Assessing Patient Health Dynamics by Comparative CT Analysis: An Automatic Approach to Organ and Body Feature Evaluation
Abstract
1. Introduction
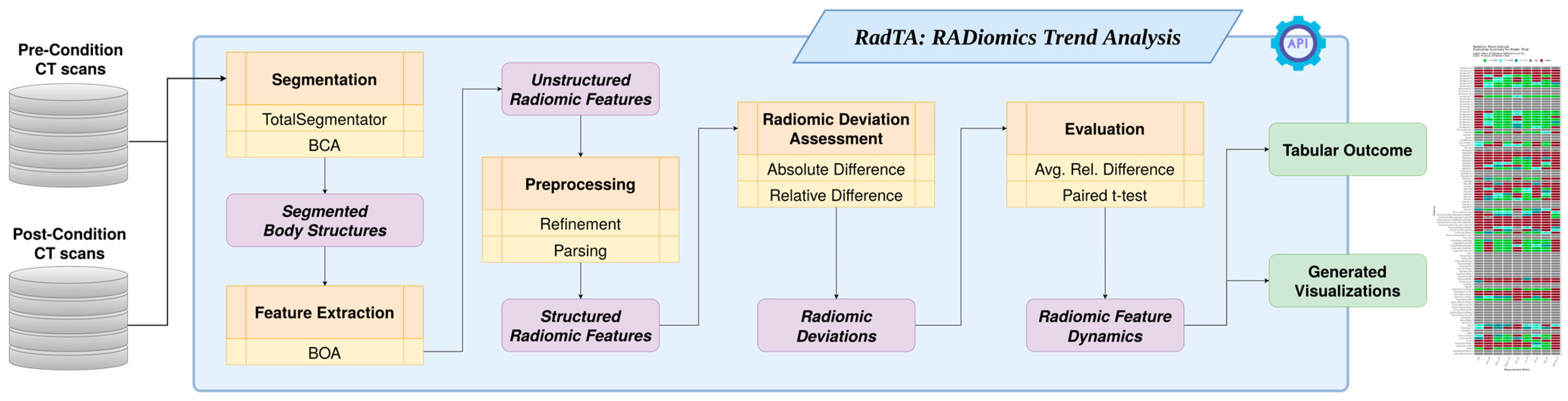
2. Materials and Methods
2.1. Command Line Interface
2.2. Structure Segmentation
2.3. Feature Extraction
2.4. Preprocessing
2.5. Radiomic Deviation Assessment
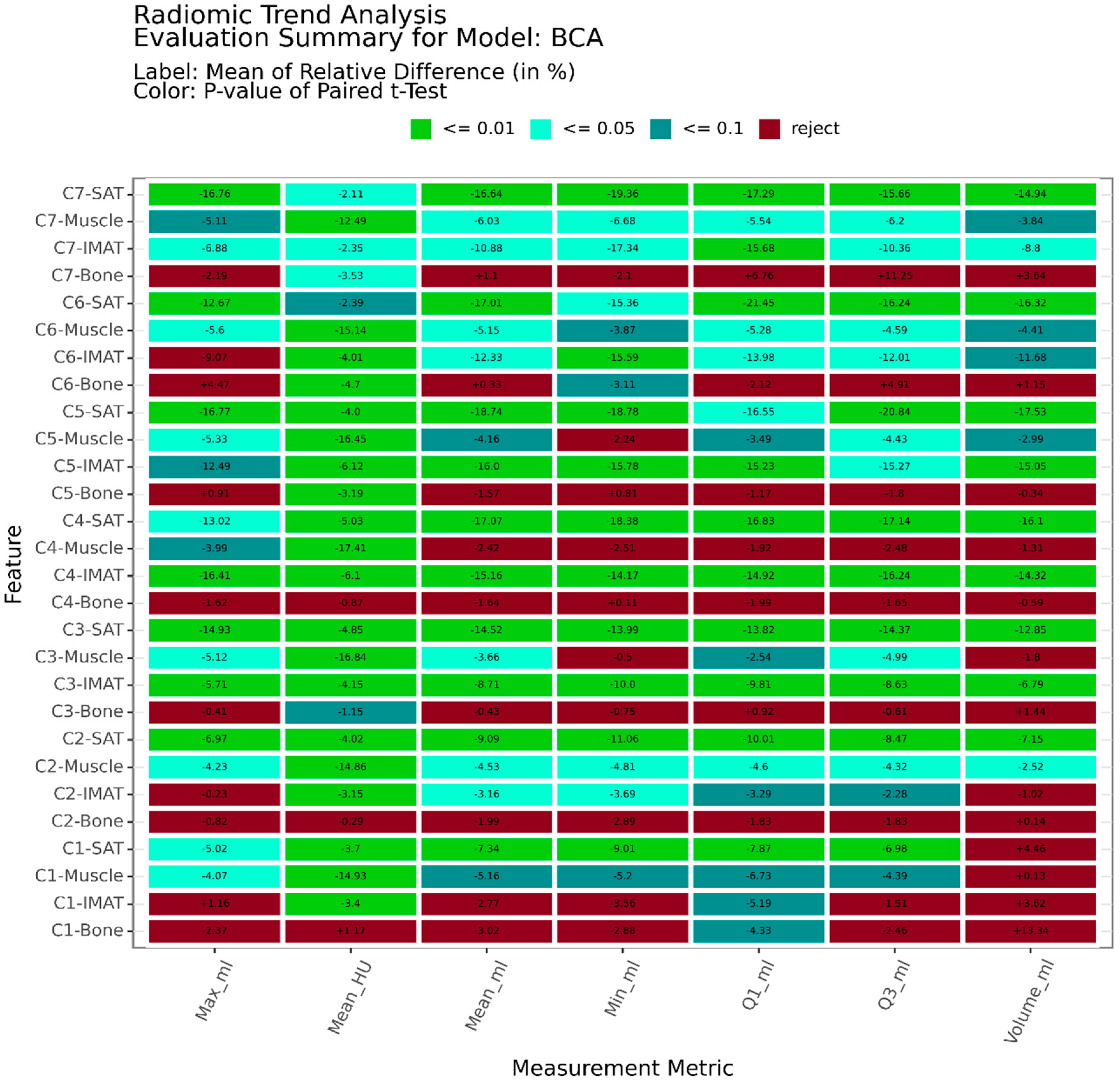
2.6. Evaluation
2.7. Use Case: Radiomic Health Dynamics in Head and Neck Squamous Cell Carcinoma
3. Results
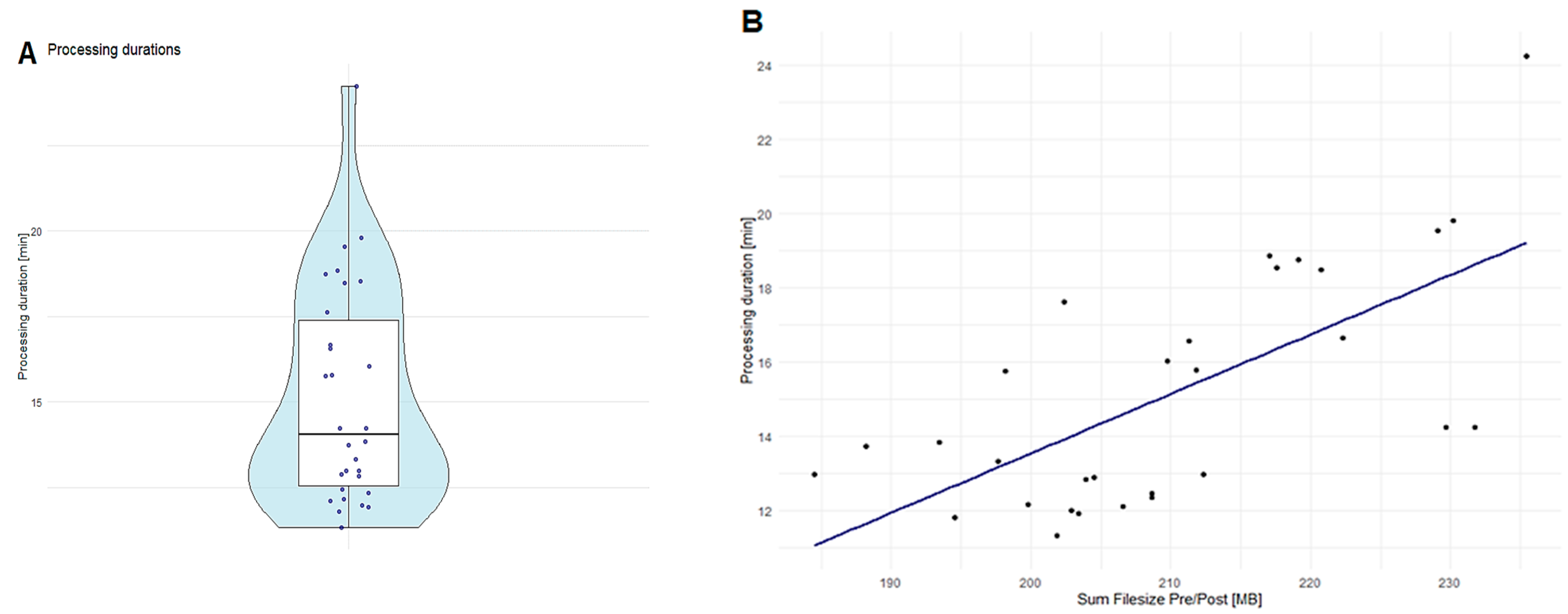
Runtime Performance Analysis of RadTA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in Health and Medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; De Jong, E.E.C.; Van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.S.; Jacobs, M.A. Deep Learning and Radiomics in Precision Medicine. Expert Rev. Precis. Med. Drug Dev. 2019, 4, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yang, P.F.; Chen, S.; Shao, Y.Y.; Xu, L.; Wu, Y.; Teng, W.; Zhou, X.Z.; Li, B.H.; Luo, C.; et al. A Delta-Radiomics Model for Preoperative Evaluation of Neoadjuvant Chemotherapy Response in High-Grade Osteosarcoma. Cancer Imaging 2020, 20, 7. [Google Scholar] [CrossRef]
- Kirienko, M.; Cozzi, L.; Rossi, A.; Voulaz, E.; Antunovic, L.; Fogliata, A.; Chiti, A.; Sollini, M. Ability of FDG PET and CT Radiomics Features to Differentiate between Primary and Metastatic Lung Lesions. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1649–1660. [Google Scholar] [CrossRef]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and Artificial Intelligence for Precision Medicine in Lung Cancer Treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef]
- Xue, C.; Zhou, Q.; Xi, H.; Zhou, J. Radiomics: A Review of Current Applications and Possibilities in the Assessment of Tumor Microenvironment. Diagn. Interv. Imaging 2023, 104, 113–122. [Google Scholar] [CrossRef]
- Lafata, K.J.; Wang, Y.; Konkel, B.; Yin, F.F.; Bashir, M.R. Radiomics: A Primer on High-Throughput Image Phenotyping. Abdom. Radiol. 2022, 47, 2986–3002. [Google Scholar] [CrossRef]
- Pereyra Pietri, M.; Farina, J.M.; Mahmoud, A.K.; Scalia, I.G.; Galasso, F.; Killian, M.E.; Suppah, M.; Kenyon, C.R.; Koepke, L.M.; Padang, R.; et al. The Prognostic Value of Artificial Intelligence to Predict Cardiac Amyloidosis in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Eur. Heart J. Digit. Health 2024, 5, 295–302. [Google Scholar] [CrossRef]
- Yip, S.S.F.; Liu, Y.; Parmar, C.; Li, Q.; Liu, S.; Qu, F.; Ye, Z.; Gillies, R.J.; Aerts, H.J.W.L. Associations between Radiologist-Defined Semantic and Automatically Computed Radiomic Features in Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 3519. [Google Scholar] [CrossRef]
- Kessler, L.G.; Barnhart, H.X.; Buckler, A.J.; Choudhury, K.R.; Kondratovich, M.V.; Toledano, A.; Guimaraes, A.R.; Filice, R.; Zhang, Z.; Sullivan, D.C. The Emerging Science of Quantitative Imaging Biomarkers Terminology and Definitions for Scientific Studies and Regulatory Submissions. Stat. Methods Med. Res. 2015, 24, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Schreiweis, B.; Pobiruchin, M.; Strotbaum, V.; Suleder, J.; Wiesner, M.; Bergh, B. Barriers and Facilitators to the Implementation of EHealth Services: Systematic Literature Analysis. J. Med. Internet Res. 2019, 21, e14197. [Google Scholar] [CrossRef] [PubMed]
- Müller, D. GitHub—Frankkramer-Lab/RadTA: RADiomics Trend Analysis for CT Scans. Available online: https://github.com/frankkramer-lab/RadTA (accessed on 24 November 2024).
- Wasserthal, J.; Breit, H.C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust Segmentation of 104 Anatomic Structures in CT Images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef] [PubMed]
- Koitka, S.; Kroll, L.; Malamutmann, E.; Oezcelik, A.; Nensa, F. Fully Automated Body Composition Analysis in Routine CT Imaging Using 3D Semantic Segmentation Convolutional Neural Networks. Eur. Radiol. 2021, 31, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. NnU-Net: A Self-Configuring Method for Deep Learning-Based Biomedical Image Segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Haubold, J.; Baldini, G.; Parmar, V.; Schaarschmidt, B.M.; Koitka, S.; Kroll, L.; Van Landeghem, N.; Umutlu, L.; Forsting, M.; Nensa, F.; et al. BOA: A CT-Based Body and Organ Analysis for Radiologists at the Point of Care. Investig. Radiol. 2024, 59, 433–441. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Bejarano, T.; De Ornelas-Couto, M. Head-and-Neck Squamous Cell Carcinoma Patients with CT Taken during Pre-Treatment, Mid-Treatment, and Post-Treatment (HNSCC-3DCT-RT) [Data Set]. Cancer Imaging Arch. 2018. [Google Scholar] [CrossRef]
- Wadden, J.J. Defining the Undefinable: The Black Box Problem in Healthcare Artificial Intelligence. J. Med. Ethics 2021, 48, 764–768. [Google Scholar] [CrossRef]
- Salmanpour, M.R.; Hosseinzadeh, M.; Rezaeijo, S.M.; Rahmim, A. Fusion-Based Tensor Radiomics Using Reproducible Features: Application to Survival Prediction in Head and Neck Cancer. Comput. Methods Programs Biomed. 2023, 240, 107714. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Leventis, D.; Spanakis, K.; Karantanas, A.H.; Kranioti, E.F. Post-Mortem CT Radiomics for the Prediction of Time since Death. Eur. Radiol. 2023, 33, 8387–8395. [Google Scholar] [CrossRef] [PubMed]
- Abler, D.; Schaer, R.; Oreiller, V.; Verma, H.; Reichenbach, J.; Aidonopoulos, O.; Evéquoz, F.; Jreige, M.; Prior, J.O.; Depeursinge, A. QuantImage v2: A Comprehensive and Integrated Physician-Centered Cloud Platform for Radiomics and Machine Learning Research. Eur. Radiol. Exp. 2023, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Multiple Significance Tests: The Bonferroni Correction. BMJ 2012, 344, e509. [Google Scholar] [CrossRef]
- Ulrich, H.; Anywar, M.; Kinast, B.; Schreiweis, B. Large-Scale Standardized Image Integration for Secondary Use Research Projects. In Studies in Health Technology and Informatics; IOS Press BV: Amsterdam, The Netherlands, 2024; Volume 310, pp. 174–178. [Google Scholar]

| Characteristic | Median (Q1, Q3); n (%) | |
|---|---|---|
| Patients | 31 (100%) | |
| Age | 65 (55, 71) | |
| Sex | ||
| F | 8 (26%) | |
| M | 23 (74%) | |
| Cancer staging | ||
| I | 2 (6.5%) | |
| II | 4 (13%) | |
| III | 9 (29%) | |
| IVA | 16 (52%) | |
| PTV volume (cm3) | 133 (40, 261) | |
| Tumor location | ||
| Larynx | 5 (16%) | |
| Nose | 3 (9.7%) | |
| Other | 8 (26%) | |
| Tongue | 11 (35%) | |
| Tonsil | 4 (13%) | |
| Performance status | ||
| ECOG 0 | 12 (39%) | |
| ECOG 1–2 | 4 (13%) | |
| None | 15 (48%) | |
| Chemotherapy medication | ||
| Cetuximab only | 2 (6.5%) | |
| Cetuximab + Cis/Carboplatin | 3 (9.7%) | |
| Cis/Carboplatin only | 14 (45%) | |
| None | 12 (39%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, D.; Voran, J.C.; Macedo, M.; Hartmann, D.; Lind, C.; Frank, D.; Schreiweis, B.; Kramer, F.; Ulrich, H. Assessing Patient Health Dynamics by Comparative CT Analysis: An Automatic Approach to Organ and Body Feature Evaluation. Diagnostics 2024, 14, 2760. https://doi.org/10.3390/diagnostics14232760
Müller D, Voran JC, Macedo M, Hartmann D, Lind C, Frank D, Schreiweis B, Kramer F, Ulrich H. Assessing Patient Health Dynamics by Comparative CT Analysis: An Automatic Approach to Organ and Body Feature Evaluation. Diagnostics. 2024; 14(23):2760. https://doi.org/10.3390/diagnostics14232760
Chicago/Turabian StyleMüller, Dominik, Jakob Christoph Voran, Mário Macedo, Dennis Hartmann, Charlotte Lind, Derk Frank, Björn Schreiweis, Frank Kramer, and Hannes Ulrich. 2024. "Assessing Patient Health Dynamics by Comparative CT Analysis: An Automatic Approach to Organ and Body Feature Evaluation" Diagnostics 14, no. 23: 2760. https://doi.org/10.3390/diagnostics14232760
APA StyleMüller, D., Voran, J. C., Macedo, M., Hartmann, D., Lind, C., Frank, D., Schreiweis, B., Kramer, F., & Ulrich, H. (2024). Assessing Patient Health Dynamics by Comparative CT Analysis: An Automatic Approach to Organ and Body Feature Evaluation. Diagnostics, 14(23), 2760. https://doi.org/10.3390/diagnostics14232760






