Correlation between Tomography Scan Findings and Clinical Presentation and Treatment Outcomes in Patients with Orbital Floor Fractures
Abstract
1. Introduction
2. Materials and Methods
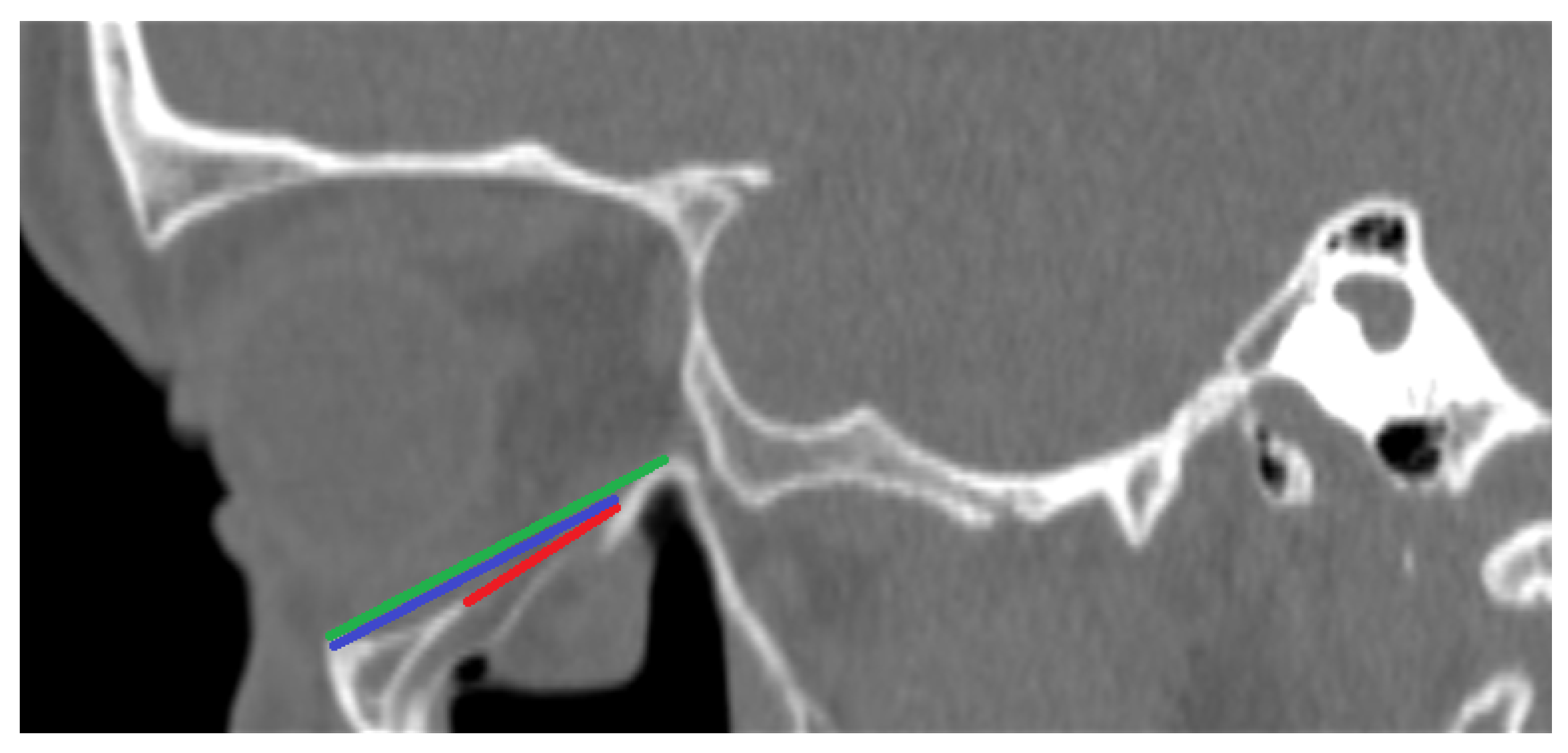
- PI—Largest length of the fracture in the sagittal plane in millimeters (See Figure 1);
- PII—Distance between the end of the fracture and the infraorbital margin in the sagittal plane in millimeters (See Figure 1);
- PIII—Distance between the infraorbital margin and the end of the orbit in the sagittal plane in millimeters (See Figure 1);
- PIV—Whether the fracture includes the medial wall of the orbit; (See Figure 2);
- PV—Relation between the fracture and the infraorbital nerve [3 possibilities: (A) the fracture does not include the infraorbital canal and (B) the fracture goes directly through the infraorbital canal, and (C) the infraorbital canal is within a fractured bone fragment] (See Figure 2);
- PVI—Largest width of the fracture in the coronal plane in millimeters (See Figure 3);
- PVII—Size of a hernia into the maxillary sinus in the coronal plane in millimeters (See Figure 3);
- PVIII—Position of the fractured bone fragments (i.e., presence or absence of a trap-door formation) (See Figure 2); and
- PIX—Displacement of the rectus inferior muscle [3 possibilities: (A) muscle not displaced, (B) muscle displaced to the level of the orbital floor, and (C) muscle displaced into the maxillary sinus] (See Figure 2).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AO | Arbeitsgemeinschaft für Osteosynthesefragen |
| CT | Computed tomography |
| BT | Before treatment |
| AT | After treatment |
| IAT | Improvement after treatment |
| AEP | Asymmetric eyeball placement |
| IOM | Impaired ocular mobility |
| PI | Parameter I |
| PII | Parameter II |
| PIII | Parameter III |
| PIV | Parameter IV |
| PV | Parameter V |
| PVI | Parameter VI |
| PVII | Parameter VII |
| PVIII | Parameter VIII |
| PIX | Parameter IX |
| PX | Parameter X |
| PXI | Parameter XI |
Appendix A
Appendix B
Appendix C
Appendix D
References
- Kryst, L. Chirurgia Szczękowo-Twarzowa. Podręcznik dla Studentów, 2nd ed.; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 1993; pp. 267–274. [Google Scholar]
- Mittermiller, P.A.; Bidwell, S.S.; Thieringer, F.M.; Cornelius, C.P.; Trickey, A.W.; Kontio, R.; Girod, S.; AO Trauma Classification Study Group. The Comprehensive AO CMF Classification System for Mandibular Fractures: A Multicenter Validation Study. Craniomaxillofac. Trauma Reconstr. 2019, 12, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Gómez Roselló, E.; Quiles Granado, A.M.; Artajona Garcia, M.; Juanpere Martí, S.; Laguillo Sala, G.; Beltrán Mármol, B.; Pedraza Gutiérrez, S. Facial fractures: Classification and highlights for a useful report. Insights Imaging 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Kirkpatrick, N.A.; Lyne, J.; Urdang, M.; Waterhouse, N. Buckling and hydraulic mechanisms in orbital blowout fractures: Fact or fiction? J. Craniofac. Surg. 2006, 17, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, F.; Steiner, M.; Becker, M.E.; Kern, M.; Wiltfang, J.; Lucius, R.; Becker, S.T. Forces charging the orbital floor after orbital trauma. J. Craniofac. Surg. 2012, 23, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Andrecovich, C.; Silverman, M.; Zhang, L.; Shkoukani, M. Biomechanic Factors Associated with Orbital Floor Fractures. JAMA Facial. Plast. Surg. 2017, 19, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.B.; Jürgens, P.C.; Gabrielli, M.A.C.; Pereira Filho, V.A. Dynamic three-dimensional finite element analysis of orbital trauma. Br. J. Oral. Maxillofac. Surg. 2021, 59, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.; Attieh, A.; Khalil, A.; Szávai, S.; Nazha, H. Biomechanical assessment of orbital fractures using patient-specific models and clinical matching. Stomatol. Oral. Maxillofac. Surg. 2021, 122, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- Wanyura, H.; Kowalczyk, P.; Samolczyk-Wanyura, D.; Stopa, Z.; Bossak, M. Finite element analysis of external loads resulting in isolated orbital floor fractures. Czasopismo Stomatologiczne 2011, 64, 476–489. [Google Scholar]
- Patel, S.; Shokri, T.; Ziai, K.; Lighthall, J.G. Controversies and contemporary management of orbital floor fractures. Craniomaxillofac Trauma Reconstr. 2022, 15, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Seen, S.; Young, S.M.; Teo, S.J.; Lang, S.S.; Amrith, S.; Lim, T.C.; Sundar, G. Permanent versus bioresorbable implants in orbital floor blowout fractures. Ophthalmic. Plast. Reconstr. Surg. 2018, 34, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Goldberg, R. Re: Permanent versus bioresorbable implants in orbital floor blowout fractures. Ophthalmic. Plast. Reconstr. Surg. 2019, 35, 202. [Google Scholar] [CrossRef] [PubMed]
- Young, S.M.; Sundar, G. Reply Re: Permanent versus bioresorbable implants in orbital floor blowout fractures. Ophthalmic. Plast. Reconstr. Surg. 2019, 35, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Bocchialini, G.; Castellani, A. Facial trauma: A retrospective study of 1262 patients. Ann. Maxillofac. Surg. 2019, 9, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wu, J.; Yang, C.; Zhang, C.; Xu, B.; Zhang, Y.; Zhang, S. Classifying and standardizing panfacial trauma according to anatomic categories and Facial Injury Severity Scale: A 10-year retrospective study. BMC Oral. Health 2021, 21, 557. [Google Scholar] [CrossRef] [PubMed]
- Seddon, H. Three types of nerve injury. Brain 1943, 66, 237–288. [Google Scholar] [CrossRef]
- Kovar, D.; Voldrich, Z.; Voska, P.; Lestak, J.; Astl, J. Indications for repositioning of blow-out fractures of the orbital floor based on new objective criteria-tissue protrusion volumometry. Biomed. Pap. 2017, 161, 403–406. [Google Scholar] [CrossRef]
- Zinn, J. Descriptio Anatomica Oculi Humani Iconibus Illustrata, Latin ed.; Goettingae: Apud viduam B. Abrahami Vandenhoeck: Electorate of Brunswick-Lüneburg, 1755.
- Koenen, L.; Waseem, M. Orbital Floor Fracture; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Gugliotta, Y.; Roccia, F.; Demo, P.G.; Rossi, M.B. Characteristics and surgical management of pure trapdoor fracture of the orbital floor in adults: A 15-year review. Oral. Maxillofac. Surg. 2022, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, T.; Iuchi, T.; Hashimoto, T.; Sueyoshi, Y.; Nagasao, T.; Isogai, N. Image analysis of the inferior rectus muscle in orbital floor fracture using cine mode magnetic resonance imaging. J. Craniomaxillofac. Surg. 2015, 43, 2066–2070. [Google Scholar] [CrossRef] [PubMed]




| Sex | Battery | Sport Injury | Traffic Accident | Fall | Other/Unknown/No Data |
|---|---|---|---|---|---|
| Male | 65.6% | 9.4% | 0 % | 15.6% | 9.4% |
| Female | 37.5% | 12.5% | 12.5% | 25 % | 12.5% |
| Total | 60% | 10 % | 2.5% | 17.5% | 10% |
| PI | PII | PIII | PIV | PVI | PVII | PVIII | PIX | PXI | |
|---|---|---|---|---|---|---|---|---|---|
| BT | 21.7 mm | 29.7 mm | 32.8 mm | 8/18 | 16.1 mm | 9.4 mm | 19/7 | 11/11/4 | 3.2 mm |
| 24 mm | 29.9 mm | 33.7 mm | 6/5 | 16.9 mm | 10.4 mm | 6/5 | 3/6/2 | 3.9 mm | |
| 0.2525 | 0.8444 | 0.4090 | 0.1729 | 0.6492 | 0.2529 | 0.2711 | 0.6869 | 0.4277 | |
| AT | 19.8 mm | 28.7 mm | 32.2 mm | 4/5 | 15.8 mm | 10.3 mm | 8/1 | 3/4/2 | 3.5 mm |
| 23.4 mm | 29.6 mm | 33.2 mm | 7/13 | 16.1 mm | 9.6 mm | 12/8 | 9/9/2 | 3.6 mm | |
| 0.0978 | 0.4992 | 0.4337 | 0.6277 | 0.8575 | 0.5691 | 0.1198 | 0.6451 | 0.9763 | |
| IAT | 23.4 mm | 30.5 mm | 32.9 mm | 3/7 | 17.0 mm | 10.1 mm | 6/4 | 5/4/1 | 2.4 mm |
| 22 mm | 29.4 mm | 32.9 mm | 8/10 | 15.8 mm | 10.0 mm | 13/5 | 6/9/3 | 3.6 mm | |
| 0.5232 | 0.2664 | 0.9736 | 0.4533 | 0.4688 | 0.9288 | 0.5070 | 0.6727 | 0.2084 |
| PI | PII | PIII | PIV | PVI | PVII | PVIII | PIX | PX | PXI | |
|---|---|---|---|---|---|---|---|---|---|---|
| AEP | 26.2 mm | 30.4 mm | 35.0 mm | 7/2 | 18.1 mm | 8.3 mm | 5/4 | 3/3/3 | 242 mm2 | 4.6 mm |
| 21.3 mm | 29.0 mm | 32.3 mm | 6/20 | 15.6 mm | 9.9 mm | 21/5 | 11/13/2 | 175 mm2 | 3.3 mm | |
| 0.0225 | 0.3176 | 0.0146 | 0.006 | 0.1670 | 0.1126 | 0.1358 | 0.1638 | 0.0439 | 0.2797 | |
| IOM | 22.7 mm | 29.9 mm | 33.2 mm | 7/12 | 15.3 mm | 9.6 mm | 15/4 | 6/10/3 | 181 mm2 | 3.3 mm |
| 22.4 mm | 28.6 mm | 32.7 mm | 6/9 | 17.5 mm | 9.2 mm | 10/5 | 7/6/2 | 203 mm2 | 4.1 mm | |
| 0.8868 | 0.3050 | 0.6266 | 0.8508 | 0.1826 | 0.6561 | 0.4203 | 0.6644 | 0.4804 | 0.4323 |
| PI | PII | PIII | PV | PVI | PVII | PVIII | PX | PXI | |
|---|---|---|---|---|---|---|---|---|---|
| BT | 21.9 mm | 29.0 mm | 32.6 mm | 1/1/6 | 15.28 mm | 9.6 mm | 5/3 | 179 mm2 | 3.6 mm |
| 22.4 mm | 29.5 mm | 33.1 mm | 5/11/15 | 16.5 mm | 9.4 mm | 21/10 | 194 mm2 | 3.6 mm | |
| 0.8416 | 0.7307 | 0.6816 | 0.3711 | 0.5254 | 0.8620 | 0.7792 | 0.6602 | 0.9880 | |
| AT | 21.4 mm | 29.4 mm | 33.7 mm | 0/1/3 | 14.6 mm | 10.7 mm | 4/0 | 185 mm2 | 4.3 mm |
| 22.7 mm | 29.5 mm | 32.8 mm | 4/9/13 | 16.4 mm | 9.8 mm | 16/10 | 192 mm2 | 3.3 mm | |
| 0.6567 | 0.9527 | 0.6007 | 0.5718 | 0.4516 | 0.5598 | 0.1287 | 0.8825 | 0.5674 | |
| IAT | 23.4 mm | 29.8 mm | 32.5 mm | 1/1/1 | 14.9 mm | 8.3 mm | 2/1 | 183 mm2 | 2.7 mm |
| 22.6 mm | 29.5 mm | 33.1 mm | 3/9/14 | 16.5 mm | 10.1 mm | 17/9 | 195 mm2 | 3.6 mm | |
| 0.8157 | 0.8884 | 0.7500 | 0.5637 | 0.5742 | 0.0031 | 0.9647 | 0.9017 | 0.6134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stopa, Ł.; Stopa, W.; Stopa, Z. Correlation between Tomography Scan Findings and Clinical Presentation and Treatment Outcomes in Patients with Orbital Floor Fractures. Diagnostics 2024, 14, 245. https://doi.org/10.3390/diagnostics14030245
Stopa Ł, Stopa W, Stopa Z. Correlation between Tomography Scan Findings and Clinical Presentation and Treatment Outcomes in Patients with Orbital Floor Fractures. Diagnostics. 2024; 14(3):245. https://doi.org/10.3390/diagnostics14030245
Chicago/Turabian StyleStopa, Łukasz, Wojciech Stopa, and Zygmunt Stopa. 2024. "Correlation between Tomography Scan Findings and Clinical Presentation and Treatment Outcomes in Patients with Orbital Floor Fractures" Diagnostics 14, no. 3: 245. https://doi.org/10.3390/diagnostics14030245
APA StyleStopa, Ł., Stopa, W., & Stopa, Z. (2024). Correlation between Tomography Scan Findings and Clinical Presentation and Treatment Outcomes in Patients with Orbital Floor Fractures. Diagnostics, 14(3), 245. https://doi.org/10.3390/diagnostics14030245






