17β-Estradiol Effects in Skeletal Muscle: A 31P MR Spectroscopic Imaging (MRSI) Study of Young Females during Early Follicular (EF) and Peri-Ovulation (PO) Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Characterization, Protocol Approvals, and Consent
2.2. MRS Protocol
2.3. Data Analysis
2.4. Evaluation of Intracellular pH
2.5. Evaluation of Intracellular Mg2+
2.6. Analysis of 17β-Estradiol
2.7. Statistical Analysis
3. Results
3.1. Voxel 31P MRSI Spectra
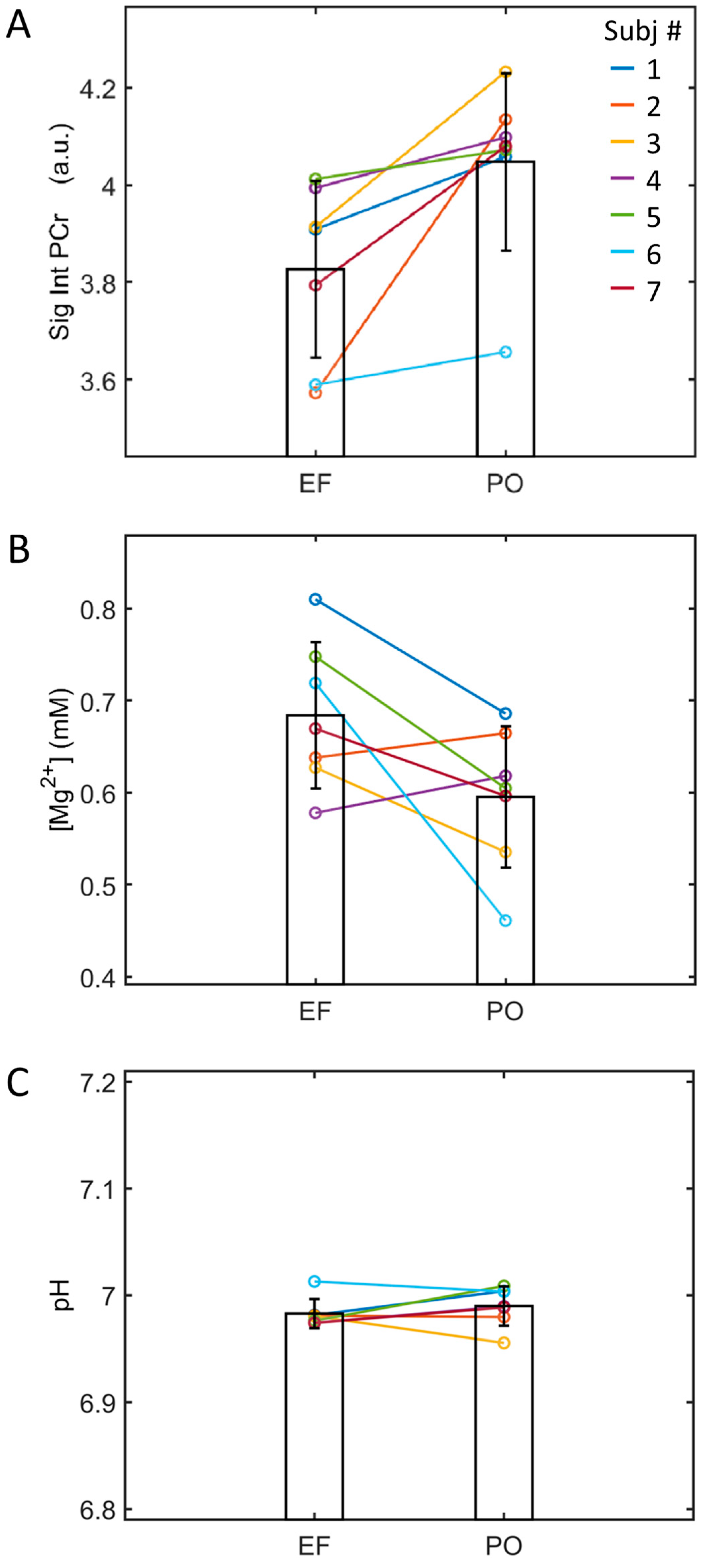
3.2. EF vs. PO 31P Spectra
3.3. EF and PO Difference in 17β-Estradiol
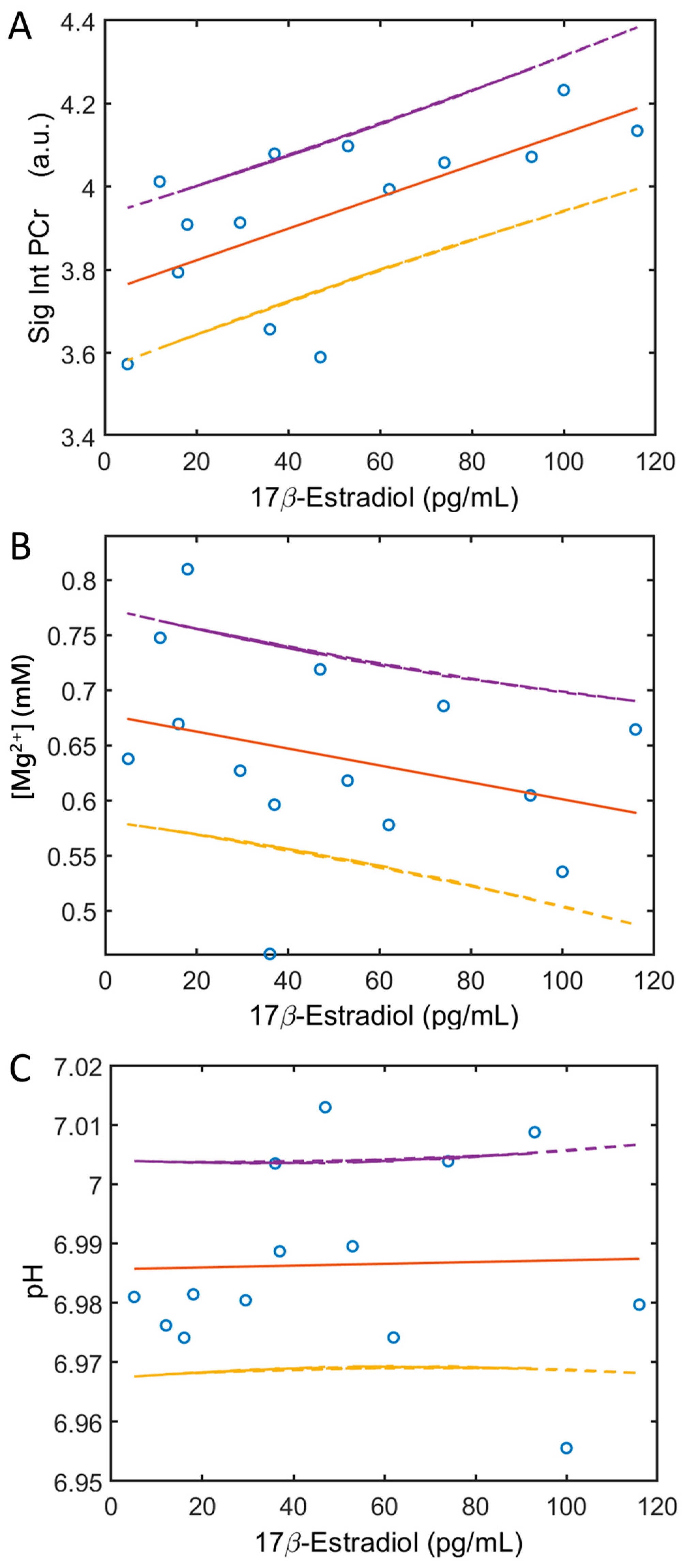
3.4. Blood 17β-Estrogen and Soleus 31P MRS Correlation
3.5. EF and PO Metabolite Correlation
4. Discussion
4.1. Major Findings
4.2. Role of PCr in Energy Metabolism
4.3. Correlation between PCr and 17β-Estradiol
4.4. Acting Sites of 17β-Estradiol
4.5. Membrane Phospholipids (MPL) Metabolites
4.6. pH and Free Mg Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| BMS | bulk magnetic susceptibility effect |
| Cr | free creatine |
| EF | early follicular |
| ER | estrogen receptors |
| GPC | glycerolphosphocholine |
| GPE | glycerolphosphoehtanolamine |
| HF | header-foot |
| MPL | membrane phospholipids |
| NAD | nicotinamide adenine dinucleotide, a combination of NAD+ and NADH |
| NTP | nucleoside triphosphates |
| PCr | phosphocreatine |
| PC | phosphocholine |
| PE | phosphoethanolamine |
| Pi | inorganic phosphate |
| PDE | phosphodiester |
| PME | phosphomonoester |
| PO | peri-ovulation |
| PTH | parathyroid hormone |
References
- Xia, H.; Scholtes, C.; Dufour, C.R.; Guluzian, C.; Giguère, V. ERRα fosters running endurance by driving myofiber aerobic transformation and fuel efficiency. Mol. Metab. 2023, 78, 101814. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef]
- Wiik, A.; Ekman, M.; Johansson, O.; Jansson, E.; Esbjörnsson, M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem. Cell Biol. 2009, 131, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ekenros, L.; Papoutsi, Z.; Fridén, C.; Dahlman Wright, K.; Lindén Hirschberg, A. Expression of sex steroid hormone receptors in human skeletal muscle during the menstrual cycle. Acta Physiol. 2017, 219, 486–493. [Google Scholar] [CrossRef]
- Wiik, A.; Glenmark, B.; Ekman, M.; Esbjörnsson-Liljedahl, M.; Johansson, O.; Bodin, K.; Enmark, E.; Jansson, E. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol. Scand. 2003, 179, 381–387. [Google Scholar] [CrossRef]
- Lin, G.; Siddiqui, R.; Lin, Z.; Blodgett, J.M.; Patel, S.N.; Truong, K.N.; Mariakakis, A. Blood glucose variance measured by continuous glucose monitors across the menstrual cycle. NPJ Digit Med. 2023, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Alshdaifat, E.; Absy, N.; Sindiani, A.; AlOsta, N.; Hijazi, H.; Amarin, Z.; Alnazly, E. Premenstrual Syndrome and Its Association with Perceived Stress: The Experience of Medical Students in Jordan. Int. J. Womens Health 2022, 14, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The role of estrogen in insulin resistance: A review of clinical and preclinical data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and Bone Mass in Middle-Aged Women: Role of Menopausal Status and Physical Activity. J. Cachexia Sarcopenia Muscle 2020, 11, 698–709. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Zitnay, J.; Kazmers, N.H.; Veerabhadraiah, S.R.; Zelada, A.C.; Honeggar, M.; Chalmers, P.N.; Henninger, H.B.; Jurynec, M.J. Estrogen and testosterone supplementation improves tendon healing and functional recovery after rotator cuff repair. J. Orthop. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, T.; Maki, J.; Ooba, H.; Eto, E.; Takahashi, K.; Kondo, T.; Ikeda, T.; Sakamoto, Y.; Mitsuhashi, T.; Masuyama, H. Protocol for a randomised, placebo-controlled, double-blinded clinical trial on the effect of oestrogen replacement on physical performance to muscle resistance exercise for older women with osteoarthritis of knee joint: The EPOK trial. BMC Geriatr. 2023, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.A.; Aladhami, A.K.; Hope, M.C.; Cotham, W.E.; Nettles, K.W.; Clegg, D.J.; Velázquez, K.T.; Enos, R.T. Skeletal Muscle Endogenous Estrogen Production Ameliorates the Metabolic Consequences of a High-Fat Diet in Male Mice. Endocrinology 2023, 164, bqad105. [Google Scholar] [CrossRef]
- Samad, N.; Nguyen, H.H.; Hashimura, H.; Pasco, J.; Kotowicz, M.; Strauss, B.J.; Ebeling, P.R.; Milat, F.; Vincent, A.J. Abnormal Trabecular Bone Score, Lower Bone Mineral Density and Lean Mass in Young Women with Premature Ovarian Insufficiency Are Prevented by Oestrogen Replacement. Front. Endocrinol. 2022, 13, 860853. [Google Scholar] [CrossRef] [PubMed]
- Chidi-Ogbolu, N.; Baar, K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front. Physiol. 2019, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Beaudart, C.; Buckinx, F.; Bruyère, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Armstrong, V.J.; Muzylak, M.; Sunters, A.; Zaman, G.; Saxon, L.K.; Price, J.S.; Lanyon, L.E. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J. Biol. Chem. 2007, 282, 20715–20727. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Dyke, J.; Schelbaum, E.; Jett, S.; Loughlin, L.; Jang, G.; Rahman, A.; Hristov, H.; Pahlajani, S.; et al. Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci. Rep. 2021, 11, 10867. [Google Scholar] [CrossRef]
- Mosconi, L.; Jett, S.; Nerattini, M.; Andy, C.; Yepez, C.B.; Zarate, C.; Carlton, C.; Kodancha, V.; Schelbaum, E.; Williams, S.; et al. In vivo Brain Estrogen Receptor Expression by Neuroendocrine Aging and Relationships with Gray Matter Volume, Bio-Energetics, and Clinical Symptomatology. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Jett, S.; Dyke, J.P.; Andy, C.; Schelbaum, E.; Jang, G.; Boneu Yepez, C.; Pahlajani, S.; Diaz, I.; Diaz Brinton, R.; Mosconi, L. Sex and menopause impact 31P-Magnetic Resonance Spectroscopy brain mitochondrial function in association with 11C-PiB PET amyloid-beta load. Sci. Rep. 2022, 12, 22087. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.A.; Kristjansen, P.E.; Brünner, N.; Clarke, R.; Spang-Thomsen, M.; Quistorff, B. Effect of estrogen withdrawal on energy-rich phosphates and prediction of estrogen dependence monitored by in vivo 31P magnetic resonance spectroscopy of four human breast cancer xenografts. Cancer Res. 1995, 55, 1664–1669. [Google Scholar] [PubMed]
- Kristensen, C.A.; Askenasy, N.; Jain, R.K.; Koretsky, A.P. Creatine and cyclocreatine treatment of human colon adenocarcinoma xenografts: 31P and 1H magnetic resonance spectroscopic studies. Br. J. Cancer 1999, 79, 278–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, J.; Sherry, A.D.; Malloy, C.R. (31)P-MRS of healthy human brain: ATP synthesis, metabolite concentrations, pH, and T1 relaxation times. NMR Biomed. 2015, 28, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sherry, A.D.; Malloy, C.R. Modular 31 P wideband inversion transfer for integrative analysis of adenosine triphosphate metabolism, T1 relaxation and molecular dynamics in skeletal muscle at 7T. Magn. Reason. Med. 2019, 81, 3440–3452. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yu, F.; Greenberg, B.M. ATP line splitting in association with reduced intracellular magnesium and pH: A brain 31 P MR spectroscopic imaging (MRSI) study of pediatric patients with myelin oligodendrocyte glycoprotein antibody-associated disorders (MOGADs). NMR Biomed. 2023, 36, e4836. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Valkovič, L.; Purvis, L.A.B.; Clarke, W.T.; Rodgers, C.T. Reproducibility of human cardiac phosphorus MRS (31 P-MRS) at 7 T. NMR Biomed. 2019, 32, e4095. [Google Scholar] [CrossRef]
- Niendorf, T.; Paul, K.; Oezerdem, C.; Graessl, A.; Klix, S.; Huelnhagen, T.; Hezel, F.; Rieger, J.; Waiczies, H.; Frahm, J.; et al. W(h)ither human cardiac and body magnetic resonance at ultrahigh fields? technical advances, practical considerations, applications, and clinical opportunities. NMR Biomed. 2016, 29, 1173–1197. [Google Scholar] [CrossRef]
- Matson, G.B.; Twieg, D.B.; Karczmar, G.S.; Lawry, T.J.; Gober, J.R.; Valenza, M.; Boska, M.D.; Weiner, M.W. Application of image-guided surface coil P-31 MR spectroscopy to human liver, heart, and kidney. Radiology 1988, 169, 541–547. [Google Scholar] [CrossRef]
- Boska, M.D.; Meyerhoff, D.J.; Twieg, D.B.; Karczmar, G.S.; Matson, G.B.; Weiner, M.W. Image-guided 31P magnetic resonance spectroscopy of normal and transplanted human kidneys. Kidney Int. 1990, 38, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Lagemaat, M.W.; Maas, M.C.; Vos, E.K.; Bitz, A.K.; Orzada, S.; Weiland, E.; van Uden, M.J.; Kobus, T.; Heerschap, A.; Scheenen, T.W. (31) P MR spectroscopic imaging of the human prostate at 7 T: T1 relaxation times, Nuclear Overhauser Effect, and spectral characterization. Magn. Reson. Med. 2015, 73, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Kobus, T.; Bitz, A.K.; van Uden, M.J.; Lagemaat, M.W.; Rothgang, E.; Orzada, S.; Heerschap, A.; Scheenen, T.W. In vivo 31P MR spectroscopic imaging of the human prostate at 7 T: Safety and feasibility. Magn. Reson. Med. 2012, 68, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Rata, M.; Giles, S.L.; de Souza, N.M.; Leach, M.O.; Payne, G.S. Comparison of three reference methods for the measurement of intracellular pH using 31P MRS in healthy volunteers and patients with lymphoma. NMR Biomed. 2014, 27, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Joncquel-Chevalier Curt, M.; Voicu, P.M.; Fontaine, M.; Dessein, A.F.; Porchet, N.; Mention-Mulliez, K.; Dobbelaere, D.; Soto-Ares, G.; Cheillan, D.; Vamecq, J. Creatine biosynthesis and transport in health and disease. Biochimie 2015, 119, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A. Creatine deficiency syndromes. Handb. Clin. Neurol. 2013, 113, 1837–1843. [Google Scholar]
- Haas, D.; Gan-Schreier, H.; Langhans, C.D.; Anninos, A.; Haege, G.; Burgard, P.; Schulze, A.; Hoffmann, G.F.; Okun, J.G. Diagnosis and therapeutic monitoring of inborn errors of creatine metabolism and transport using liquid chromatography-tandem mass spectrometry in urine, plasma and CSF. Gene 2014, 538, 188–194. [Google Scholar] [CrossRef]
- van den Broek, N.M.; Ciapaite, J.; Nicolay, K.; Prompers, J.J. Comparison of in vivo postexercise phosphocreatine recovery and resting ATP synthesis flux for the assessment of skeletal muscle mitochondrial function. Am. J. Physiol. Cell Physiol. 2010, 299, C1136-43. [Google Scholar] [CrossRef]
- Kan, H.E.; Veltien, A.; Arnts, H.; Nabuurs, C.I.; Luijten, B.; de Haan, A.; Wieringa, B.; Heerschap, A. Gated dynamic 31P MRS shows reduced contractile phosphocreatine breakdown in mice deficient in cytosolic creatine kinase and adenylate kinase. NMR Biomed. 2009, 22, 523–531. [Google Scholar] [CrossRef]
- Hendriks, A.D.; van der Kemp, W.J.M.; Luijten, P.R.; Petridou, N.; Klomp, D.W.J. SNR optimized 31 P functional MRS to detect mitochondrial and extracellular pH change during visual stimulation. NMR Biomed. 2019, 32, e4137. [Google Scholar] [CrossRef]
- van de Bank, B.L.; Maas, M.C.; Bains, L.J.; Heerschap, A.; Scheenen, T.W.J. Is visual activation associated with changes in cerebral high-energy phosphate levels? Brain Struct Funct. 2018, 223, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Pohmann, R.; Speck, O.; Scheffler, K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn. Reson. Med. 2016, 75, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Soedirdjo, S.D.H.; Rodriguez, L.A., 2nd; Chung, Y.C.; Casey, E.; Dhaher, Y.Y. Sex hormone-mediated change on muscle activation deactivation dynamics in young eumenorrheic women. Front. Physiol. 2023, 14, 1104578. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Przepiórska, K.; Pietrzak, B.A.; Kajta, M. Emerging Evidence on Membrane Estrogen Receptors as Novel Therapeutic Targets for Central Nervous System Pathologies. Int. J. Mol. Sci. 2023, 24, 4043. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.L.; Ribas, V.; Moore, T.M.; Zhou, Z. ERα in the Control of Mitochondrial Function and Metabolic Health. Trends Mol. Med. 2021, 27, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]
- Hevener, A.L.; Ribas, V.; Moore, T.M.; Zhou, Z. The Impact of Skeletal Muscle ERα on Mitochondrial Function and Metabolic Health. Endocrinology. 2020, 161, bqz017. [Google Scholar] [CrossRef]
- Marin, R.; Canerina-Amaro, A.; Hernandez-Abad, L.G.; Ferrer, I.; Quinto-Alemany, D.; Mesa-Herrera, F.; Ferri, C.; Puertas-Avendaño, R.A.; Diaz, M. Lipid raft ER signalosome malfunctions in menopause and Alzheimer’s disease. Front. Biosci. 2017, 9, 111–126. [Google Scholar] [CrossRef]
- Das, N.; Ren, J.; Spence, J.; Chapman, S.B. Phosphate Brain Energy Metabolism and Cognition in Alzheimer’s Disease: A Spectroscopy Study Using Whole-Brain Volume-Coil 31Phosphorus Magnetic Resonance Spectroscopy at 7Tesla. Front Neurosci. 2021, 15, 641739. [Google Scholar] [CrossRef]
- Moolman, J.A. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc. Res. 2006, 69, 777–780. [Google Scholar] [CrossRef]
- Iotti, S.; Frassineti, C.; Alderighi, L.; Sabatini, A.; Vacca, A.; Barbiroli, B. In vivo (31)P-MRS assessment of cytosolic [Mg(2+)] in the human skeletal muscle in different metabolic conditions. Magn. Reson. Imaging 2000, 18, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Porri, D.; Biesalski, H.K.; Limitone, A.; Bertuzzo, L.; Cena, H. Effect of magnesium supplementation on women’s health and well-being. NFS J. 2021, 23, 30–36. [Google Scholar] [CrossRef]
- Sonu, Y.; Avinash, S.S.; Sreekantha Arun Kumar, K.; Malathi, M.; Shivashankara, A.R. Effect of Oestrogen on Altering the Serum and Urinary Levels of Calcium, Phosphate and Magnesium in Hysterectomised Women Compared to Natural Menopausal South Indian Women: A Case Control Study. Indian J. Clin. Biochem. 2016, 31, 326–331. [Google Scholar] [CrossRef] [PubMed]
- McNair, P.; Christiansen, C.; Transbøl, I. Effect of menopause and estrogen substitutional therapy on magnesium metabolism. Min. Electrolyte Metab. 1984, 10, 84–87. [Google Scholar]
- McCully, K.K.; Malucelli, E.; Iotti, S. Increase of free Mg2+ in the skeletal muscle of chronic fatigue syndrome patients. Dyn Med. 2006, 5, 1. [Google Scholar] [CrossRef]
- Chronic Fatigue Syndrome. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/chronic-fatigue-syndrome (accessed on 4 December 2023).
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Kirchberg, F.; Mori, T.A.; Huang, R.C.; Beilin, L.J.; Hellmuth, C.; Oddy, W.H. Lipidomics Reveals Associations of Phospholipids with Obesity and Insulin Resistance in Young Adults. J. Clin. Endocrinol. Metab. 2016, 101, 871–879. [Google Scholar] [CrossRef]
- Syme, C.; Czajkowski, S.; Shin, J.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Strug, L.; et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation 2016, 134, 1629–1636. [Google Scholar] [CrossRef]
- Liu, J.; de Vries, P.S.; Del Greco, M.F.; Johansson, Å.; Schraut, K.E.; Hayward, C.; van Dijk, K.W.; Franco, O.H.; Hicks, A.A.; Vitart, V.; et al. A multi-omics study of circulating phospholipid markers of blood pressure. Sci. Rep. 2022, 12, 574. [Google Scholar] [CrossRef] [PubMed]
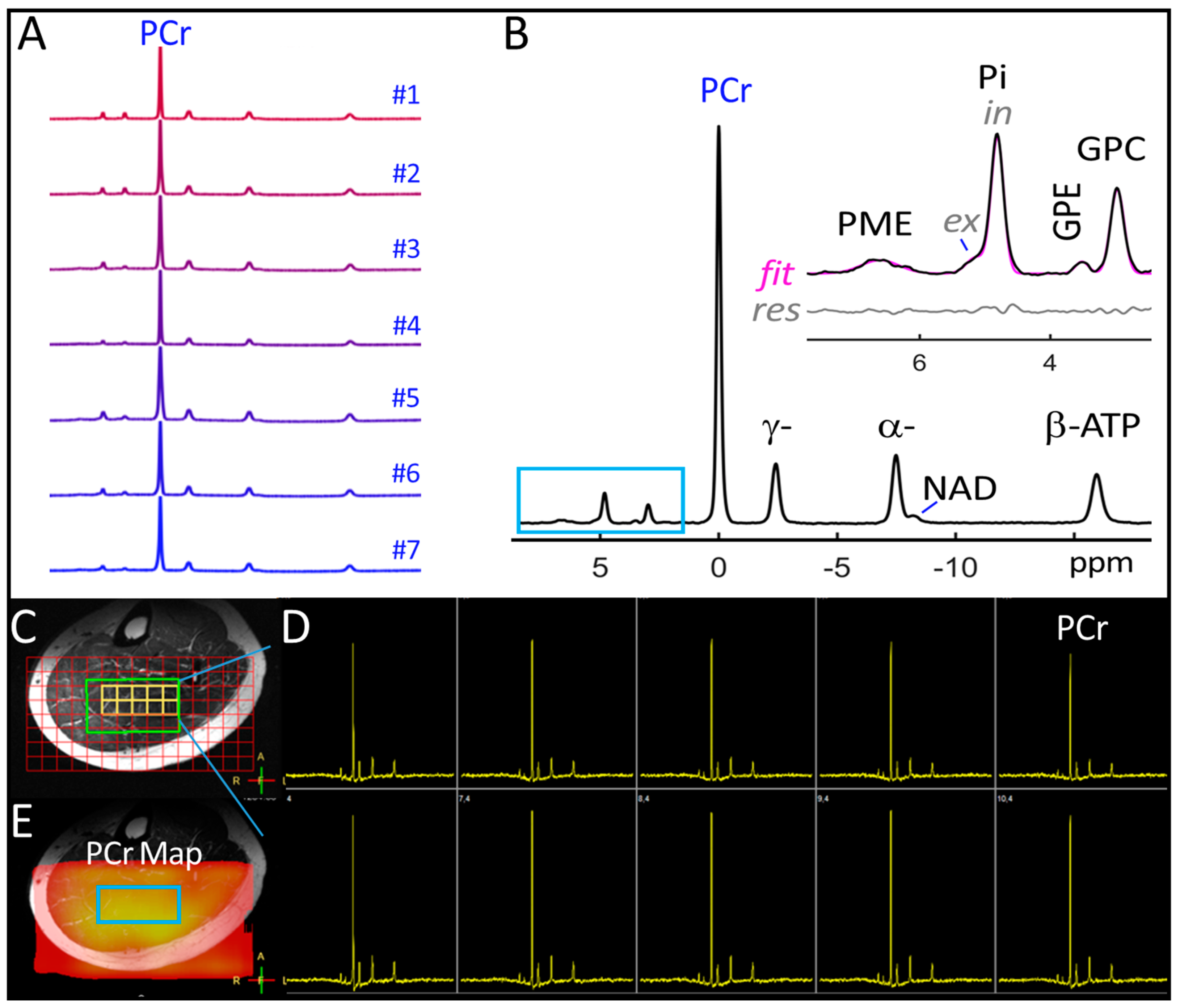
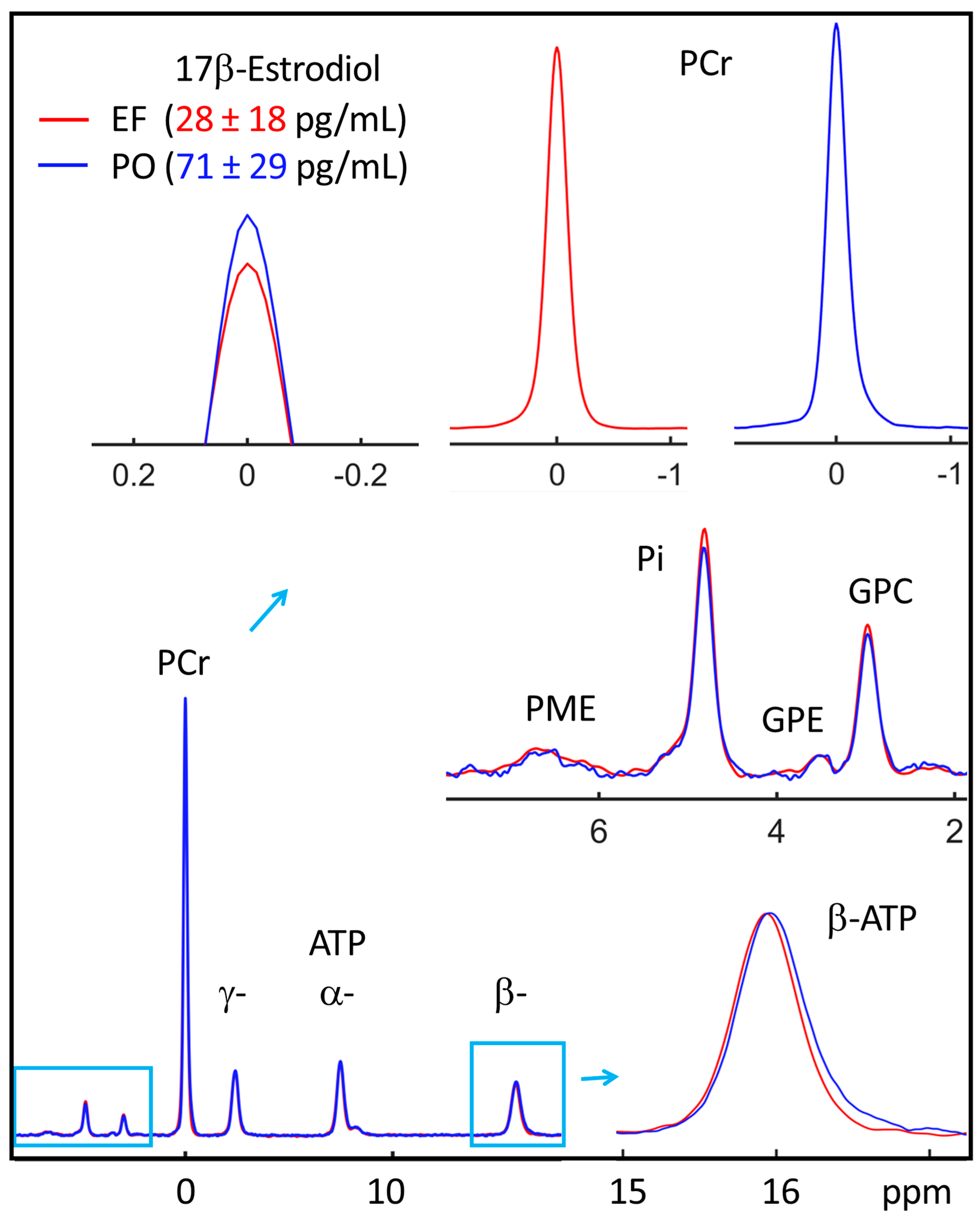

| Metabolite-to-ATP Ratio (a.u.) | ||||||
|---|---|---|---|---|---|---|
| δ (ppm) | EF | PO | ||||
| PME | 6.63 | ± 0.21 | 0.10 | ± 0.04 | 0.07 | ± 0.03 |
| Pi(ex) | 5.14 | ± 0.06 | 0.06 | ± 0.03 | 0.05 | ± 0.02 |
| Pi(in) | 4.81 | ± 0.02 | 0.30 | ± 0.08 | 0.31 | ± 0.09 |
| GPE | 3.51 | ± 0.04 | 0.03 | ± 0.01 | 0.03 | ± 0.01 |
| GPC | 2.97 | ± 0.01 | 0.19 | ± 0.10 | 0.20 | ± 0.10 |
| PCr | [0] | 3.82 | ± 0.18 | 4.05 | ± 0.18 * | |
| γ-ATP | −2.41 | ± 0.01 | [1.0] | [1.0] | ||
| α-ATP | −7.48 | ± 0.02 | 1.08 | ± 0.08 | 1.09 | ± 0.08 |
| NAD | −8.06 | ± 0.10 | 0.27 | ± 0.05 | 0.33 | ± 0.14 |
| β-ATP | −15.95 | ± 0.03 | 1.16 | ± 0.07 | 1.25 | ± 0.08 |
| pH | 6.983 | ± 0.014 | 6.990 | ± 0.018 | ||
| Mg (mM) | 0.68 | ± 0.08 | 0.60 | ± 0.08 | ||
| Metabolites | p-Value | r-Value |
|---|---|---|
| PME | 0.951 | −0.018 |
| Pi(ex) | 0.985 | −0.005 |
| Pi(in) | 0.332 | 0.280 |
| GPE | 0.625 | −0.143 |
| GPC | 0.766 | 0.088 |
| PCr | 0.014 * | 0.638 |
| γ-ATP | - | − |
| α-ATP | 0.295 | 0.301 |
| NAD | 0.458 | −0.216 |
| β-ATP | 0.158 | 0.398 |
| pH | 0.910 | 0.033 |
| Mg | 0.290 | −0.304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Rodriguez, L., II; Johnson, T.; Henning, A.; Dhaher, Y.Y. 17β-Estradiol Effects in Skeletal Muscle: A 31P MR Spectroscopic Imaging (MRSI) Study of Young Females during Early Follicular (EF) and Peri-Ovulation (PO) Phases. Diagnostics 2024, 14, 235. https://doi.org/10.3390/diagnostics14030235
Ren J, Rodriguez L II, Johnson T, Henning A, Dhaher YY. 17β-Estradiol Effects in Skeletal Muscle: A 31P MR Spectroscopic Imaging (MRSI) Study of Young Females during Early Follicular (EF) and Peri-Ovulation (PO) Phases. Diagnostics. 2024; 14(3):235. https://doi.org/10.3390/diagnostics14030235
Chicago/Turabian StyleRen, Jimin, Luis Rodriguez, II, Talon Johnson, Anke Henning, and Yasin Y. Dhaher. 2024. "17β-Estradiol Effects in Skeletal Muscle: A 31P MR Spectroscopic Imaging (MRSI) Study of Young Females during Early Follicular (EF) and Peri-Ovulation (PO) Phases" Diagnostics 14, no. 3: 235. https://doi.org/10.3390/diagnostics14030235
APA StyleRen, J., Rodriguez, L., II, Johnson, T., Henning, A., & Dhaher, Y. Y. (2024). 17β-Estradiol Effects in Skeletal Muscle: A 31P MR Spectroscopic Imaging (MRSI) Study of Young Females during Early Follicular (EF) and Peri-Ovulation (PO) Phases. Diagnostics, 14(3), 235. https://doi.org/10.3390/diagnostics14030235






