Abstract
Background and Clinical Significance: Sarcopenia, characterized by muscle loss and fat infiltration, poses a significant health burden for aging populations. Quantitative Color 2D and 3D radiodensitometry provides a powerful tool to monitor muscle quality and quantity through CT imaging. This study assessed the impact of a ten-year-long home-bed gym exercise intervention on muscle quality in an elderly subject using CT-derived radiodensitometric analysis. The study involved two comparative analyses: Study A, which compared knee-to-ankle CT scans of the subject between 2013 and 2023; and Study B, which compared the subject’s 2023 thigh CT scan with a cohort of 2500 elderly Icelandic individuals from the AGES-Reykjavik study. Case Presentation: A 70-year-old male began a home-based Full-Body In-Bed Gym exercise program in 2013. Quantitative muscle volume and radiodensity measurements were performed using CT at baseline and after ten years. Results: Study A shows significant improvements in muscle volume observed in the knee-to-ankle region, while a slower decline in radiodensity was noted, indicating substantial preservation of muscle quality despite the expected decay of ten-year aging. For instance, muscle volume increased by 15% in the left Soleus muscle and by 6% in the right Soleus muscle, while the average radiodensity decreased by 12–17 HU. The subject’s thigh muscle quality at 80-years-old is above the AGES-Reykjavik’s cohort average, with reduced fat infiltration. Conclusions: Long-term home Full-Body In-Bed Gym, a low-impact exercise, can mitigate aging sarcopenia, as evidenced by improved tissue radiodensity and muscle mass substantial preservation. This suggests potential applications in personalized healthcare strategies to enhance muscle preservation among aging populations.
1. Introduction
Sarcopenia, the age-related decline in muscle strength and mass, significantly impacts the elderly population’s quality of life, increasing risks of falls, frailty, and mortality [1].
Studies demonstrate that maintaining moderate to high levels of physical activity throughout life is the most effective countermeasure against age-related decline in muscle health and function [1]. However, a significant portion of the global population remains largely sedentary, a trend that only intensifies with age. This widespread lack of physical activity accelerates the natural decline associated with aging, contributing to conditions like sarcopenia, reduced mobility, and overall poorer quality of life in older adults [2].
As a result of the growing interest in diagnosing and monitoring sarcopenia, quantitative radiodensitometry using computed tomography (CT), which measures tissue density based on its attenuation of X-rays during a CT scan, has become a valuable and reliable tool. This technique allows for a detailed assessment of muscle quality, including muscle density and the degree of fat infiltration, providing critical insights into both muscle mass and structural integrity [3].
This study addresses a significant research gap by providing long-term evidence about the effectiveness of accessible home-based exercise programs, specifically the Full-Body In-Bed Gym, in mitigating sarcopenia in elderly populations. The Full-Body In-Bed Gym is a home-based exercise protocol designed to address muscle weakness and atrophy, particularly in aging or impaired populations [4,5,6]. Inspired by established cardiovascular and respiratory rehabilitation protocols for surgical patients, this regimen adapts those principles into a short (10–20 min) sequence of 10–15 simple, tool-free exercises that can be performed entirely in bed. The exercises include movements such as hand gripping, ankle flexion, arm and leg cycling, spinal stretching, and progressive weight-bearing activities, all aimed at enhancing muscle strength, mobility, and overall physical fitness. Starting with as few as 3–5 repetitions, participants gradually increase to 15–20 repetitions as they build endurance. Over time, the speed and intensity of exercises are adjusted to maximize their benefits.
The program has been shown to be effective in improving quality of life and reducing pain in elderly individuals, with participants reporting significant enhancements after two months of consistent practice. Furthermore, this program not only offers a practical method for preserving muscle mass and function in the elderly, but it also provides a significant exercise opportunity for economically disadvantaged populations, who may not be able to afford expensive equipment or gym memberships [4,5,6].
Despite the growing recognition of exercise as a key intervention to combat muscle loss, there is limited data on the sustained impact of such programs. Here, we explore the application of quantitative radiodensitometry paired with 2D and 3D imaging to acquire information about the average X-ray absorption properties of soft tissue and their distributions as Hounsfield Units (HU). Indeed, while quantitative radiodensitometry, including 2D and 3D imaging, has shown promise in assessing muscle health, its application in aging populations has not been well explored.
This case report aimed to fill these gaps by utilizing a 10-year longitudinal study with CT imaging to monitor muscle health outcomes, offering a cost-effective and accessible solution for preserving muscle mass and improving quality of life in older adults.
The aim of this case report was twofold: (1) to support the fact that the Full-Body In-Bed Gym program can combat sarcopenia; and (2) to provide further evidence supporting the effectiveness of 2D and 3D quantitative radiodensitometry as a monitoring tool for muscle health in aging populations.
2. Case Presentation
2.1. Study Design
This study focused on a 70-year-old male subject who undertook a home-based exercise regimen for over 10 years. The main objectives were to assess changes in muscle volume and quality over time using radiodensitometry. The study was divided into two parts: (1) Study A compared CT scans of the knee-to-ankle region from 2013 and then 2023; while (2) Study B compared data from a cross-section of a 2023 thigh CT scan with the data from a cohort of 2700 age-matched individuals from the AGES-Reykjavik study.
2.2. Intervention
The home-based Full-Body In-Bed Gym program employed here consisted of 10 exercises performed six times per week. The Full-Body In-Bed Gym program allows individuals to engage in various exercises from the comfort of their beds, making it particularly appealing for those with mobility limitations or those who prefer to exercise without getting out of bed. This program typically involves a series of low-impact exercises that can be performed while lying down, sitting, or even standing next to the bed. Exercises may include strength training and stretching activities. The subject performed 10 repetitions of each exercise starting in 2013, which he gradually increased in intensity and volume over time. Each session lasted between 5 and 15 min.
Figure 1 shows one of the ten home Full-Body In-Bed Gym protocol exercises. The figure was reprinted with permission from the reference Maccarone et al. 2023 [4].

Figure 1.
An example of the ten exercises which constitute the home-based Full-Body In-Bed Gym protocol.
2.3. Imaging Protocol
2.3.1. Study A: Knee-to-Ankle Comparison (2013 vs. 2023)
CT scans were conducted using a Philips iCT 256 scanner. Baseline scans from 2013 were compared to scans from 2023, focusing on the region from the knee to the ankle. Both scans were acquired at 120 kVp, with a voxel size of 0.665 mm and slice thickness of 0.335 mm. Radiodensity and muscle volume were measured using custom segmentation software, analyzing three major muscle groups: Tibialis anterior, Soleus, and Gastrocnemius muscles.
2.3.2. Study B: Thigh Comparison: Longitudinal Case Study (2013–2023) vs. AGES-Reykjavik Population [7,8]
Images were captured from the mid-thigh region using a voltage of 120 kVp. This study compared the 2023 CT scan of the subject’s thigh to those of a reference population of 2500 age-matched individuals from the AGES-Reykjavik cohort. Radiodensity was measured for fat (−200 to −10 HU), connective (−10 to 40 HU), and muscle (40 to 200 HU) tissues. The case study values were plotted against the AGES-Reykjavik cohort group to assess relative muscle quality and fat infiltration.
2.4. Medical History of the Octogenarian
The case study subject was a male, born in Abano Terme, Padua, Italy, on 23 February 1943. His medical history was collected at the beginning of the study, with the main pathological events including:
In 1969, the subject experienced a vehicular accident that resulted in fractures to both legs and ankles. A significant complication from the incident was the rupture of his spleen, which necessitated surgical removal to address the internal bleeding. He survived as a result of the intravenous infusion of fluids and blood from donors. Unfortunately, during his subsequent convalescence, he developed transfusion-induced hepatitis, which delayed the recovery of the skeletal lesions.
Over the next 40 years, he experienced only two episodes of viral flu and has not developed any COVID-19 infections to date. He has received five doses of anti-COVID vaccines.
Despite having no family history of hypertension and only passive smoking exposure, he was diagnosed with asymptomatic arterial hypertension at the age of 40. He delayed treatment, which ultimately resulted in severe coronary artery disease.
From his medical history recently collected at the Hospital of Rovigo, Italy, we report the following:
An 80-year-old man with a history of hypertension and mixed dyslipidemia, treated pharmacologically since age 45, began experiencing symptomatic effort angina at age 68. Coronary angiography revealed significant disease in the right coronary artery (RCA), left circumflex artery (LCx), and first diagonal branch (D1), leading to percutaneous coronary intervention (PCI) and a drug-eluting stent (DES) placement. Three years later, he developed a symptomatic popliteal artery aneurysm requiring surgical exclusion. At a 10-year follow-up, a transthoracic echocardiogram indicated chronic ischemic/hypertensive cardiomyopathy with preserved left ventricular function. Holter monitoring showed initial conduction system impairment, including first- and second-degree atrioventricular blocks and frequent ectopic beats. Follow-up coronary angiography demonstrated good patency of the previously implanted DES. His optimized outpatient cardiology regimen included aspirin 100 mg, Irbesartan 300 mg once a day, calcium antagonist 10 mg once a day, and rosuvastatin 10 mg.
Despite this strong evidence of a slowly progressive cardiomyopathy, the subject still performs all regular activities of daily living, engages in light voluntary exercise (home-based Full-Body In-Bed Gym), and undertakes heavy gardening work.
2.5. Quantitative Image Analysis
In Study A we compared volumetric CT data taken from knee-to-ankle joints (Figure 2).
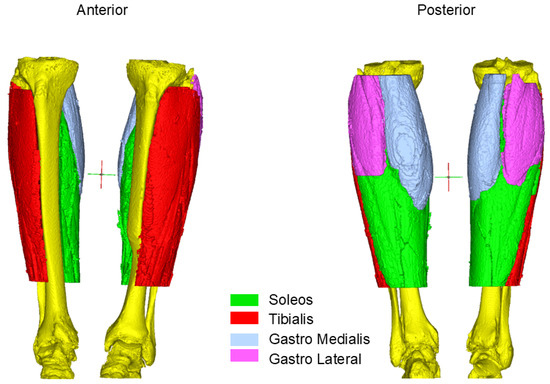
Figure 2.
Muscle segmentation of the Octogenarian: Different region of interest from the first time point in 2013 when he was 70-years-old.
CT images were analyzed using Mimics 26.0 segmentation software (Materialize, Belgium).
To assess the effect of the intervention, a 3D model of the calves was reconstructed from the two datasets. An initial thresholding between 0 and 150 HU was performed. To regularize the borders and eliminate floating pixels, a closing gap operation with a gap size of 4 pixels followed by an erosion of 2 pixels was performed. These operations resulted in a 3D model of the muscles in the calves, including the intramuscular fat and other tissues inside muscle bundles. The four principal muscles were separated with a manual splitting tool following their borders over the darker background, and the volume of each bundle was extracted. At this point, the histogram of each muscle was drawn, and the HU spanned from −200 to 200. The contribution of different tissue types was extracted: fat (−200 to −10 HU), connective tissue (−10 to 40 HU), and muscle (40 to 200 HU) [9]. The average HU was calculated and stored for further comparison.
In Study B, we analyzed a mid-thigh CT image cross-section of the Octogenarian (Figure 3). We calculated the average radiodensity of connective, muscle, and fat tissues, extracted with the same threshold introduced before (Figure 4), and compared it with age-matched controls from the AGES-Reykjavik cohort database. The amount of fat computed in this manner evaluated both intramuscular and subcutaneous fat. In the AGES cohort, we analyzed the same parameters from 2700 individuals aged 65 to 95 [7,8].
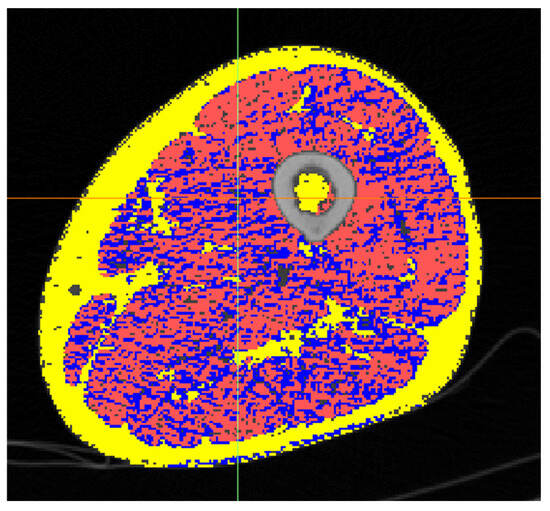
Figure 3.
Cross-section of the mid-thigh of the Octogenarian. Fat in yellow, connective in blue and muscle in red.
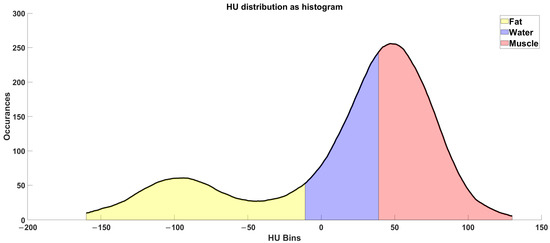
Figure 4.
Radio densiometric profile of the cross-section of the mid-thigh of the Octogenarian in 2023, where the areas of fat, connective, and muscle tissues are colored, respectively, in yellow, blue and red.
2.6. Results
2.6.1. Study A: Knee-to-Ankle Muscle Volume and Density Changes (2013 vs. 2023)
Data analysis revealed that muscle volume increased across all studied groups of muscles within the distal part of the leg, particularly in the Soleus and Tibialis anterior muscles. The left Soleus muscle showed a 15% increase, while the right Soleus muscle increased by 6% (Table 1). Despite the increases in muscle volume, the muscle radiodensity decreased (Table 2); however, this decline occurred at a slower pace than reported in the literature about the average muscle radiodensity decay that accompanies 10 years of aging (see discussion). Indeed, the radiodensity data revealed a moderate decline in muscle density and increased fat infiltration. Nonetheless, the fat infiltration of the subject remained lower than that reported in the literature [10,11,12,13,14,15,16,17,18,19,20].

Table 1.
Muscles bundle volumes and their percent change in response to the intervention.

Table 2.
Tissue radiodensity by muscular bundle before (rows 1–4) and after (rows 5–8) the intervention. Values in HU.
The analysis also revealed that total muscle volume increased in both legs over the ten years of intervention (15% left leg and 6% right leg). However, not all muscles underwent the same changes: left leg distal muscles (Soleus and Tibialis muscles) experienced an increase in volume, whereas on the right leg their volume slightly decreased (Table 1, rows 1 to 4). On the other hand, an opposite scenario was revealed for the Gastrocnemius muscles. The left volume decreased less than 10% in volume while the right Gastrocnemius muscle gained almost 65% in volume laterally (Table 1, rows 5 to 8). A graphical representation of these results is pictured in Figure 5.
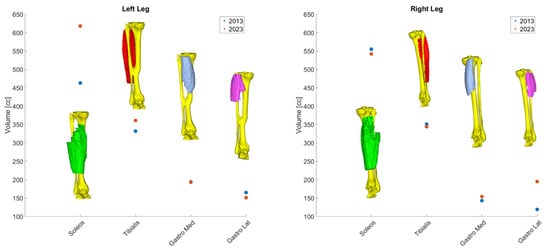
Figure 5.
Volumetric changes in muscular bundle by leg.
On the other hand, average muscle radiodensity increased from 12 to 17 HU, whereas the average fat and connective tissues radiodensity increased from 5 to 17 HU.
2.6.2. Study B: Thigh Muscle Quality Compared to AGES-Reykjavik Population
The radiodensitometric profile extracted from the cross-section of the midthigh is reported in Figure 6. The different tissues are highlighted with different colors. The extracted averages are 61.25 HU for muscle (red), 23.20 HU for connective tissue (blue), and −81.70 HU for fat (yellow).
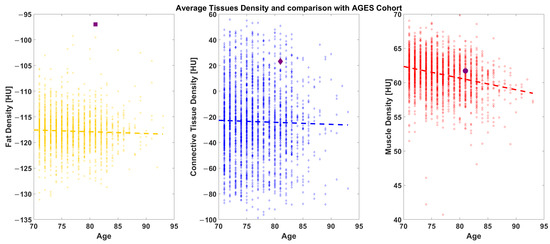
Figure 6.
AGES comparison: The Octogenarian (reported as a black symbol) is shown against a cohort of 2700 age-matched individuals ranging from 70 to 95-years-old.
Compared to the AGES-Reykjavik population, the Octogenarian’s 2023 thigh CT scan revealed a higher-than-average muscle density and reduced fat infiltration. Indeed, when the subject’s average values are plotted with that of the AGES population, the data place the subject above average in muscular density and at a lower subcutaneous fat density (Figure 6).
3. Discussion
This study demonstrated the effectiveness of a long-term, home-based exercise protocol in mitigating sarcopenia, as evidenced by the preservation of muscle volume and the reduced fat infiltration in the muscle of our exercising elderly subject. Quantitative radiodensitometry using CT imaging has proven to be a valuable tool for assessing long-term muscle quality and quantity.
The home-based Full-Body In-Bed Gym program demonstrated significant benefits in preserving muscle mass and quality. The subject was in his seventies (“Septuagenarian”) when the intervention began and is now in his eighties (“Octogenarian”). The unbalanced volume changes (i.e., differences in left and right legs), reported in Figure 4, may be a consequence of the right-side dominancy of the subject’s posture.
Additionally, the Full-Body In-Bed Gym program contributed to improved quality of life, reduced pain, and a decreased risk of sarcopenia in the elderly sedentary individual [4]. This suggests that similar exercise programs could be effective for a broader aging population. An added benefit of the home-based Full-Body In-Bed Gym is that this safe approach is highly cost-effective.
Compared to data in the literature, the subject maintained higher than average radiodensity values and better muscle quality, highlighting the potential of persevering low-medium intensity exercise to slow muscle decay associated with aging. Our findings are consistent with previous research, showing the efficacy of exercise interventions in combatting sarcopenia, whether due to aging and neuromuscular disorders [10,11,12,13,14,15,16,17,18,19,20], or tumors [21,22,23]. Specifically, quantitative radiodensitometry has proven to be a reliable method for assessing both muscle mass and quality, allowing for precise monitoring of age-related and exercise-related changes.
Muscle type also plays an important role in age-related muscle degeneration. Age-related atrophy has proven to affect various muscles fibers differently. For example, type II fibers, which are more prevalent in the locomotor muscles of the lower body (e.g., Gastrocnemius muscle), experience greater rates of atrophic change while only moderate changes occur among type I fibers, characteristic of the muscles involved in a postural role (e.g., Soleus muscle) [24,25,26,27]. On the other hand, hypoplasia has also been found to contribute partially to muscle mass reduction [24]. Some reports suggest that hypoplasia and atrophy contribute equally to muscle loss [27]. Indeed, studies have highlighted the critical importance of both processes, although there remains some controversy over which one plays the more significant role [28,29,30,31,32,33,34,35,36,37,38,39].
Similar results were found in a previous study: Ikezoe et al. (2011) found that muscle thickness, measured by ultrasound, decreases with aging based on the type of muscle, and that life-space assessment (LSA) correlates only with the Soleus muscle [40]. Reimers et al. (2012) demonstrated the impact of aging and athletic activity on a large, mixed cohort. They found that in athletes, calf thickness remained stable with age, although fat content increased. However, in non-athletic males, the thickness of the Gastrocnemius and Soleus muscles decreased significantly—by 16% between the ages of 20 and 70—while the Tibialis muscle showed only a 7% reduction [41]. On the other hand, Trappe et al. (1996) reported that elite distance runners and lesser trained/untrained subjects have the same calf cross-sectional area [42]. In addition, Klitgaard et al. (1990) found that elderly swimmers and runners had similar calf cross-sectional areas compared to an age-matched less active control group [43].
Exercise plays a crucial role in the prevention and management of sarcopenia. Numerous studies in the literature demonstrate that regular exercise programs, particularly resistance and strength training, can significantly reduce the risk of sarcopenia by improving muscle mass, strength, and mobility [44]. However, in low-income countries, where access to fitness facilities and equipment is limited, the challenge lies in implementing sustainable and accessible interventions [45,46,47,48,49,50]. Low-cost solutions, such as bodyweight exercises or home-based programs, have proven effective in promoting muscle health even in resource-limited settings [7,51,52,53]. Simple, safe, and adaptable protocols, such as the Full-Body In-Bed Gym, could serve as valuable strategies to combat sarcopenia and enhance quality of life, particularly in economically vulnerable populations.
Future research should expand on these results by studying more extensive cohorts and incorporating diverse exercise regimens to validate the benefits of such interventions further [2]. Additionally, the role of quantitative imaging in tracking muscle health warrants further exploration to enhance diagnostic and therapeutic strategies for sarcopenia.
4. Conclusions
CT-based quantitative radiodensitometry is a valuable method for tracking muscle health over time, particularly in aging populations. The Full-Body In-Bed Gym program, designed for home use, showed promising results in maintaining muscle mass and quality, which could contribute to improved quality of life and reduced sarcopenia risk among sedentary older adults. These findings highlight the potential for similar low-cost exercise programs to serve as practical and scalable interventions, benefiting both individual health outcomes and societal healthcare systems. Furthermore, quantitative radiodensitometry paired with 2D and 3D imaging is an excellent way to assess muscle quality and would be useful for diagnoses of muscle pathologies and follow-ups of muscle treatment regimes.
Author Contributions
U.C., P.G., M.Q. and S.M.; methodology, R.F., P.G., G.B., M.Q., T.B., A.M., B.R., S.Z., A.P., U.C., M.C.M. and S.M.; formal analysis, R.F., P.G., G.B., M.Q., T.B. and A.M.; investigation, R.F., P.G., G.B., M.Q., T.B., A.M., B.R., S.Z., A.P., U.C., M.C.M. and S.M.; data curation, R.F., P.G., G.B., M.Q., T.B. and A.M.; writing—original draft preparation, R.F., P.G., G.B., M.Q., T.B., A.M., U.C. and M.C.M.; writing—review and editing, R.F., P.G., G.B., M.Q., T.B., A.M., B.R., S.Z., A.P., U.C., M.C.M. and S.M.; visualization R.F., P.G., G.B., M.Q., T.B., A.M., U.C. and M.C.M.; supervision, U.C., P.G., M.Q. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Detailed data supporting the findings of this study can be obtained from the corresponding author.
Acknowledgments
We thank the A&C M-C Foundation for Translational Myology, Padua, Italy, and the Physical Medicine and Rehabilitation School, University of Padova, Padua, Italy, for their contribution to the publication expenses.
Conflicts of Interest
Author Aldo Morra was employed by the company Synlab IRCCS SDN S.p.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sánchez-Sánchez, J.L.; He, L.; Morales, J.S.; de Souto Barreto, P.; Jiménez-Pavón, D.; Carbonell-Baeza, A.; Casas-Herrero, Á.; Gallardo-Gómez, D.; Lucia, A.; Del Pozo Cruz, B.; et al. Association of physical behaviours with sarcopenia in older adults: A systematic review and meta-analysis of observational studies. Lancet Healthy Longev. 2024, 5, e108–e119. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Status Report on Physical Activity 2022. Available online: http://www.who.int/publications/i/item/9789240059153 (accessed on 25 October 2024).
- Ricciardi, C.; Edmunds, K.J.; Recenti, M.; Sigurdsson, S.; Gudnason, V.; Carraro, U.; Gargiulo, P. Assessing cardiovascular risks from a mid-thigh CT image: A tree-based machine learning approach using radiodensitometric distributions. Sci. Rep. 2020, 10, 2863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maccarone, M.C.; Caregnato, A.; Regazzo, G.; Carriero, A.; Casellato, G.; Finamoni, C.; Jirillo, R.; Laskova, O.; Marigo, E.; Sánchez, D.Y.; et al. Effects of the Full-Body in-Bed Gym program on quality of life, pain and risk of sarcopenia in elderly sedentary individuals: Preliminary positive results of a Padua prospective observational study. Eur. J. Transl. Myol. 2023, 33, 11780. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, M.; Baccaglini, T.; Morra, A. Quantitative 3D-CT imaging of sarcopenia mitigation in the elderly: Evidence from a case report. Eur. J. Transl. Myol. 2024, 34, 12715. [Google Scholar] [CrossRef]
- Carraro, U.; Alberty, M.S.; Anton, S.; Barbieri, E.; Bersch, I.; Bosco, G.; Coraci, D.; Gargiulo, P.; Gentil, P.; Gorgey, A.S.; et al. State of art of mobility medicine: Some more abstracts and evidence that the success of Pdm3 is based on extra-session relationships. Eur. J. Transl. Myol. 2024, 34, 12492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ziebart, C.; MacDermid, J.; Bryant, D.; Szekeres, M.; Suh, N. Hands Up Program: Results of a feasibility study of a randomized controlled trial of a bone health exercise and education program for adults aged 50–65 post distal radius fracture. PLoS ONE 2024, 19, e0313013. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Nitsche, M.A.; Ren, X.; Wang, D.; Wang, L. Top-down and bottom-up stimulation techniques combined with action observation treatment in stroke rehabilitation: A perspective. Front. Neurol. 2023, 14, 1156987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaabene, H.; Prieske, O.; Herz, M.; Moran, J.; Höhne, J.; Kliegl, R.; Ramirez-Campillo, R.; Behm, D.G.; Hortobágyi, T.; Granacher, U. Home-based exercise programmes improve physical fitness of healthy older adults: A PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Res. Rev. 2021, 67, 101265. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, R.; Thorgeirsson, G.; Einarsson, H.; Ólafsson, Ö.; Aspelund, T.; Harris, T.B.; Launer, L.; Gudnason, V. Prevalence of heart failure in the elderly and future projections: The AGES-Reykjavík study. Scand Cardiovasc. J. 2017, 51, 183–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edmunds, K.J.; Okonkwo, O.C.; Sigurdsson, S.; Lose, S.R.; Gudnason, V.; Carraro, U.; Gargiulo, P. Soft tissue radiodensity parameters mediate the relationship between self-reported physical activity and lower extremity function in AGES-Reykjavík participants. Sci. Rep. 2021, 11, 20173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edmunds, K.; Gíslason, M.; Sigurðsson, S.; Guðnason, V.; Harris, T.; Carraro, U.; Gargiulo, P. Advanced quantitative methods in correlating sarcopenic muscle degeneration with lower extremity function biometrics and comorbidities. PLoS ONE 2018, 13, e0193241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallagher, D.; Kuznia, P.; Heshka, S.; Albu, J.; Heymsfield, S.B.; Goodpaster, B.; Visser, M.; Harris, T.B. Adipose tissue in muscle: A novel depot similar in size to visceral adipose tissue. Am. J. Clin. Nutr. 2005, 81, 903–910. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carraro, U.; Edmunds, K.J.; Gargiulo, P. 3D False Color Computed Tomography for Diagnosis and Follow-Up of Permanent Denervated Human Muscles Submitted to Home-Based Functional Electrical Stimulation. Eur. J. Transl. Myol. 2015, 25, 5133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Buckinx, F.; Reginster, J.Y.; Dardenne, N.; Croisiser, J.L.; Kaux, J.F.; Beaudart, C.; Slomian, J.; Bruyère, O. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: A cross-sectional study. BMC Musculoskelet. Disord. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boutin, R.D.; Yao, L.; Canter, R.J.; Lenchik, L. Sarcopenia: Current Concepts and Imaging Implications. Am. J. Roentgenol. 2015, 205, W255–W266. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.; Edmunds, K.; Levi, C.; Lin, L.; Cheng, X.; Aviv, R.; Kleinig, T.; Butcher, K.; Zhang, J.; Parsons, M.; et al. Cost-effectiveness of targeted thrombolytic therapy for stroke patients using multi-modal CT compared to usual practice. PLoS ONE 2018, 13, e0206203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Recenti, M.C.; Edmunds, K.J.; Gislason, M.K.; Sigurdsson, S.; Carraro, U.; Gargiulo, P. Healthy Aging Within an Image: Using Muscle Radiodensitometry and Lifestyle Factors to Predict Diabetes and Hypertension. IEEE J. Biomed. Health Inform. 2021, 25, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Oba, H.; Matsui, Y.; Arai, H.; Watanabe, T.; Iida, H.; Mizuno, T.; Yamashita, S.; Ishizuka, S.; Suzuki, Y.; Hiraiwa, H.; et al. Evaluation of muscle quality and quantity for the assessment of sarcopenia using mid-thigh computed tomography: A cohort study. BMC Geriatr. 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, Y.W.; Hong, N.; Na, J.C.; Han, W.K.; Rhee, Y. Computed Tomography-Derived Skeletal Muscle Radiodensity Is an Early, Sensitive Marker of Age-Related Musculoskeletal Changes in Healthy Adults. Endocrinol. Metab. 2021, 36, 1201–1210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heymsfield, S.B.; Adamek, M.; Gonzalez, M.C.; Jia, G.; Thomas, D.M. Assessing skeletal muscle mass: Historical overview and state of the art. J. Cachexia Sarcopenia Muscle 2014, 5, 9–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mortellaro, S.; Triggiani, S.; Mascaretti, F.; Galloni, M.; Garrone, O.; Carrafiello, G.; Ghidini, M. Quantitative and Qualitative Radiological Assessment of Sarcopenia and Cachexia in Cancer Patients: A Systematic Review. J. Pers. Med. 2024, 14, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J.; Vandervoort, A.A.; Brown, W.F. Effects of ageing on the motor unit: A brief review. Can. J. Appl. Physiol. 1993, 18, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Grogan, B.F.; Hsu, J.R.; Skeletal Trauma Research Consortium. Volumetric muscle loss. J. Am. Acad. Orthop. Surg. 2011, 19 (Suppl. S1), S35–S37. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Pellegrino, M.A.; Bottinelli, R.; Franchi, M.V.; Narici, M.V. Loss of neuromuscular junction integrity and muscle atrophy in skeletal muscle disuse. Ageing Res. Rev. 2023, 83, 101810. [Google Scholar] [CrossRef] [PubMed]
- Minetto, M.A.; Busso, C.; Gamerro, G.; Lalli, P.; Massazza, G.; Invernizzi, M. Quantitative assessment of volumetric muscle loss: Dual-energy X-ray absorptiometry and ultrasonography. Curr. Opin. Pharmacol. 2021, 57, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.; Prisby, R.; Varanasi, V.; Zhou, J.; Pan, Z.; Brotto, M. Old and new biomarkers for volumetric muscle loss. Curr. Opin. Pharmacol. 2021, 59, 61–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corona, B.T.; Wenke, J.C.; Ward, C.L. Pathophysiology of Volumetric Muscle Loss Injury. Cells Tissues Organs 2016, 202, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Dirks, M.L.; van Loon, L.J. Skeletal muscle atrophy during short-term disuse: Implications for age-related sarcopenia. Ageing Res. Rev. 2013, 12, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.D.; Harder, T.; Saxe, H. Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: An ultrasound study. J. Neurol. Sci. 1998, 159, 60–66, Erratum in J. Neurol. Sci. 1999, 162, 211. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikezoe, T.; Mori, N.; Nakamura, M.; Ichihashi, N. Age-related muscle atrophy in the lower extremities and daily physical activity in elderly women. Arch. Gerontol. Geriatr. 2011, 53, e153–e157. [Google Scholar] [CrossRef]
- Reimers, C.D.; Knapp, G.; Reimers, A.K. Does Physical Activity Increase Life Expectancy? A Review of the Literature. J. Aging Res. 2012, 2012, 243958. [Google Scholar] [CrossRef]
- Trappe, S.W.; Costill, D.L.; Goodpaster, B.H.; Pearson, D.R. Calf muscle strength in former elite distance runners. Scand. J. Med. Sci. Sports 1996, 6, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, H.; Mantoni, M.; Schiaffino, S.; Ausoni, S.; Gorza, L.; Laurent-Winter, C.; Schnohr, P.; Saltin, B. Function, morphology and protein expression of ageing skeletal muscle: A cross-sectional study of elderly men with different training backgrounds. Acta Physiol. Scand. 1990, 140, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, L.; Williams, J.; Townsend, N.; Mikkelsen, B.; Roberts, N.; Foster, C.; Wickramasinghe, K. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: A systematic review. Lancet Glob. Health 2017, 5, e277–e289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velez, M.; Lugo-Agudelo, L.H.; Patiño Lugo, D.F.; Glenton, C.; Posada, A.M.; Mesa Franco, L.F.; Negrini, S.; Kiekens, C.; Spir Brunal, M.A.; Roberg, A.B.; et al. Factors that influence the provision of home-based rehabilitation services for people needing rehabilitation: A qualitative evidence synthesis. Cochrane Database Syst. Rev. 2023, 2, CD014823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahmood, A.; Nayak, P.; English, C.; Deshmukh, A.; Shashikiran, U.; Manikandan, N.; Solomon, J.M. Adherence to home exercises and rehabilitation (ADHERE) after stroke in low-to-middle-income countries: A randomized controlled trial. Top. Stroke Rehabil. 2022, 29, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Kreiger, N. Nutrition and physical activity interventions for low-income populations. Can. J. Diet Pract. Res. 2007, 68, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Gothi, D.; Joshi, J.M. Pulmonary rehabilitation in resource poor settings. Indian J. Chest Dis. Allied Sci. 2011, 53, 163–172. [Google Scholar] [PubMed]
- Vlachopoulos, S.P. Contextual Measurement of Sources of Exercise Amotivation: The Revised Amotivation Toward Exercise Scale-2. J. Sport Exerc. Psychol. 2024, 46, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Mittaz Hager, A.G.; Mathieu, N.; Lenoble-Hoskovec, C.; Swanenburg, J.; de Bie, R.; Hilfiker, R. Effects of three home-based exercise programmes regarding falls, quality of life and exercise-adherence in older adults at risk of falling: Protocol for a randomized controlled trial. BMC Geriatr. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Adjetey, C.; Karnon, B.; Falck, R.S.; Balasubramaniam, H.; Buschert, K.; Davis, J.C. Cost-effectiveness of exercise versus multimodal interventions that include exercise to prevent falls among community-dwelling older adults: A systematic review and meta-analysis. Maturitas 2023, 169, 16–31. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).