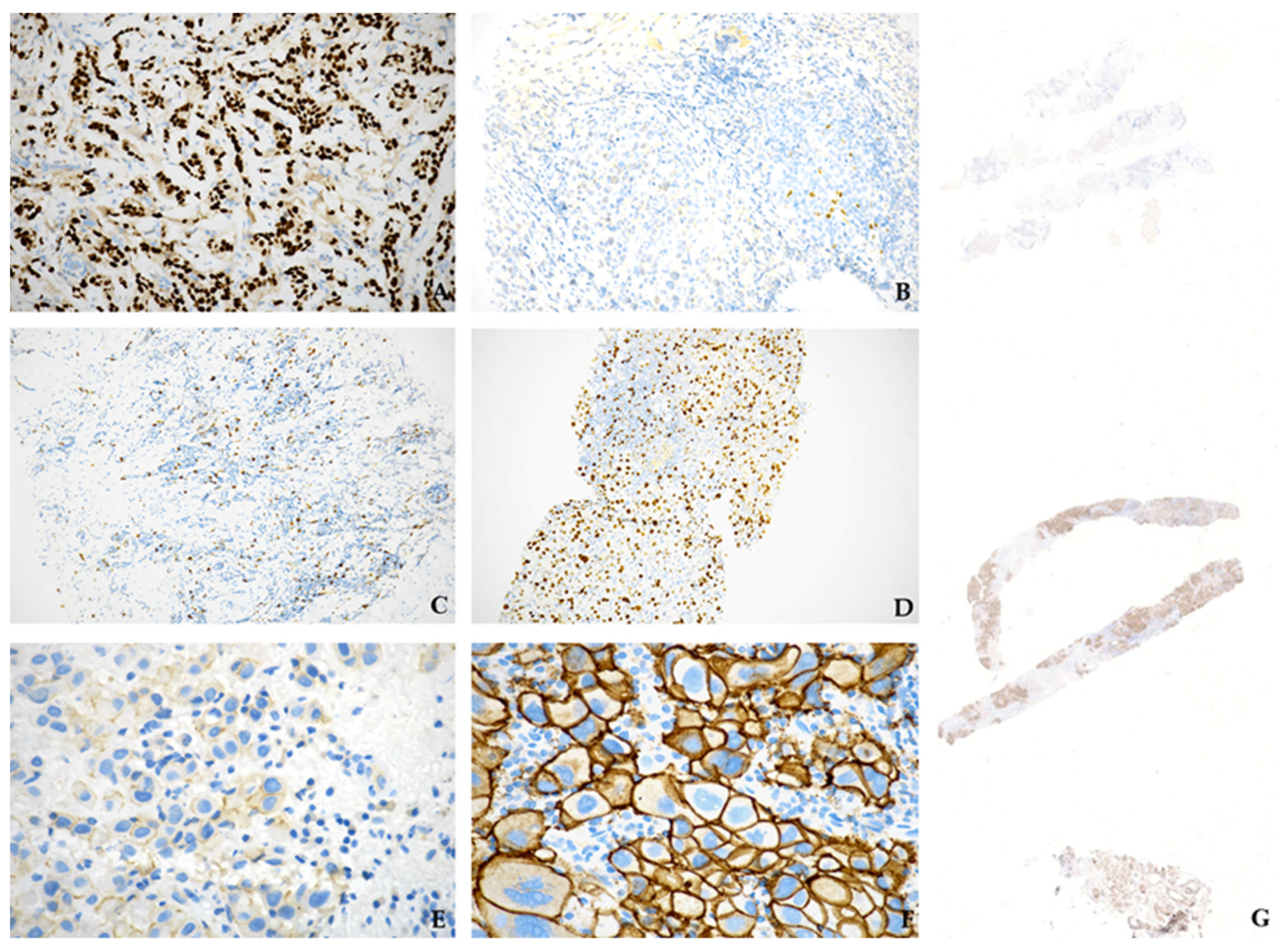
| Parameter | No. of Cases (%) | Parameter | No. of Cases (%) |
|---|---|---|---|
| Sex | N. of lesions (PT) | ||
| F | 29 (96%) | Single | 29 (96%) |
| M | 1 (4%) | Multiple | 1 (4%) |
| Age | Histology (PT) | ||
| 35–81 y.o., median 59 | NST | 16 (52%) | |
| ≤59 | 13 (43%) | ILC | 3 (10%) |
| >59 | 17 (57%) | Mixed NST/ILC | 6 (19%) |
| Other variant | 6 (19%) | ||
| Size | |||
| 0.5–6 cm, median 2.2 cm | Nuclear grading (PT) | ||
| ≤2 cm | 12 (43%) | G2 | 21 (68%) |
| >2 cm | 16 (57%) | G3 | 10 (32%) |
| Laterality | Necrosis (PT) | ||
| Right | 23 (74%) | Absent | 29 (94%) |
| Left | 8 (26%) | Present | 2 (6%) |
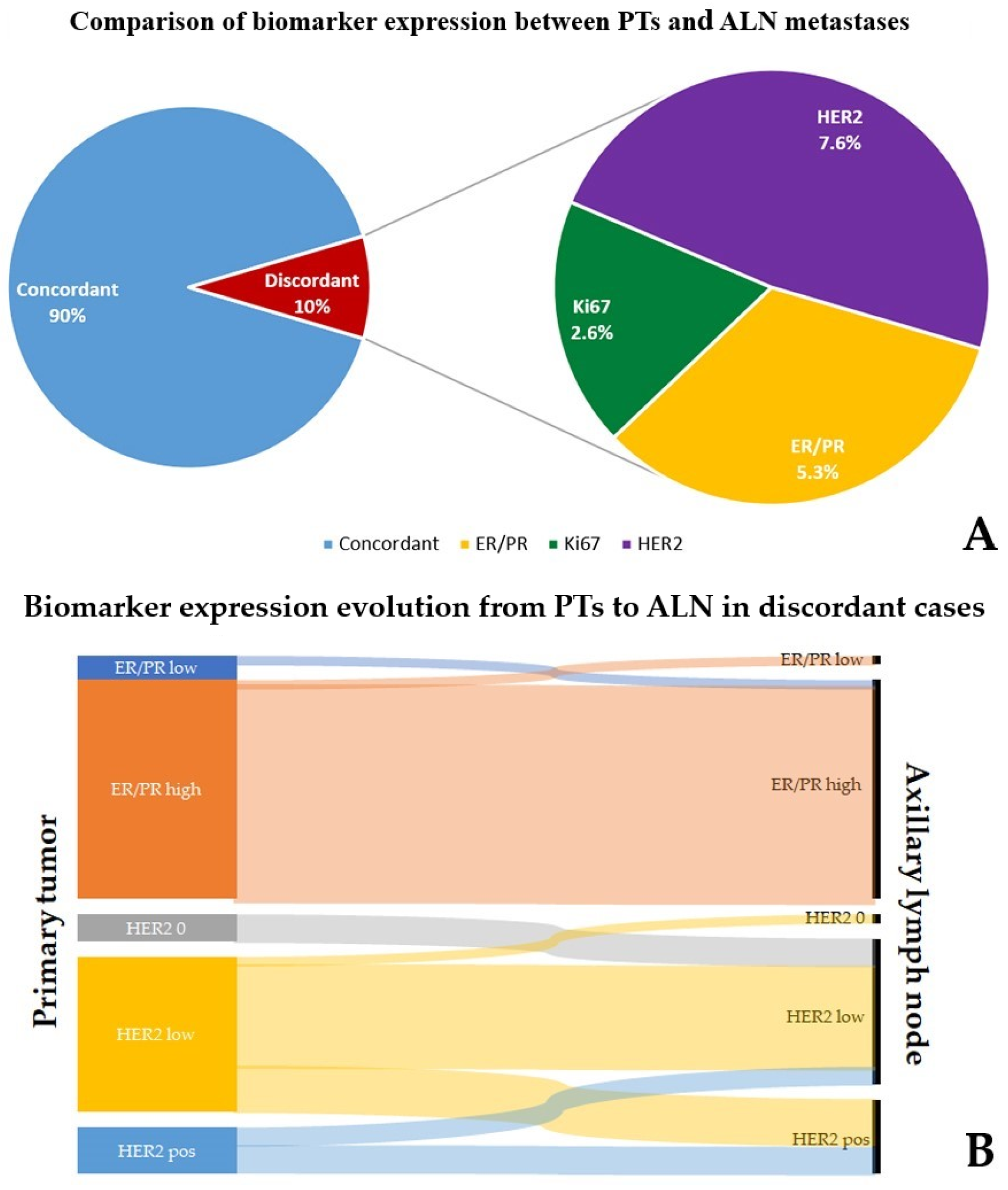
| Pathologically Relevant | ||||||||
|---|---|---|---|---|---|---|---|---|
| ER/PR | Ki67 (≤15% vs. ≥30%) | HER2 | ||||||
| Increased | Reduced | |||||||
| Low to high | 8 | Low to high | 5 | 0 → 1+ | 1 | 2+ (FISH neg.) → 1+ | 1 | |
| High to low | 8 | High to low | 1 | 0 → 2+ (FISH neg.) | 2 | 2+ (FISH neg.) → 0 | 1 | |
| 1+ → 2+ (FISH neg.) | 11 | 3+ → 1 + | 1 | |||||
| 1+ → 3+ | 3 | 2+(FISH +)/3+ → 1+ | 1 | |||||
| 2+ (FISH neg.) → 3+ | 1 | |||||||
| 2+ (FISH neg.) → 2+ (FISH +) | 1 | |||||||
| Total | 16 | Total | 6 | Total | 19 | Total | 4 | |
| Clinically Relevant | ||||||||
| ER/PR | HER2 | |||||||
| Increased | Reduced | |||||||
| Low to high | 1 | 0 → 1+ | 1 | 3+ → 1+ | 1 | |||
| High to low | 1 | 0 → 2+ (FISH neg.) | 2 | 2+ (FISH +)/3+ → 1+ | 1 | |||
| 1+ → 3+ | 3 | |||||||
| 2+ (FISH neg.) → 3+ | 1 | |||||||
| 2+ (FISH neg.) → 2+ (FISH+) | 1 | |||||||
| Total | 2 | Total | 8 | Total | 2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marletta, S.; Giorlandino, A.; Cavallo, E.; Dello Spedale Venti, M.; Leone, G.; Tranchina, M.G.; Gullotti, L.; Bonanno, C.L.; Spoto, G.; Falzone, G.; et al. Discordance of Biomarker Expression Profile between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis in Preoperative Core Needle Biopsy. Diagnostics 2024, 14, 259. https://doi.org/10.3390/diagnostics14030259
Marletta S, Giorlandino A, Cavallo E, Dello Spedale Venti M, Leone G, Tranchina MG, Gullotti L, Bonanno CL, Spoto G, Falzone G, et al. Discordance of Biomarker Expression Profile between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis in Preoperative Core Needle Biopsy. Diagnostics. 2024; 14(3):259. https://doi.org/10.3390/diagnostics14030259
Chicago/Turabian StyleMarletta, Stefano, Alexandra Giorlandino, Enrico Cavallo, Michele Dello Spedale Venti, Giorgia Leone, Maria Grazia Tranchina, Lucia Gullotti, Claudia Lucia Bonanno, Graziana Spoto, Giusi Falzone, and et al. 2024. "Discordance of Biomarker Expression Profile between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis in Preoperative Core Needle Biopsy" Diagnostics 14, no. 3: 259. https://doi.org/10.3390/diagnostics14030259
APA StyleMarletta, S., Giorlandino, A., Cavallo, E., Dello Spedale Venti, M., Leone, G., Tranchina, M. G., Gullotti, L., Bonanno, C. L., Spoto, G., Falzone, G., Tornabene, I., Trovato, C., Baron, M. M., Di Mauro, G., Falsaperna, L., Angelico, G., Pafumi, S., & Rizzo, A. (2024). Discordance of Biomarker Expression Profile between Primary Breast Cancer and Synchronous Axillary Lymph Node Metastasis in Preoperative Core Needle Biopsy. Diagnostics, 14(3), 259. https://doi.org/10.3390/diagnostics14030259







