Abstract
Background: An accurate status determination of breast cancer biomarkers (ER, PR, HER2, Ki67) is crucial for guiding patient management. The “gold standard” for assessing these biomarkers in FFPE tissue is IHC, which faces challenges in standardization and exhibits substantial variability. In this study, we compare the concordance of a new commercial RT-qPCR kit with IHC in determining BC biomarker status. Methods: The performance was evaluated using 634 FFPE specimens, which underwent histological analysis in accordance with standard of care methods. HER2 2+ tumors were referred to ISH testing. An immunoreactive score of ≥2/12 was considered positive for ER/PR and 20% staining was used as a cut-off for Ki67 high/low score. RT-qPCR and results calling were performed according to the manufacturer’s instructions. Results: High concordance with IHC was seen for all markers (93.2% for ER, 87.1% for PR, 93.9% for HER2, 77.9% for Ki67 and 80.1% for proliferative signature (assessed against Ki67 IHC)). Conclusions: By assessing the concordance with the results obtained through IHC, we sought to demonstrate the reliability and utility of the kit for precise BC subtyping. Our findings suggest that the kit provides a highly precise and accurate quantitative assessment of BC biomarkers.
Keywords:
APIS Breast Cancer Subtyping Kit; breast cancer; clinical performance; ERBB2; HER2-low; ESR1; PGR; MKI67; FFPE; molecular subtyping; RT-qPCR 1. Introduction
In clinical practice, the molecular subtyping of breast cancer (BC) relies on immunohistochemistry (IHC) to measure the protein expression of estrogen receptor ER/ESR1, progesterone receptor PR/PGR, human epidermal growth factor receptor 2 HER2/ERBB2, and marker of proliferation Ki67/MKI67 [1]. By providing prognostic and predictive information, these biomarkers play a critical role in optimizing treatment outcomes [2,3,4,5].
Concerns regarding the accuracy and reproducibility of IHC testing persist due to high inter-and intra-laboratory variability, with ER and HER2 testing yielding up to 21.4% false-negative and up to 14.5% false-positive results, respectively [6]. To enhance reliability, guidelines have been introduced for HER2 and ER/PR testing [7,8], but challenges remain with the Ki67 marker’s inconsistent assessment, hindering its precise, routine diagnostic use for classifying breast carcinomas into Luminal A and Luminal B subtypes. Variability, even when using the same antibody and when assessed by the same pathologist, underscores the need for standardization in Ki67 evaluation [9], as misclassification can have significant clinical implications. Consequently, alternative assays that offer enhanced robustness, standardization, and precision in assessing the expression of Ki67, HER2, ER, and PR are of benefit.
To address this need, the APIS BC Subtyping Kit has been developed. This in vitro molecular diagnostic test employs RT-qPCR to precisely determine the mRNA expression levels of ESR1, PGR, ERBB2 and MKI67. By determining the status (positive/high or negative/low) of these crucial markers, the kit accurately classifies the tumor into a specific molecular subtype. The kit also detects three additional proliferative markers, generating a proliferative score encompassing all cell cycle stages. Data analysis is performed using accessible web-based software, presenting a clear and informative output for all marker results. With automated result calling, the need for pathologists to interpret results is reduced, thereby alleviating their increasing workload [10]. Consequently, this streamlined process facilitates faster clinical decision-making for oncologists.
Herein, we report the development and validation of the APIS BC Subtyping Kit, assessing clinical performance, reliability, and reproducibility across numerous testing sites. The APIS BC Subtyping Kit represents a promising advancement towards standardized, precise, and reliable assessment of ER, PR, HER2, and Ki67 expression, holding the potential to improve breast cancer diagnostic pathways.
2. Materials and Methods
2.1. Clinical Performance Evaluation
2.1.1. Specimen Collection
A total of n = 920 invasive BC formalin-fixed paraffin-embedded (FFPE) specimens obtained through core needle biopsy (CNB) or resection were collected in two cohorts (cohort 1 N = 650; cohort 2 N = 270) at a single institution (MVZ Prof. Dr. med. A. Niendorf Pathologie Hamburg-West GmbH) and processed in accordance with approval from the Ethics Committee (EC) of the Hamburg Medical Association (Ethics Approval Vote PV2946). All specimens were collected from different patients.
2.1.2. Pathological Evaluation
Tumor grading, tumor typing, and immunohistochemistry (ER, PR, HER2, Ki67) (IHC) were performed for all specimens by MVZ Prof. Dr. med. A. Niendorf Pathologie Hamburg-West GmbH. IHC was performed and analyzed by two board-certified pathologists according to the institute’s standardized protocols and observing the ASCO/CAP guidelines [7,8]. Tumors with an HER2 score of 2+ were referred to in situ hybridization (ISH) to determine ERBB2 amplification, with the ISH result replaced the IHC result in determining the HER2 status (positive/negative) of the tumor. Tumors with an immunoreactive score (IRS) of ≥2/12 for ER/PR by Remmele and Stegner were considered positive. The score was determined by factoring in both the percentage of positive cells and the intensity of the staining reaction [11]. Ki67 expression was considered high when ≥20% of the nuclei stained positively.
2.1.3. RNA Extraction
Total RNA was extracted from an at least 6 µm FFPE tissue section with a tumor content ≥ 20%, using the RNeasy® DSP FFPE Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instruction. The RNeasy DSP FFPE Kit was validated by APIS as an RNA extraction method to be used in conjunction with the APIS BC Subtyping Kit. For each specimen, RNA within the eluate was fluorometrically quantified using Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), normalized to 2.5 ng/µL and stored at −80 °C until use.
2.1.4. Gene Expression by RT-qPCR
The mRNA expression levels of ESR1, PGR, ERBB2, and MKI67, as well as three additional targets encompassing the proliferative signature (CCNA2, KIF23, and PCNA) and two reference genes (IPO8 and PUM1), were determined by RT-qPCR using the APIS BC Subtyping Kit (APIS Assay Technologies, Manchester, UK).
The APIS BC Subtyping Kit comprises four reaction mixes, each consisting of up to three assays (pairs of primers and a probe specific to the respective target sequence). Each assay is labeled with target-specific fluorophores, allowing for the simultaneous detection of all targets. RT-qPCR was performed using the QuantStudio™ 5 Dx real time PCR system (QS5™Dx; Thermo Fisher Scientific). An instrument-specific locked run template was used to ensure that correct reaction settings were applied, as per the APIS BC Subtyping Kit Instructions for Use.
Each patient specimen was analyzed with each assay in duplicate using 10 ng of RNA per reaction. Each RT-qPCR included up to 10 patient specimens and 1 replicate of positive and negative control per assay.
Normalized gene expression levels (ΔCt) for each specimen were determined by subtracting the average cycle threshold (Ct) value of the duplicate measurements of the target of interest from the mean Ct value of the duplicate measurements of the reference genes.
Binary target calls (positive/negative) are based on clinically validated target- and device-specific ΔCt cut-off values. ΔCt cut-off values were established by employing a binary classifier logistic regression model trained using the ΔCt expression data as input and the IHC status input as the binary classifier. The value that achieved an equal balance between the sensitivity and specificity of the assay, and demonstrated the highest performance, was chosen as the ΔCt cut-off for each target.
A logistic model using ΔCt values of MKI67, CCNA2, PCNA, and KIF23 was used to calculate a proliferation score (between 0 and 1); a value less than 0.5 was reported as low proliferation, and greater than 0.5 as high proliferation. The combination of ESR1, PGR, ERBB2, and MKI67 status was used to determine the molecular subtype, based on a definition by the St Gallen working group [12] (Table 1).

Table 1.
Subtype calling algorithm (St Gallen 2013). Gene names associated with each subtype are presented in italics.
2.1.5. Statistical Analysis
The run validity and result calling were performed using APIS BC Subtyping Kit analysis software (APIS Assay Technologies). The software uses the QS5 Dx result file, providing run and sample validity information, normalized gene expression levels (ΔCt), binary marker status, proliferation measurements, and tumor subtype.
The agreement between the APIS BC Subtyping Kit and the reference method was evaluated by constructing 2 × 2 cross-tables, and calculating the overall percent agreement (OPA), positive percent agreement (PPA; diagnostic sensitivity), negative percent agreement (NPA; diagnostic specificity), positive predictive value (PPV), and negative predictive value (NPV), alongside their respective two-sided 95% confidence intervals (95% CIs). JMP 16.1.0 software (SAS® Institute) was used for all statistical analyses.
2.2. Analytical Precision and Reproducibility
2.2.1. Study Design
The precision of the APIS BC Subtyping Kit was determined in accordance with the standard methods provided in CLSI EP05-A3 guidelines [13]. The study was carried out across three testing sites: APIS Assay Technologies (site 1), and two independent molecular laboratories—MDNA Life Sciences UK Ltd. (Site 2; Gateshead, UK) and The North East Innovation Lab, Newcastle upon Tyne Hospitals NHS Foundation Trust (site 3). Prior to the study start, all operators were trained and deemed proficient in the use of the APIS BC Subtyping Kit. During the study, each site performed repeated measurements with the APIS BC Subtyping Kit according to the predefined study plan. At each site, two different APIS BC Subtyping Kit lots were tested, with a total of three kit lots used across all sites. The study comprised five non-consecutive days for sites 2 and 3, and 10 non-consecutive days for site 1, resulting in a generation of 100 measurements per sample. Full details of the study design can be found in Supplementary File S1.
2.2.2. Samples
The samples used in this study were prepared by extracting total RNA from clinical FFPE breast cancer tissue blocks, as described above. A total of six RNA samples were contrived, representing negative, low-positive and mid-positive expression levels for each target. Samples were divided into single use aliquots and distributed to all study sites.
2.2.3. Gene Expression by RT-qPCR
The mRNA expression levels of each target were measured as described above. Each sample was measured in a single replicate. Binary target calls were determined for each measurement.
2.2.4. Statistical Analysis
The precision and reproducibility of the APIS BC Subtyping Kit were assessed on binary (single marker status) and semi-quantitative (∆Ct) levels. To assess the precision of single-marker status calling (positive/high; negative/low), the proportion of results in agreement with the expected target call was reported alongside corresponding 95% CIs. The output of the proliferative signature was also used to summarize the hit rate for the proliferative score, using Ki67 status as a reference.
For quantitative precision assessment, variance component analysis was carried out using JMP 16.1.0 software (SAS Institute Inc., Cary, NC, USA), utilizing main effects models and the Restricted Maximum Likelihood (REML) estimates only. The factors were nested in the following order: Site, Operator, Instrument, Lot, and Run/Day. The variability associated with each factor was reported in terms of standard deviation (SD), and the percent of total variance, where the variability corresponding to each component is calculated as a percentage out of the sum of all variance components. Estimates of reproducibility were based on the analysis of the full dataset (three sites). Repeatability was generated using replicate measurements of the same samples using an identical PCR layout, with two operators and two instruments, at Site 1 over 10 days.
3. Results
3.1. Clinical Performance Evaluation
3.1.1. Patient Population
Out of the 920 patient specimens, 229 were excluded from further analysis due to not meeting inclusion criteria (the reasons for withdrawal are detailed in Supplementary File S2). The inclusion criteria consisted of confirmed invasive breast cancer specimens (CNB or resected FFPE tissue) with >20% tumor content, not older than 18 months, and with documented ethics and informed consent or a waiver for research. An additional 54 specimens were excluded due to insufficient RNA. Testing with the APIS BC Subtyping Kit was conducted at two different locations: APIS Assay Technologies (259 specimens; cohort 2) and MVZ Prof. Dr. med. A. Niendorf Pathologie Hamburg-West GmbH (378 specimens; cohort 1) (Figure 1).
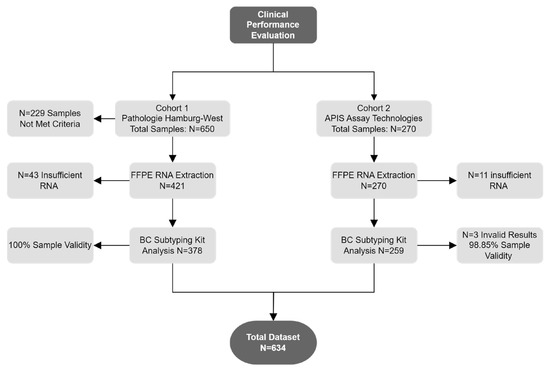
Figure 1.
Schematic of specimens used for clinical performance evaluation.
The characteristics of cohort 1 and cohort 2 patient populations were broadly similar for histological type and grade in order to keep the confounding factors to a minimum. Cases included invasive breast cancers of the histological subtypes NST (no special type, ductal carcinoma) and lobular carcinoma, of low, intermediate, or high grade, with or without expression of the estrogen and/or progesterone receptor, of HER2 positive or negative status, and of high or low proliferation rate (Ki67) to avoid sample bias. Due to a low prevalence in the study population, HER2-positive tumors make up the smallest group of patients in cohort 1. Cohort 2 comprised only CNB specimens. Within cohort 1, 62.4% of specimens were collected via CNB and 37.6% via resection. The basic clinicopathological characteristics of the tumors used for analysis are listed in Table 2.

Table 2.
Clinicopathological characteristics of the specimens used for analysis from cohort 1 and cohort 2. Results for ER, PR, HER2, and KI67 as listed in this table are reported based on immunohistochemistry.
3.1.2. Agreement between APIS Breast Cancer Subtyping Kit and IHC
The overall agreement between mRNA expression levels determined by APIS BC Subtyping Kit and IHC is shown in Table 3. A strong correlation between ∆Ct values and the percentage of staining with IHC was observed for ESR1/ER (R2 = 0.664, p < 0.0001) (Figure 2A) and PGR/PR (R2 = 0.663, p < 0.0001) (Figure 2B). A moderate level of correlation was noted between MKI67 ΔCt and the percentage of staining with IHC (R2 = 0.414, p < 0.0001) (Figure 2C), and between Ki67 IHC staining and proliferation score (R2 = 0.472, p < 0.0001) (Figure 2D). A clear stratification of ΔCt by HER2 IHC status was noted (Figure 3). Patients assigned HER2 2+ status were classified into negative and positive groups by the APIS BC Subtyping Kit in line with the results obtained from ISH testing. This result is of utmost importance, as the anti-HER2 neo-adjuvant therapy decision is based on the results provided by this test. The APIS BC Subtyping Kit accurately detects HER2 expression (with IHC 2+/ISH+ and IHC 3+ cases defined as positive). ERBB2 mRNA expression was detected by the APIS BC Subtyping Kit in a subset of patients with 0 and 1+ IHC HER2 scores, highlighting the continuous nature of ERBB2 expression, and providing an opportunity to enhance HER2 stratification into a HER2-low category. Notably, the majority of cases showing disagreement between ER and PR calls made by APIS BC Subtyping Kit and IHC status were found to be in close proximity to their respective ΔCt cut-off values. Considering the binary nature of positive and negative results for ER and PR and the inherent variability of quantification methods, the biological implications of this discordant calling require further investigation.

Table 3.
Contingency tables and agreement analysis for results generated by the APIS BC Subtyping Kit compared to results by IHC/ISH for each marker. Two MKI67 and 20 ERBB2 cases were excluded from the individual marker analysis due to lack of IHC and ISH data, respectively. Gene names and protein names are presented in italics and normal text, respectively.
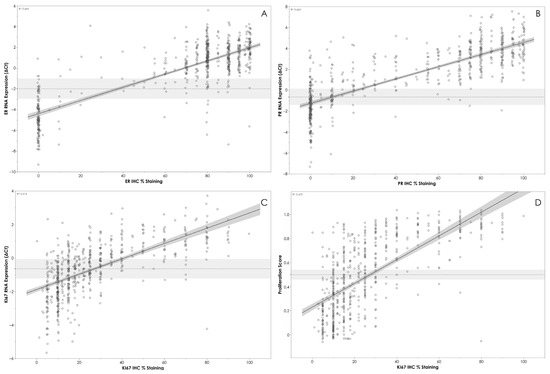
Figure 2.
(A) APIS BC Subtyping Kit results (ΔCt) for ER plotted against ER IHC %. (B) APIS BC Subtyping Kit results (ΔCt) for PR plotted against PR IHC %. (C) APIS BC Subtyping Kit results (ΔCt) for KI67 plotted against KI67 IHC %. (D) APIS BC Subtyping Kit Proliferation Score plotted against KI67 IHC %. Individual target cut-offs are indicated by the dashed line. Shading around cut-off lines indicates assay’s precision. The majority of discordant calls are close to the assay cut-off.
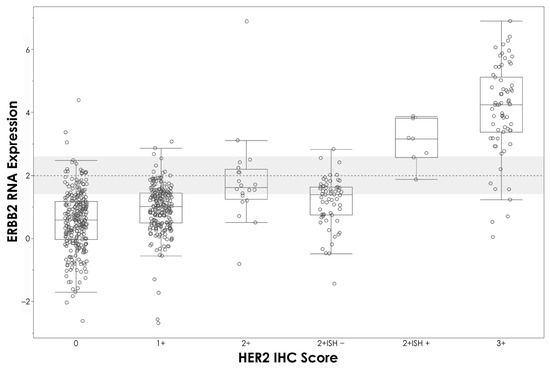
Figure 3.
APIS BC Subtyping Kit results (ΔCt) plotted against HER2 IHC score. HER2 cut-off is indicated by the dashed line.
3.1.3. Subtype Calling Agreement
For each tumor, the subtype call by the APIS BC Subtyping Kit was compared to the subtype assigned by the pathologist, based on the IHC/ISH test results. A high overall percent agreement for every subtype was shown (71.59%). It is important to note that this result is most likely driven by the differences in MKI67 RNA and Ki67 protein expression. Indeed, when taking into consideration ER, PR, and HER2 status only, the overall percent agreement in subtype calls was shown to be notably higher (89.56%). The PPA, NPA, and OPA for each subtype, along with 95% CIs, are shown in Table 4 and the full breakdown of subtype calling by each method is displayed in Figure 4.

Table 4.
Contingency tables and agreement analysis for subtype calls generated by the APIS BC Subtyping Kit compared to calls made by pathologists based on IHC/ISH results.
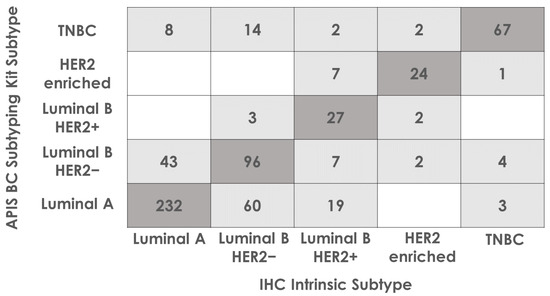
Figure 4.
Subtype calling agreement between APIS BC Subtyping Kit and the intrinsic subtype determined by the pathologist based on IHC results. Eleven cases have been removed due to having no assigned IHC subtype. Cases where APIS BC Subtyping Kit calls agreed with IHC intrinsic subtypes are highlighted in dark grey. The remaining cases are highlighted light grey.
3.1.4. Agreement between CNB and Resected FFPE Specimens
The clinical performance of the APIS BC Subtyping Kit was assessed with both sample types: CNB (N = 492) and resection (N = 142). All specimens were collected from different patients. Overall, an agreement of over 85% was achieved for both CNB and resected sample types for ER, PR, and HER2. Performance was comparable for the two sample types, across all markers (Table 5).

Table 5.
Contingency tables and agreement analysis for results from the APIS BC Subtyping Kit compared to results from IHC/ISH for each marker for the dataset as split by sample type (CNB and resected tissue). Gene names and protein names are presented in italics and normal text, respectively.
3.2. Analytical Precision and Reproducibility
3.2.1. Analytical Precision
Analytical precision was calculated, taking into consideration data generated by Site 1. The variability attributed to factors such as operator, instrument, kit lot, and run (including between-day variability) was assessed and reported as standard deviation (SD) and as a percentage of the total variance. These percentages represent the contribution of each factor to the overall variance (Table 6).

Table 6.
ΔCt values and variability components attributed to each variable. Reported in terms of SD and %Total Variance. Analysis of data generated by Site 1—APIS Assay Technologies.
3.2.2. Inter-site Reproducibility
Between-site reproducibility was calculated across all three sites involved in the study. The primary source of variability was the replicates within each run, while inter-instrument variance had minimal impact on assay variation. The between-site analysis revealed excellent reproducibility in quantitative measurements, with a total standard deviation (SD) ranging from 0.00 to 0.22 for positive samples (Table 7).

Table 7.
Total variance and standard deviation (SD) for ΔCt across all study factors (site, operator, instrument, lot, and run).
3.2.3. Binary Single Marker Agreement
The proportion of results in agreement with the expected result was reported along with the corresponding 95% CIs displaying excellent concordance (Table 8); 100% reproducibility was achieved for ER, PR, and HER2 for all tested samples (negative/low-positive/mid-positive), and for most Ki67 samples (87% agreement for negative samples).

Table 8.
APIS BC Subtyping Kit binary precision—proportion of correct calls for each target and proliferation score across all sites.
4. Discussion
The accurate and reliable determination of ER, PR, HER2, and Ki67 expression is crucial for precise breast cancer diagnosis and effective patient management. However, the reliability of current “gold standard” methods such as IHC, particularly for Ki67, is limited due to significant variations observed within and between laboratories [9]. To overcome these limitations, the APIS BC Subtyping Kit aims to determine the status of these markers accurately and consistently, enabling the delivery of precise and effective patient management decisions.
Indeed, the APIS BC Subtyping Kit has demonstrated a strong agreement with IHC for all markers, exhibiting excellent accuracy and repeatability. Furthermore, its performance across multiple testing sites highlights the potential to enhance the reproducibility of assessing these markers in a diagnostic setting. The ability of the APIS BC Subtyping Kit to achieve highly repeatable and reproducible results is advantageous compared to current methods that rely on IHC and are prone to inconsistencies. The APIS BC Subtyping Kit minimizes the reliance on highly trained and experienced personnel for accurate results interpretation, thus improving the efficiency and reliability of breast cancer management.
Our study’s limitations include its retrospective design, relying on specimens procured from a singular institution across two cohorts. Despite this, no substantial differences were noted between these cohorts. Cohort one was curated to maintain an even representation of all BC subtypes, whereas cohort two aimed to mirror the real-world distribution of BC subtypes, inadvertently yielding a greater number of HER2-negative specimens compared to HER2-positive specimens.
Here, we found that the APIS BC Subtyping Kit exhibited a diagnostic sensitivity (PPA) of over 90% for ESR1, PGR, and ERBB2. We found that the discrepancies were mainly seen in tumors with RNA levels close to the ∆Ct cut-offs and lower protein expression detected by IHC. This finding underscores the significance of employing semi-quantitative methods in result reporting, especially since previous studies indicated that low-ER-expressing tumors often present characteristics more similar to ER-negative cancers. As is still not clear what clinical implications these discrepancies may have, it is important to incorporate clinicopathological characteristics, such as grade, number of nodes, and tumor size, in patient management decisions [14]. Further explorations of the links between semi-quantitative mRNA expression and treatment response are required to demonstrate its clinical value for therapeutic decision-making.
Higher discordance was observed for PGR/PR measurements, which aligns with the heterogeneous distribution of PR staining and the resulting increased variability across different tissue sections [15]. These discrepancies are likely attributed to measurement uncertainty and tumor heterogeneity. It is important to note that due to the nature of the assay, RT-qPCR was performed on a separate section that, although spatially close to the section used for IHC, inherently exhibits heterogeneity. Alternative RT-qPCR-based kits already available on the market utilizing RNA extracted from the same tissue section could be used for comparison, helping to address potential discrepancies arising from tumor heterogeneity.
The agreement between IHC and RT-qPCR for MKI67/Ki67 was moderate with a diagnostic sensitivity (PPA) of 79.86%, indicating that expression at the RNA level does not always translate to observable protein by IHC. However, Ki67 staining reproducibility has been widely discussed in the literature [9,16], with significant inter- and intra-laboratory variability observed for this marker. Challenges of IHC processing and analytical issues in scoring IHC slides have resulted in a “Grey Zone” for Ki67 scoring being suggested, where only IHC values below 5% or above 30% should be considered as low or high expression, respectively, as reproducibility within this range is particularly poor [16]. A substantial overlap in RT-qPCR results between tumors categorized as high and low with IHC can be observed, although the populations are clearly distinct, as higher ΔCt values correspond to increased IHC % staining. Consistent with previous studies, most of the discordant cases for this marker were identified as false positive (i.e., false high) cases [15].
It is worth noting that Ki67 IHC analysis might selectively focus on staining hotspots during image analysis, whereas RT-qPCR evaluates the average MKI67 score across an entire FFPE section [17]. This averaging process across the section may contribute to the differences observed between mRNA and IHC results.
The current routine diagnostic assessment for BC markers involves the use of IHC, and when necessary, additional reflex testing for HER2 using ISH [8]. However, in the clinical setting, there is a clear need for a rapid and accurate testing method. Notably, the APIS BC Subtyping Kit provides a single-resolution ERBB2/HER2 detection method, removing the requirement for additional reflex testing and thus providing valuable information to healthcare providers in a rapid manner, reducing overall turnaround time and aiding fast patient management decisions. As demonstrated in Figure 3, the APIS BC Subtyping Kit correctly assigned positive status to 6 out of 7 HER2 2+ ISH positive tumors, and negative status to 57 out of 62 HER2 2+ ISH negative tumors.
The quantification of HER2-low in breast cancer has garnered considerable interest due to emerging evidence of the efficacy of targeted therapies in this patient subgroup [18]. Trastuzumab deruxtecan (T-Dxd), an antibody-drug conjugate (ADC) targeted at HER2, has recently gained approval in the USA and Europe for treating HER2-low breast cancer, which is currently defined as IHC scores of 1+ or 2+ without ERBB2 ISH amplification. However, data from the DAISY phase II trial reported a response to T-Dxd treatment of 29.7% in tumors with HER2 IHC 0 score (vs. 37.5% in HER2-low patients) [19]. In fact, our dataset shows a substantial overlap in ERBB2 expression across all HER2 “negative” tumors, including those classified as HER2 IHC 0 score, highlighting the continuous nature of ERBB2 expression and higher sensitivity of RT-qPCR-based detection approaches. Furthermore, these results confirm that IHC stratification may not be an appropriate method for predicting the response to novel anti-HER2 therapies, such as T-Dxd. Understanding the correlation between APIS BC Subtyping Kit ERBB2 mRNA expression and the response to anti-HER2 treatments could aid in stratifying patients into responder and non-responder groups, potentially reducing unnecessary severe side effects and offering a more personalized treatment approach. The implementation of additional ∆Ct cut-offs could allow the further stratification of ERBB2 expression into negative, low, and high status; however, in order to validate this approach, additional studies showing a correlation of the expression level to anti-HER2 treatment response would be required.
The inclusion of the novel proliferation signature within the kit presents a potential avenue for advancing the clinical utility of MKI67 in the measurement of tumor proliferation. Proliferation status is a commonly used factor in determining the need for chemotherapy in patients with luminal breast cancer, in cases where patients have low-risk features such as node status [18]. The inclusion of three additional targets (CCNA2, PCNA, and KIF23) within the APIS BC Subtyping Kit, known to be associated with breast cancer proliferation [20,21,22,23,24] and expressed across all cell cycle stages, allows for the generation of a more complete proliferation measure. While this score is correlated with Ki67 staining, establishing a correlation between RNA measurement and clinical outcome would provide valuable information. The ability to consistently determine tumor proliferation would be a valuable addition to the pathology evaluation of breast cancer patients, providing clinicians with a reliable measure of proliferation. Various multigene tests addressing this issue exist. Oncotype Dx® (Exact Sciences, Madison, WI, USA), Prosigna® (NanoString Technologies, Seattle, WA, USA), EndoPredict® (Myriad Genetics, Salt Lake City, UT, USA), and MammaPrint® (Agendia, Irvine, CA, USA) provide risk scores with prognostic information for distant recurrence in patients with Luminal A and Luminal B tumors. The risk determined by these tests (high/low) is often used to guide chemotherapy treatment decisions [25]. However, these tests are often centralized and very expensive, and therefore only used as an additional test for those cases that require further assessment.
The exploration of the prognostic potential of the APIS BC Subtyping Kit’s proliferation signature by testing its utility in predicting treatment outcomes and identifying subtypes in comparison to the commercially available tests will be important to provide a centralized and accessible cost-effective alternative.
Subtype calls by the APIS BC Subtyping Kits demonstrated an overall percent agreement for every subtype of over 75%. The lowest performance was identified in distinguishing Luminal B HER2- and Luminal A subtypes. The primary distinction between these two subtypes revolves around the expression level of Ki67. As previously discussed, Ki67 IHC results should be interpreted with caution; rather than distinct high and low populations, Ki67 expression (both at the protein and mRNA level) shows a considerable overlap (Figure 2C). Given this uncertainty in distinguishing between Ki67 high and low expression, some ambiguity should also be anticipated when differentiating Luminal B HER2- and Luminal A tumors. Different breast cancer subtypes exhibit different clinicopathological features, recurrence patterns, and survival outcomes. Molecular subtyping, based on biomarker status, can provide clinical information that could be used to facilitate breast cancer management decisions.
Once validated, the APIS BC Subtyping Kit could provide information on individual biomarker status, BC subtype, and recurrence risk in one test. This would distinguish it from the aforementioned tests, which do not always report individual biomarker status, and therefore cannot be used for the initial biomarker assessment. Future studies are required to compare the subtype calls generated by the APIS BC Subtyping test and other molecular classifiers (e.g., the Prosigna test).
The APIS Breast Cancer Subtyping Kit can provide information about the predictive and prognostic power of the examined parameters, especially on small biopsy material in, e.g., neoadjuvant settings with planned cytotoxic chemotherapy or concerning proliferation rate in endocrine challenge (neoadjuvant endocrine therapy).
5. Conclusions
The APIS Breast Cancer Subtyping Kit provides a safe, highly accurate, and reproducible method for the assessment of breast cancer biomarkers and molecular subtypes in patients with invasive breast cancer, using resected and CNB FFPE tissues. The kit proved its reliability when used by clinical scientists in real clinical settings, exhibiting potential to improve the quality of primary breast cancer diagnostics. By providing results in a timely manner, the APIS BC Subtyping Kit holds potential to improve routine practice, by saving pathologists time. Results of immunohistochemistry could be validated and controlled (also for the purpose of quality assurance) by the APIS BC Subtyping Kit or even be replaced in settings where routine IHC diagnostic is not feasible or where results could be provided faster using the APIS BC Subtyping Kit.
Further clinical investigations are required to validate the APIS BC Subtyping Kit’s prognostic potential and establish appropriate thresholds for the semi-quantitative evaluation of ERBB2/HER2. The reproducibility and semi-quantitative capabilities of the APIS BC Subtyping Kit could offer researchers a valuable opportunity to uncover meaningful associations between marker expression and treatment response, potentially improving personalized management strategies for breast cancer patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14030241/s1, File S1: Repeatability and Reproducibility Study Design; File S2: Withdrawn Samples.
Author Contributions
Conceptualization, A.N., S.R. and Z.P.; methodology, S.R., L.G. and N.D.; software, A.G (Agustin Guardiola).; validation, All.; formal analysis, S.R, A.G. (Agustin Guardiola), and A.G. (Anna Gasior); investigation, A.N.; resources, All.; data curation, D.H., A.-S.W., Z.P., A.G. (Agustin Guardiola) and A.N.; writing—original draft preparation, A.-S.W. and A.G. (Anna Gasior); writing—review and editing, A.-S.W., A.G. (Anna Gasior), J.G. and A.N.; visualization, A.G. (Anna Gasior); supervision, A.N.; project administration, D.H. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Hamburg Medical Council (PV 2946; date of approval: 22 May 2008 [initial application], 26 February 2015 [update of study documents and continuation]).
Informed Consent Statement
Patient consent was waived in line with the ethical approval.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We would like to thank colleagues at Pathologie-Hamburg West who have contributed to the generation of the data through RT-qPCR, IHC, and histological review of tissue. We would also like to thank the North East Innovation Hub at NHS Newcastle Upon Tyne Hospitals and MDNA Life Sciences for conducting the BC Subtyping Kit reproducibility study.
Conflicts of Interest
Authors J.G., S.R., L.G., N.D., A.G. (Agustin Guardiola), A.G. (Anna Gasior), and Z.P. are employed by APIS Assay Technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Perou, C.M.; Sorlie, T.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; Fluge, O.; et al. Molecular portraits. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Bevers, T.B.; Helvie, M.; Bonaccio, E.; Calhoun, K.E.; Daly, M.B.; Farrar, W.B.; Garber, J.E.; Gray, R.; Greenberg, C.C.; Greenup, R.; et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Penault-Llorca, F.; Poortmans, P.; Thompson, A.; Zackrisson, S.; Cardoso, F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi7–vi23. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, M.; Floris, G.; Galant, C.; Lambein, K.; Libbrecht, L. A plea for appraisal and appreciation of immunohistochemistry in the assessment of prognostic and predictive markers in invasive breast cancer. Breast 2018, 37, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 2010, 134, e48–e72. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Hanna, W.; Jenkins, R.B.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef]
- Focke, C.M.; Bürger, H.; van Diest, P.J.; Finsterbusch, K.; Gläser, D.; Korsching, E.; Decker, T.; Anders, M.; Bollmann, R.; Eiting, F.; et al. Interlaboratory variability of Ki67 staining in breast cancer. Eur. J. Cancer 2017, 84, 219–227. [Google Scholar] [CrossRef]
- Märkl, B.; Füzesi, L.; Huss, R.; Bauer, S.; Schaller, T. Number of pathologists in Germany: Comparison with European countries, USA, and Canada. Virchows Arch. 2021, 478, 335–341. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Precision of Quantitative Measurement Procedures, Approved Guideline, 3rd ed.; CLSI Document EP05-A3; Clinical Laboratory Standards Institute: Berwyn, PA, USA, 2014; Volume 34, p. 13. [Google Scholar]
- Yoder, R.; Kimler, B.F.; Staley, J.M.; Schwensen, K.; Wang, Y.Y.; Finke, K.; O’dea, A.; Nye, L.; Elia, M.; Crane, G.; et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer 2022, 8, 80. [Google Scholar] [CrossRef]
- Caselli, E.; Pelliccia, C.; Teti, V.; Bellezza, G.; Mandarano, M.; Ferri, I.; Hartmann, K.; Laible, M.; Sahin, U.; Varga, Z.; et al. Looking for more reliable biomarkers in breast cancer: Comparison between routine methods and RT-qPCR. PLoS ONE 2021, 23, e0255580. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. JNCI J. Natl. Cancer Inst. 2020, 113, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer Working group. JNCI J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 23, 66. [Google Scholar] [CrossRef]
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: The phase 2 DAISY trial. Nat. Med. 2023, 29, 2110–2120. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Y.; Mi, Y.; Gu, L.; Sang, M.; Geng, C. Identification of core genes and potential molecular mechanisms in breast cancer using bioinformatics analysis. Pathol.-Res. Pract. 2019, 215, 152436. [Google Scholar] [CrossRef]
- Li, T.-F.; Zeng, H.-J.; Shan, Z.; Ye, R.-Y.; Cheang, T.-Y.; Zhang, Y.-J.; Lu, S.-H.; Zhang, Q.; Shao, N.; Lin, Y. Overexpression of kinesin superfamily members as prognostic biomarkers of breast cancer. Cancer Cell Int. 2020, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, T.; Wang, X.; Liao, X.; Han, C.; Yang, C.; Su, K.; Cao, W.; Gong, Y.; Chen, Z.; et al. Distinct Diagnostic and Prognostic Values of Kinesin Family Member Genes Expression in Patients with Breast Cancer. J. Pharmacol. Exp. Ther. 2018, 24, 9442–9464. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, G.; Xiao, Y.; Zhang, T.; Su, F.; Chang, R.; Ling, X.; Bai, Y. Upregulated cyclins may be novel genes for triple-negative breast cancer based on bioinformatic analysis. Breast Cancer 2020, 27, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.N.; Ismaila, N.; McShane, L.M.; Andre, F.; Collyar, D.E.; Gonzalez-Angulo, A.M.; Hammond, E.H.; Kuderer, N.M.; Liu, M.C.; Mennel, R.G.; et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women with Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 1134–1150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).